Abstract
Neural stem/precursor cells (NPCs) that reside within germinal niches of the adult CNS have more complex roles than previously expected. In addition to their well-documented neurogenic functions, emerging evidence indicates that NPCs exert non-neurogenic functions that contribute to the regulation and preservation of tissue homeostasis under both physiological and pathological conditions. In this issue of the JCI, Mohammad et al. found that DCs efficiently patrol the CNS only when the germinal niche of the subventricular zone functions properly. Indeed, DCs traveled from the ventricles along the rostral migratory stream to the olfactory bulb (a cervical lymph node access point) to dampen anti-CNS immune responses. The authors’ findings further support a non-neurogenic role for NPCs in maintaining tissue homeostasis and promoting tissue protection in the adult brain.
Neurogenesis: A tale of two germinal niches
In the adult rodent CNS, lifelong neurogenesis — the process of neuron generation from neural stem/progenitor cells (NPCs) — primarily occurs in two distinct areas of the brain (i.e., germinal niches), the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles (1, 2). Depending on the germinal niche, NPCs have distinct fates. Adult NPCs generated in the SGZ migrate a short distance into the granule cell layer of the dentate gyrus (DG) and become indistinguishable from preexisting cells, an activity that is considered necessary for modulating and refining the neuronal circuits involved in hippocampus-dependent memory processing and behavior (1–3). Newly formed NPCs from the SVZ migrate along the rostral migratory stream (RMS) to the olfactory bulb (OB), where they integrate within the granule and glomerular cell layers to maintain and reorganize the OB system (1, 2). Recent compelling evidence challenges the limited view that neurogenic areas of the brain act solely as sources of newly formed neurons for replacement of neuronal cells in the hippocampus and OB (4). In fact, the exclusive neurogenic role of the SVZ has been questioned due to recent data clearly indicating that adult OB neurogenesis might not have any functional significance in humans. In adult humans, 700 new neurons are added to the hippocampus each day (corresponding to an annual turnover of 1.75% of the neurons within the renewing fraction); however, retrospective birth dating has established that the majority of OB neurons are of the same age as the individual, and that additional neurons in the adult human OB account for less than 1% of the total neurons exchanged over a century (4).
Not only for neurogenesis: SVZ-derived NPCs exhibit non-neurogenic functions
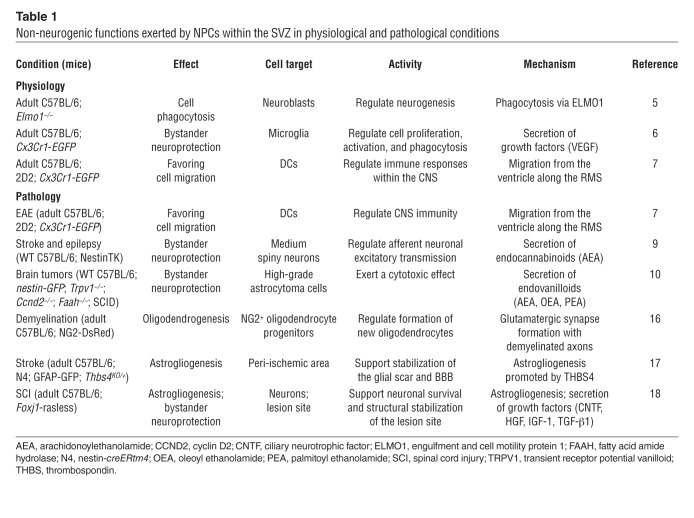
How can we explain the apparent paradox of NPCs being produced by the SVZ, yet no evident neuron turnover in the OB? One thought-provoking explanation comes from recent studies indicating that adult NPCs residing within the SVZ exert non-neurogenic functions — such as protecting and regulating homeostasis — as alternatives to cell replacement, in both physiological and pathological conditions (Table 1 and Figure 1). For example, it has been shown that SVZ-derived NPCs have phagocytic activity toward maturing neurons, which requires the intracellular engulfment protein ELMO1 to promote Rac activation downstream of phagocytic receptors (5). Additionally, SVZ-derived NPCs have been described as having a secretory protein profile (including secretion of VEGF) distinct from other brain cells and capable of modulating activation, proliferation, and phagocytosis of microglia (6).
Table 1.
Non-neurogenic functions exerted by NPCs within the SVZ in physiological and pathological conditions
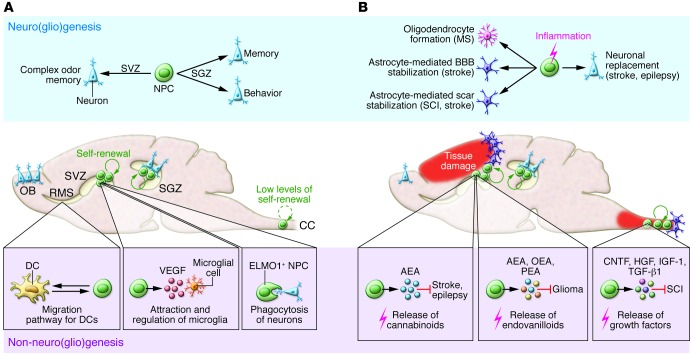
Figure 1. NPC functions during physiological and pathological conditions.
(A) In physiological (homeostatic) conditions, NPCs reside within 2 main neurogenic niches, the SVZ and the SGZ, and undergo neurogenesis to maintain specific CNS circuits in the OB and in the DG. Concurrently, NPCs might exert non-neurogenic functions, such as controlling microglial cell behavior, through secretion of VEGF, and phagocytosis of maturing neurons. A recently reported non-neurogenic SVZ NPC function is the ability to act as scaffolding cells for the proper migration of DCs patrolling the CNS, allowing DC trafficking from the ventricles toward cervical lymph nodes. (B) In maladaptive (stressful) conditions, such as those occurring during pathology/inflammation, NPCs might also exert neurogenic and non-neurogenic functions aimed at maintaining or reestablishing dysfunctional CNS circuits. Depending on the pathological process, neurogenic functions are finalized to generate new neurons capable of integrating into functional circuits, whereas non-neurogenic functions are mainly aimed at limiting and/or preventing tissue damage and promoting tissue recovery. Non-neurogenic tissue restoration operates via a bimodal mechanism of action. On the one hand, SVZ NPCs do sense and contrast danger signals in order to avoid harmful inflammatory reactions through the production of soluble factors, such as cannabinoids, endovanilloids, and growth factors. On the other hand, SVZ NPCs generate new astrocytes that stabilize the glial scar and the BBB and new oligodendrocytes that promote remyelination. AEA, arachidonoylethanolamide; CC, central canal; CNTF, ciliary neurotrophic factor; OEA, oleoyl ethanolamide; PEA, palmitoyl ethanolamide; SCI, spinal cord injury.
In this issue of the JCI, Mohammad and colleagues present evidence of an additional non-neurogenic homeostatic mechanism occurring within the SVZ niche (7). Using pharmacological and toxic methods, the authors identified and characterized the RMS as a pathway for DC trafficking through the CNS to the systemic immune compartment. Ablation of NPCs and the RMS with the antiproliferative agent cytosine-β-D-arabinofuranoside (ARA-c) led to DC retention in the murine brain. Furthermore, this pathway directly modulated Treg function in the cervical lymph nodes and reduced CNS-confined immune reactions. Finally, disruption of immune cell trafficking in the brains of 2D2 mice via localized infusion of the drug fingolimod, which inhibits T cell trafficking, resulted in reduced CNS immune tolerance, enhanced anti-CNS autoimmune responses, and CNS-associated inflammatory diseases such as EAE (7). While chemorepulsive factor gradients seem to guide NPC trafficking toward the OB (8), it remains to be determined whether and how DCs and NPCs physically interact along the RMS.
SVZ NPCs: Guardians of the brain
Emerging data certainly support the concept that SVZ NPCs act as guardians of the brain. In fact, NPCs are capable of sensing and contrasting danger signals to trigger an inflammatory process involving the innate arm of the immune system (Figure 1). Apart from the aforementioned relationship between SVZ NPCs and DCs, a recent study indicates that SVZ NPCs might also protect striatal neurons from glutamatergic excitotoxicity by releasing endogenous endocannabinoids (e.g., arachidonoylethanolamide [AEA]), which are capable of binding to their respective neuronal receptors (CB1 and CB2) (9). This NPC-mediated protection is tuned up during CNS-compartmentalized inflammatory insults, such as those occurring in the early phase of ischemic stroke and epilepsy. In addition, SVZ NPCs may mediate suppression of high-grade astrocytomas (HGAs) by releasing endovanilloids that activate the transient receptor potential vanilloid subfamily member-1 (TRPV1) on HGA cells, thus triggering cell death and prolonging overall survival (10). Together, these data help to explain previous results in support of the concept of therapeutic plasticity of transplanted SVZ-derived NPCs. Various approaches have consistently shown that transplanted SVZ-derived NPCs, while remaining undifferentiated, promote CNS tissue healing via secretion of immunomodulatory and neuroprotective molecules capable of reducing detrimental responses (the so-called bystander effect) (11–14).
In addition to these newly appreciated effector functions, it is remarkable that during CNS-confined pathological processes, SVZ NPCs might also alter their neurogenic differentiation default pattern in order to confine and limit tissue damage. In toxin-induced demyelination of the corpus callosum, less than 4% of newly formed SVZ-derived cells differentiate into myelinating oligodendrocytes (15); however, newly formed SVZ-derived glial precursors can promote remyelination by forming functional glutamatergic synapses with demyelinated axons (16). Astrocyte production from the postnatal SVZ niche in response to localized photothrombotic/ischemic cortical injury (controlled by the Notch modulator thrombospondin 4 [THBS4]) has been found to stabilize the blood-brain barrier (BBB) (17). In spinal cord injury (SCI), NPC-derived astrocytes stabilize the scar and are required to restrict secondary enlargement of the lesion and further axonal loss (18). Nonetheless, NPC progeny also appear to exert a neuroprotective effect required for survival of neurons adjacent to the SCI-associated lesion. In an experimental model of epilepsy, SVZ-derived cells that migrate toward the hippocampus have been described to terminally differentiate into glial cells, but not neuronal cells (19). This process might be protective, because in temporal lobe epilepsy, newly derived neurons aberrantly migrate and integrate in the dentate hilus, exacerbating hippocampal epileptic activity (20).
Conclusions
Replacement of damaged cells does not appear to be the sole operating mechanism of SVZ-derived NPCs, and it is likely that the neurogenic and non-neurogenic behaviors of SVZ NPCs are influenced by specific characteristics of the microenvironment. Strategically, cells of the SVZ are in communication with two different microenvironments, due their contact with both blood vessels and cerebrospinal fluid (CSF) by apical processes (8, 21–23). Furthermore, the SVZ is very close to crucial areas of the midbrain — including basal ganglia and striatal structures — that contain GABAergic neurons capable of efficiently regulating and modulating interconnections among several cortical and subcortical brain areas (24). Finally, prior studies have demonstrated that inflammation occurring as a consequence of autoimmunity and/or traumatic and ischemic injuries might alter NPC proliferation and differentiation characteristics in a non–cell-autonomous fashion (25).
The study by Mohammad and colleagues describes a role for SVZ-derived NPCs in regulating immune trafficking in the CNS (7). It is tempting to speculate that endogenous SVZ NPCs maintain and/or restore CNS homeostasis through both neurogenic and non-neurogenic functions. In addition to differentiating into neuronal cells, NPCs are capable of sensing danger signals coming from the periphery and producing a response settled to restraining conditions that might prove noxious for proper neural cell function.
Acknowledgments
This work was supported in part by TargetBrain (EU Framework 7 project HEALTH-F2-2012–279017) and NEUROKINE network (EU Framework 7 ITN project).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2014;124(3):970–973. doi:10.1172/JCI74419.
See the related article beginning on page 1228.
References
- 1.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/S0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura T, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Z, et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol. 2011;13(9):1076–1083. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosher KI, et al. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15(11):1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammad MG, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest. 2014;124(3):1228–1241. doi: 10.1172/JCI71544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311(5761):629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 9.Butti E, et al. Subventricular zone neural progenitors protect striatal neurons from glutamatergic excitotoxicity. Brain. 2012;135(pt 11):3320–3335. doi: 10.1093/brain/aws194. [DOI] [PubMed] [Google Scholar]
- 10.Stock K, et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat Med. 2012;18(8):1232–1238. doi: 10.1038/nm.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev. 2006;7(5):395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 12.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20(11):1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 13.Pluchino S, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 14.Bacigaluppi M, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132(pt 8):2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 15.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13(3):287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benner EJ, et al. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497(7449):369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabelstrom H, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342(6158):637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 19.Parent JM, von dem Bussche N, Lowenstein DH. Prolonged seizures recruit caudal subventricular zone glial progenitors into the injured hippocampus. Hippocampus. 2006;16(3):321–328. doi: 10.1002/hipo.20166. [DOI] [PubMed] [Google Scholar]
- 20.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 24.Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2(5):467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 25.Pluchino S, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131(pt 10):2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]