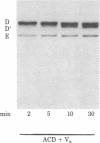
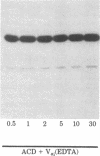
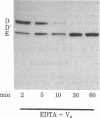
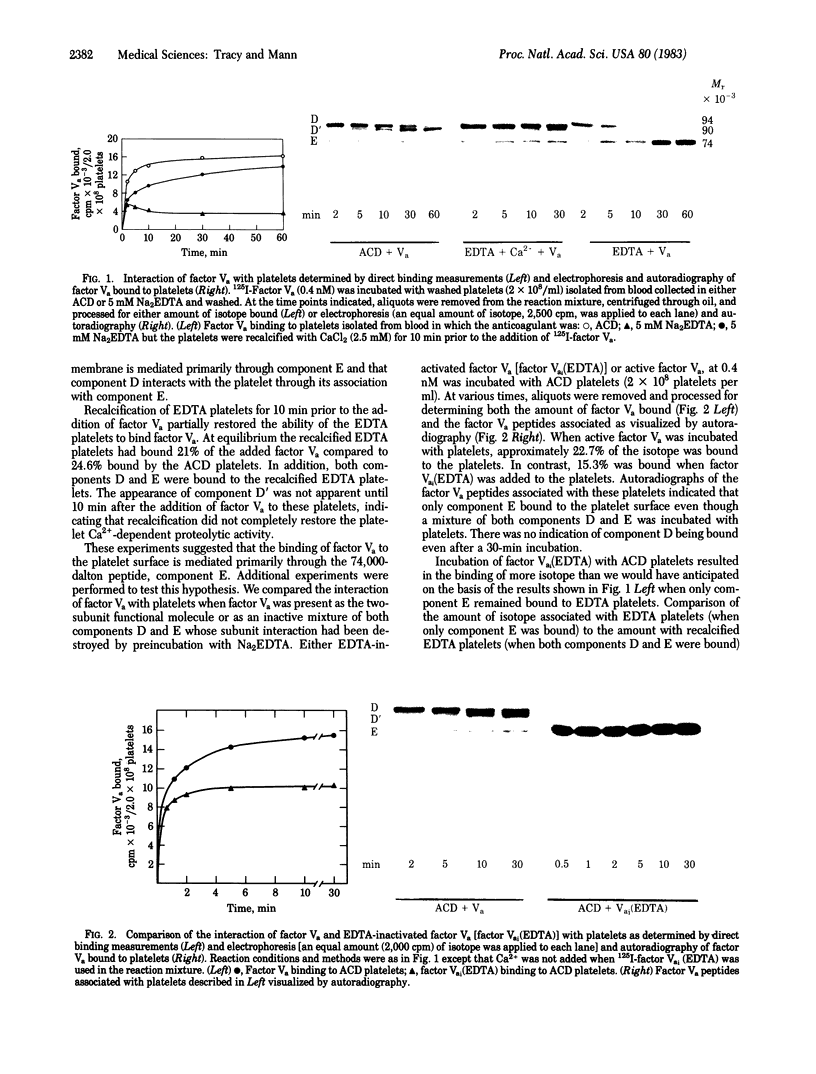
Abstract
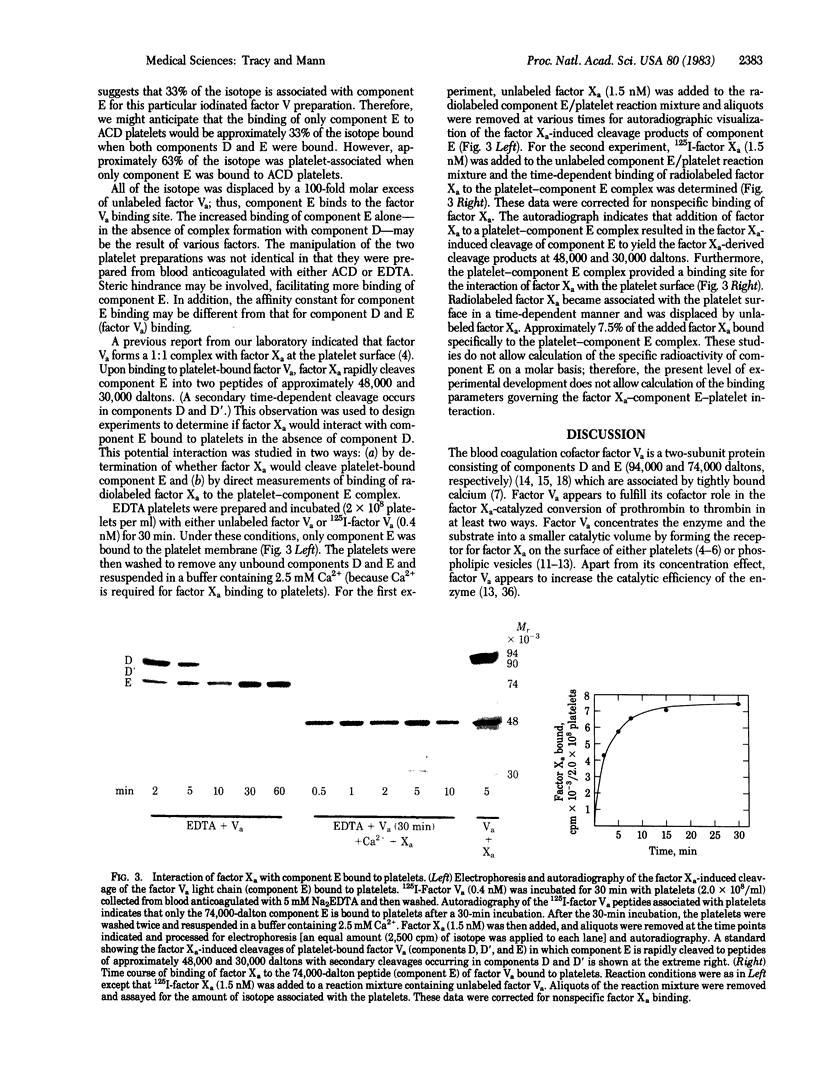
The blood coagulation protein factor Va forms the receptor for the serine protease factor Xa on the platelet surface. This membrane-bound complex of factor Va and factor Xa plus Ca2+ comprises the prothrombinase complex, the enzyme that catalyzes the proteolytic conversion of prothrombin to the clotting enzyme thrombin. Factor Va is a two-subunit protein composed of component D (Mr = 94,000) and component E (Mr = 74,000); subunit interaction is Ca2+ dependent. Factor Va bound to platelets consists of three peptides: component D, component E, and component D'(Mr = 90,000) which appears as the result of a platelet-associated protease cleavage of component D. The present studies were undertaken to determine which peptide(s) mediates the binding of factor Va to the platelet membrane surface and which peptide(s) serves as the binding site for factor Xa. These interactions were assessed by direct measurements of radiolabeled factor Va and factor Xa binding to platelets as well as autoradiographic visualization of the factor Va peptides associated with the platelet. Experiments were performed to determine the interaction of components D and E with platelets under reaction conditions in which components D and E were present as either the intact, functional two-subunit protein or as nonfunctional discrete peptides dissociated by the addition of Na2EDTA. The results suggest that component E mediates the binding of factor Va to the platelet and also serves as the binding site for the interaction of factor Xa with platelet-bound factor Va.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj S. P., Mann K. G. Simultaneous purification of bovine prothrombin and factor X. Activation of prothrombin by trypsin-activated factor X. J Biol Chem. 1973 Nov 25;248(22):7729–7741. [PubMed] [Google Scholar]
- Bloom J. W., Nesheim M. E., Mann K. G. Phospholipid-binding properties of bovine factor V and factor Va. Biochemistry. 1979 Oct 2;18(20):4419–4425. doi: 10.1021/bi00587a023. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K. Basic mechanisms in blood coagulation. Annu Rev Biochem. 1975;44:799–829. doi: 10.1146/annurev.bi.44.070175.004055. [DOI] [PubMed] [Google Scholar]
- Downing M. R., Butkowski R. J., Clark M. M., Mann K. G. Human prothrombin activation. J Biol Chem. 1975 Dec 10;250(23):8897–8906. [PubMed] [Google Scholar]
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–973. [PubMed] [Google Scholar]
- Fujikawa K., Davie E. W. Bovine factor X (Stuart factor). Methods Enzymol. 1976;45:89–95. doi: 10.1016/s0076-6879(76)45013-9. [DOI] [PubMed] [Google Scholar]
- Heldebrant C. M., Butkowski R. J., Bajaj S. P., Mann K. G. The activation of prothrombin. II. Partial reactions, physical and chemical characterization of the intermediates of activation. J Biol Chem. 1973 Oct 25;248(20):7149–7163. [PubMed] [Google Scholar]
- Hibbard L. S., Mann K. G. The calcium-binding properties of bovine factor V. J Biol Chem. 1980 Jan 25;255(2):638–645. [PubMed] [Google Scholar]
- Howard J. B., Nelsestuen G. L. Isolation and characterization of vitamin K-dependent region of bovine blood clotting factor X. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1281–1285. doi: 10.1073/pnas.72.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. M., Johnson T. F., Hanahan D. J. Studies on bovine factor X. I. Large-sclae purification of the bovine plasma protein possessing factor X activity. Biochemistry. 1968 Dec;7(12):4492–4505. doi: 10.1021/bi00852a046. [DOI] [PubMed] [Google Scholar]
- Kane W. H., Majerus P. W. The interaction of human coagulation factor Va with platelets. J Biol Chem. 1982 Apr 10;257(7):3963–3969. [PubMed] [Google Scholar]
- Kane W. H., Mruk J. S., Majerus P. W. Activation of coagulation factor V by a platelet protease. J Clin Invest. 1982 Nov;70(5):1092–1100. doi: 10.1172/JCI110697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad R. L., Uhteg L. C., Vogel C. N., Kingdon H. S., Mann K. G. Preparation and partial characterization of two forms of bovine thrombin. Biochem Biophys Res Commun. 1975 Sep 16;66(2):482–489. doi: 10.1016/0006-291x(75)90536-7. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Nesheim M. E., Tracy P. B. Molecular weight of undegraded plasma factor V. Biochemistry. 1981 Jan 6;20(1):28–33. doi: 10.1021/bi00504a005. [DOI] [PubMed] [Google Scholar]
- Mann K. G. Prothrombin. Methods Enzymol. 1976;45:123–156. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Kane W. H., Hofmann S. L., Stanford N., Majerus P. W. Deficiency of factor Xa-factor Va binding sites on the platelets of a patient with a bleeding disorder. Blood. 1979 Nov;54(5):1015–1022. [PubMed] [Google Scholar]
- Nelsestuen G. L., Lim T. K. Equilibria involved in prothrombin- and blood-clotting factor X-membrane binding. Biochemistry. 1977 Sep 20;16(19):4164–4171. doi: 10.1021/bi00638a005. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Canfield W. M., Kisiel W., Mann K. G. Studies of the capacity of factor Xa to protect factor Va from inactivation by activated protein C. J Biol Chem. 1982 Feb 10;257(3):1443–1447. [PubMed] [Google Scholar]
- Nesheim M. E., Eid S., Mann K. G. Assembly of the prothrombinase complex in the absence of prothrombin. J Biol Chem. 1981 Oct 10;256(19):9874–9882. [PubMed] [Google Scholar]
- Nesheim M. E., Katzmann J. A., Tracy P. B., Mann K. G. Factor V. Methods Enzymol. 1981;80(Pt 100):249–274. doi: 10.1016/s0076-6879(81)80023-7. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Mann K. G. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979 Feb 25;254(4):1326–1334. [PubMed] [Google Scholar]
- Nesheim M. E., Myrmel K. H., Hibbard L., Mann K. G. Isolation and characterization of single chain bovine factor V. J Biol Chem. 1979 Jan 25;254(2):508–517. [PubMed] [Google Scholar]
- Nesheim M. E., Prendergast F. G., Mann K. G. Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry. 1979 Mar 20;18(6):996–1003. doi: 10.1021/bi00573a010. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Taswell J. B., Mann K. G. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979 Nov 10;254(21):10952–10962. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Orloff K. G., Michaeli D. Fibrin-induced release of platelet serotonin. Am J Physiol. 1976 Aug;231(2):344–350. doi: 10.1152/ajplegacy.1976.231.2.344. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. I. Characterization of the reaction products formed during the activation of bovine prothrombin. J Biol Chem. 1974 Jan 25;249(2):594–605. [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Rosing J., Tans G., Govers-Riemslag J. W., Zwaal R. F., Hemker H. C. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980 Jan 10;255(1):274–283. [PubMed] [Google Scholar]
- Sakon M., Kambayashi J., Ohno H., Kosaki G. Two forms of Ca++-activated neutral protease in platelets. Thromb Res. 1981 Nov 1;24(3):207–214. doi: 10.1016/0049-3848(81)90090-6. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Suttie J. W. Vitamin K-dependent formation of gamma-carboxyglutamic acid. Annu Rev Biochem. 1977;46:157–172. doi: 10.1146/annurev.bi.46.070177.001105. [DOI] [PubMed] [Google Scholar]
- Suttie J. W., Jackson C. M. Prothrombin structure, activation, and biosynthesis. Physiol Rev. 1977 Jan;57(1):1–70. doi: 10.1152/physrev.1977.57.1.1. [DOI] [PubMed] [Google Scholar]
- Tracy P. B., Nesheim M. E., Mann K. G. Coordinate binding of factor Va and factor Xa to the unstimulated platelet. J Biol Chem. 1981 Jan 25;256(2):743–751. [PubMed] [Google Scholar]
- Tracy P. B., Nesheim M. E., Mann K. G. Proteolytic alterations of factor Va bound to platelets. J Biol Chem. 1983 Jan 10;258(1):662–669. [PubMed] [Google Scholar]
- Tracy P. B., Peterson J. M., Nesheim M. E., McDuffie F. C., Mann K. G. Interaction of coagulation factor V and factor Va with platelets. J Biol Chem. 1979 Oct 25;254(20):10354–10361. [PubMed] [Google Scholar]
- Truglia J. A., Stracher A. Purification and characterization of a calcium dependent sulfhydryl protease from human platelets. Biochem Biophys Res Commun. 1981 May 29;100(2):814–822. doi: 10.1016/s0006-291x(81)80247-1. [DOI] [PubMed] [Google Scholar]
- Tucker M. M., Foster W. B., Katzmann J. A., Mann K. G. A monoclonal antibody which inhibits the factor Va:factor Xa interaction. J Biol Chem. 1983 Jan 25;258(2):1210–1214. [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]