Summary points
Increasing age is the most important risk factor for developing prostate cancer
The most effective way to reduce prostate cancer incidence is to reduce prostate specific antigen (PSA) testing or raise thresholds that define abnormality
Prostate cancer screening with the PSA blood test results in at most a small reduction in prostate cancer mortality and leads to considerable diagnostic and treatment related harms
Physicians should recommend against PSA screening for prostate cancer
Most men with prostate cancer detected by PSA testing have tumours that will not cause health problems (overdiagnosed), but almost all undergo early treatment (overtreated)
Prostate cancer is an important health problem. More than 40 000 incident cases of prostate cancer and more than 10 000 prostate cancer related deaths occur each year in the United Kingdom.1 Prevention, detection, and treatment of localized prostate cancer remain controversial. Although screening for prostate cancer with serum prostate specific antigen (PSA) testing is not approved in the UK, informal screening is common and has led to a threefold increase in the incidence of prostate cancer. In the United States, since the introduction of PSA screening more than 1.3 million men have been diagnosed with prostate cancer and one million of these have undergone treatment.2 We update a previous review, highlighting new findings that deal with clinical questions and future research needs for the prevention, detection, and treatment of clinically localized prostate cancer.3
Who is at risk of prostate cancer?
Established risk factors for prostate cancer include increasing age, black ethnic origin, and a family history of prostate cancer in a close male relative. The last two factors convey modest risk compared with age. Prostate cancer is rare before 50 years of age, and about 80% of cases and 90% of deaths occur in men over 65.3 Lower urinary tract symptoms (poor flow, urgency, frequency, hesitancy, nocturia) are common in older men but not related to prostate cancer development. Testosterone supplementation for hypogonadism does not clearly increase prostate cancer development. Although prostate cancer rates show regional variations and differences between socioeconomic groups, these are mainly due to differences in rates of PSA testing.1
The greatest risk factor associated with a prostate cancer diagnosis is undergoing a PSA blood test.2 3 Using lower thresholds to indicate abnormality and obtaining larger numbers of tissue core samples sets off a cascade of events that more than doubles the incidence of prostate cancer through tumors that would otherwise never come to clinical attention (overdiagnosis).
Can prostate cancer be prevented?
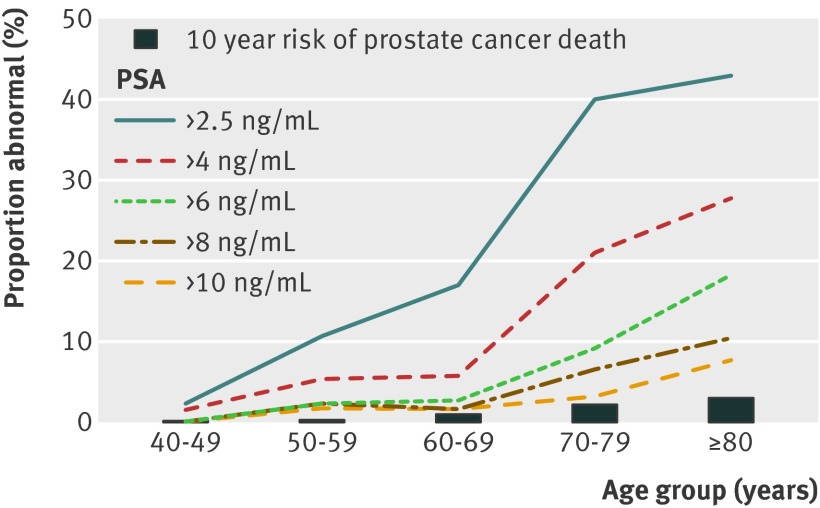
Epidemiologic evidence indicates that the most effective way to reduce prostate cancer incidence is to decrease PSA testing, raise thresholds used to define an abnormal PSA result (fig 1 ),4 and lower the number of tissue core samples obtained in men undergoing a prostate biopsy. In men who receive a PSA test, use of risk calculators that take other clinical parameters (age, prostate volume, free to total PSA ratio) into account or a triage test may help identify men who could avoid a prostate biopsy and detection of clinically insignificant disease, while still detecting potentially lethal disease that requires intervention.5 6
Fig 1 Impact of age on the proportion of men found to have an abnormal serum prostate specific antigen concentration depending on threshold used. The bars represent the 10 year risk of a prostate cancer related death in each age group4
The only other strategy shown to reduce prostate cancer incidence involves 5-α reductase inhibitors (5ARI), which are approved for treating symptoms of benign prostate enlargement. Large randomized controlled trials (RCTs) and a systematic review have shown that 5ARI reduces prostate cancer incidence but may increase the detection of high risk cancers.7 These drugs are not approved for prostate cancer prevention. RCTs indicate that antioxidants, particularly vitamin E and selenium, do not reduce prostate cancer incidence and should not be used.8 Other widely used options including aspirin, statins, low fat or soy based diets, and aerobic or weight based exercise are not clearly effective.
Should men be screened for prostate cancer with the PSA blood test?
Although such screening is common, the UK National Screening Committee and the US Preventive Services Task Force (USPSTF) recommend against it because the benefit is at best small (fig 2 ) and not greater than the harms.9 10 Both groups recognize that some men will continue to request screening and some physicians continue to offer it. All major organizations advise that PSA testing should not be conducted without an informed discussion of benefits and harms.
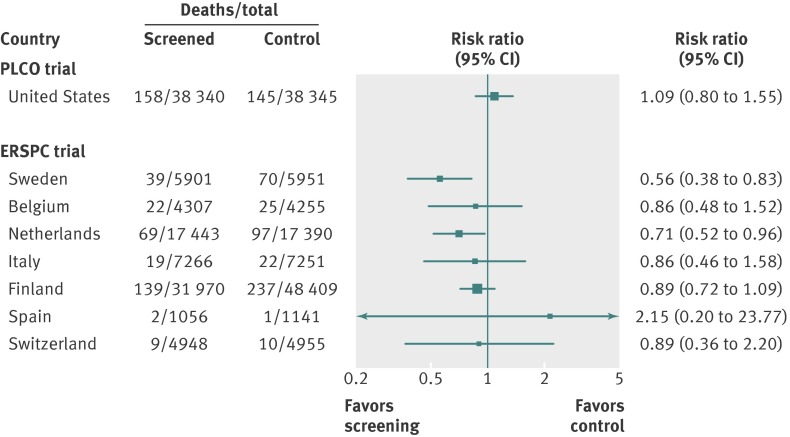
Fig 2 Forest plot showing the risk ratio with 95% confidence intervals from the two (of five) randomized prostate cancer screening trials judged to be at least “fair methodological quality” and of “low risk of bias” (PLCO and ERSPC). The vertical line represents no benefit10
The goal of screening programs is to reduce disease specific and overall morbidity and mortality, not just to detect and treat more disease. This goal is difficult to achieve because screening tests are applied to people without symptoms in the hope of preventing future health problems. Although any potential benefit occurs in just a few people, all are subjected to the harms of screening.
The effect of PSA screening on prostate cancer and overall mortality has been investigated in five large long term randomized trials.10 11 None has reported a reduction in overall mortality. Only the European Randomized Study of Screening for Prostate Cancer (ERSPC), which included results from seven countries using different screening protocols, identified a significant reduction in death from prostate cancer. Positive results mainly came from data from two countries that found large effects using PSA screening every two to four years. Although the precise lifetime effect of PSA screening on prostate cancer mortality remains uncertain, the reduction in prostate cancer mortality after 10-14 years is 0-1 per 1000 men screened, even for men in the optimal age range of 55-69 years.11
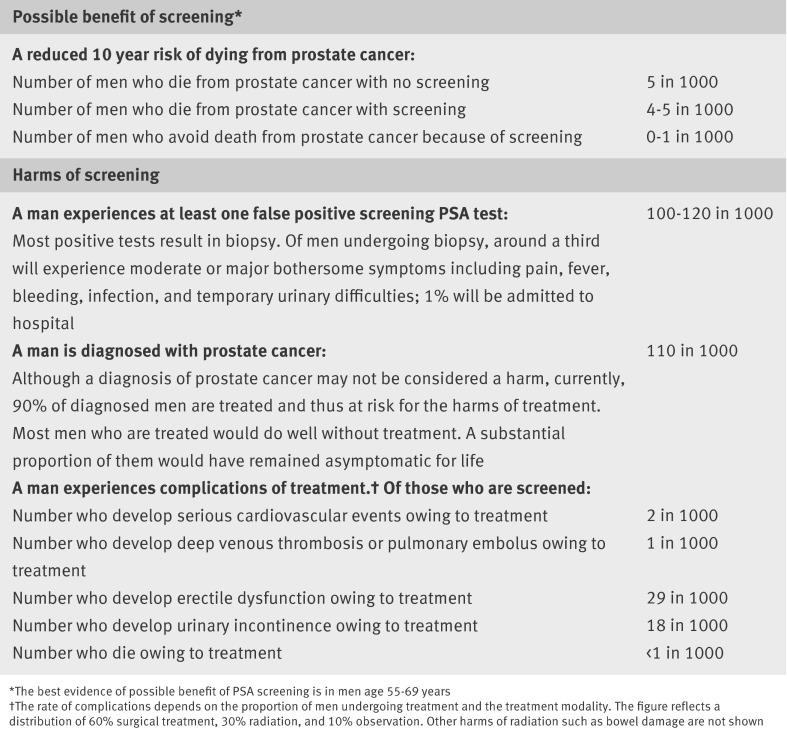
A raised PSA blood test initiates a cascade of diagnostic and treatment events that have harms and should be considered before testing (fig 3 ). Over 10 years, 15-20% of men will have a PSA test result that triggers a biopsy. About 80% of PSA tests will be false positive results at widely used cut-off points (2.5-4.0 ng/mL). Harms and errors related to the diagnostic transrectal prostate biopsy are common and often serious. The sampling needles are inserted through the (contaminated) rectum, with inconsistent use of local anesthetic. The UK Prostate Testing for Cancer and Treatment (ProtecT) study and other studies have shown that around a third of men having a prostate biopsy will have moderate or major bothersome symptoms, including pain, fever, bleeding, temporary urinary difficulties, and infection (with sepsis rates of 2-4% from multiresistant bacteria); 1% will be admitted to hospital and nearly 0.1% will die.12
Fig 3 Benefits and harms of screening men aged 55-69 years* with a prostate specific antigen (PSA) test every 1-4 years for 10 years. Calculations rely on assumptions and are imprecise. Estimates should be considered in the full context of clinical decision making and used to stimulate shared decision making10
Transrectal prostate biopsies are limited by random and systematic errors. Random errors occur because eight to 12 samples are taken randomly (the location of the cancer is unknown) and systematic ones occur because certain parts of the gland are sampled in preference to others. As a result, indolent disease is detected by chance and clinically important cancers missed. These errors lead to poor risk attribution; a man with low risk cancer on transrectal ultrasound guided biopsy has a one in three chance of harboring higher grade disease, with the prospect of undertreatment.12 This error in risk stratification can be used to advise against observation or active surveillance and recommend surgery or radiotherapy.13
There has been little progress in finding new blood or urinary biomarkers that could complement or replace PSA because biomarker development and validation depend on the inaccurate PSA-transrectal biopsy pathway. Imaging, such as multi-parametric magnetic resonance imaging (MRI), has shown promise, especially as it does not detect small low grade cancers.14 The exact role for MRI is being evaluated in the PROstate Mr Imaging Study (PROMIS) using a reference standard biopsy test—template prostate mapping—that systematically samples the whole prostate.15
The major harms of PSA screening relate to treatment of detected cancers—about half of those detected would never cause clinical problems in a man’s lifetime even in the absence of treatment (overdiagnosis).10 11 Men with such cancers cannot benefit from detection and treatment and can only be harmed. Around one in three men over the age of 50 years has prostate cancer that is unlikely to cause harm during their lifetime; this greatly exceeds the 3% lifetime risk of dying of prostate cancer even if left untreated. The use of surgery or radiotherapy to treat most of these men leads to overtreatment. Treatment related harms are common and include serious perioperative complications (rarely death) and long term erectile, urinary, and bowel dysfunction.
Comparing harms and benefits of any screening test involves trade-offs that can be difficult to quantify and often involve differing preferences and values. A recent decision modelling analysis attempted to derive a common metric to assess the benefits and harms of screening and subsequent treatment by assessing quality of life and quality adjusted life years gained or lost. It used selected findings from the European screening trial and other published data.16 The analysis found that PSA screening may reduce or increase quality adjusted survival, depending on patient values for different health states. The findings were sensitive to assumptions used. The small reported gains in quality adjusted survival would probably have disappeared if mortality and morbidity results from all screening and treatment trials had been incorporated.
What treatments should I recommend for clinically localized prostate cancer?
Treatment options for clinically localized prostate cancer include watchful waiting or observation, active surveillance, radical prostatectomy (RP), external beam radiation therapy, interstitial radiation implants (brachytherapy), cryoablation, androgen deprivation therapy (ADT), high intensity focused ultrasound, and focal therapy.3 Treatment recommendations and selection involve patients’ values and their weighting of the trade-offs in benefits and harms, as well as physician preferences. A multidisciplinary approach that includes the patient’s primary care provider and incorporates the latest findings from randomized trials or higher quality observational studies can help a patient make a well informed decision. Comparative effectiveness remains controversial, partly because few randomized treatment trials with mortality outcomes (only one in the PSA era) have been completed. Trials have been difficult to complete owing to the large size and long term follow-up needed, as well as patients’ and clinicians’ reluctance to participate. Despite evidence indicating excellent long term disease specific survival with observation, nearly 90% of men with PSA detected prostate cancer in the US receive early treatment. In the UK, 3000-4000 RPs are carried out each year, and two to three times this number of men undergo radiotherapy.17 In the UK, 60% of men diagnosed with low risk disease undergo immediate treatment; this figure is 70-90% in the US.18
Two randomized trials compared RP to observation but were conducted before widespread PSA testing.19 20 One failed to find a difference in overall mortality after more than 20 years.21 The Scandinavian Prostate Cancer Group-4 trial (SPCG-4 trial) demonstrated absolute differences in all cause and prostate cancer related mortality at 15 years of 6.6% and 6.1%, respectively, in favor of RP. Benefits were confined to men under 65 years.20 Another randomized trial comparing external beam radiotherapy with watchful waiting, also in men diagnosed before PSA testing, reported no significant difference in overall or prostate cancer related mortality over at least 16 years.22 Prostate cancer mortality in these two trials was much higher than in men with PSA detected prostate cancer.
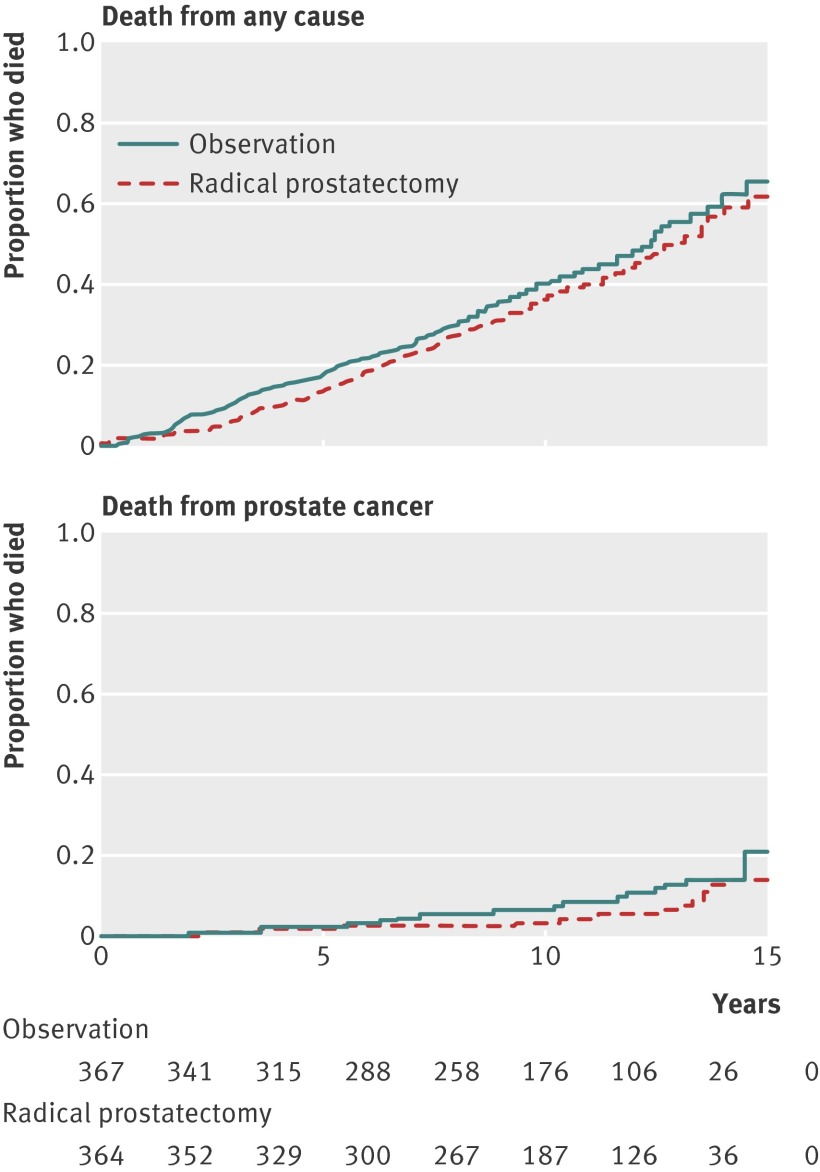
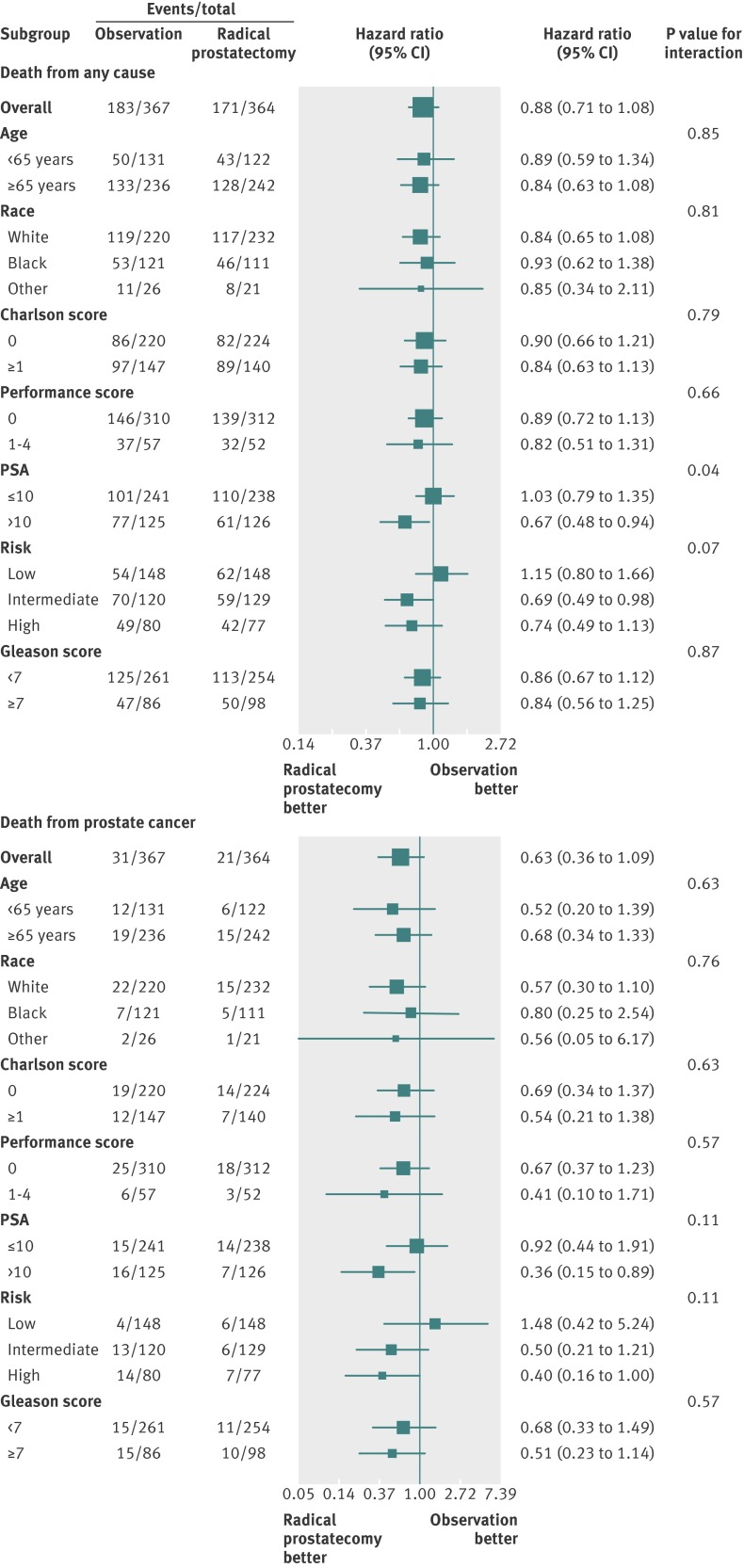
The Prostate cancer Intervention Versus Observation Trial (PIVOT) found that RP did not significantly reduce all cause or prostate cancer mortality compared with observation over 15 years (fig 4 ).23 Absolute differences were less than 3%. Treatment effects did not differ according to age, race, comorbidity, health status, or cancer grade. In men with PSA values of 10 ng/mL or less or those with low risk disease, prostate cancer mortality was rare (<3%) in men managed by observation. Radical prostatectomy was possibly associated with reduced all cause and prostate cancer mortality in men with PSA greater than 10 ng/mL and high risk disease (fig 5 ). Complications within 30 days of surgery occurred in 21.4% of men and included one death. Patient reported urinary incontinence and erectile dysfunction, but not bowel dysfunction, were more common at two years in men randomized to surgery.
Fig 4 Kaplan-Meier curves showing death from any cause and death from prostate cancer in the Prostate Cancer Intervention versus Observation Trial (PIVOT) comparing radical prostatectomy with observation24
Fig 5 Forest plots demonstrating subgroup effects (hazard ratio with 95% confidence intervals and P value for interaction) in the Prostate Cancer Intervention versus Observation Trial (PIVOT) comparing radical prostatectomy with observation. The vertical line indicates no effect. The size of the boxes indicates the weight of the effects24
The current practice of repeated PSA testing, lower PSA thresholds triggering biopsies, obtaining more tissue biopsy samples, and repeating biopsies after initial negative findings increases detection of smaller volume indolent cancers.24 Therefore, in men diagnosed currently, RP will probably result in smaller absolute reductions in metastases and mortality and a longer time needed to identify a reduction than were reported in PIVOT or SPCG-4.
In contrast to observation, active surveillance involves repeat prostate biopsies every one to two years, in addition to PSA testing and digital rectal examination every three to four months. If these indicate reclassification to a higher risk status, treatment with curative intent is often started. Clinical experience with active surveillance suggests there is an estimated risk of metastasis of less than 1% at two to eight years.25 5ARI has shown promising results in reducing risk reclassification on active surveillance, but follow-up is too short to make definitive recommendations.21 Active surveillance is being compared with surgery or radiotherapy in the UK’s ProtecT randomized trial.26
No randomized trials have assessed mortality outcomes of external beam radiotherapy or brachytherapy versus other management strategies in men with PSA detected prostate cancer. Treatment of localized prostate cancer is not an approved indication for ADT.
Radical surgery, external beam radiotherapy, or interstitial radiotherapy can cause serious harm because they treat the whole prostate regardless of risk, volume, or location of the cancer. This leads to collateral damage to the external urinary sphincter, neurovascular bundles, and rectal mucosa. Although rates of side effects vary between treatment modalities, urinary incontinence occurs in 10-20%, erectile dysfunction in 50%, and rectal toxicity (diarrhea, bleeding, pain) in 10-20%.27 Despite refinements in radiotherapy (conformal, intensity modulation, robotic radiosurgery) and radical prostatectomy (laparoscopic or robot assisted) these harms have not changed. No large long term RCTs have evaluated the clinical and cost effectiveness outcomes of these newer techniques. ADT is associated with an increased risk for impotence, hot flashes, metabolic syndrome, gynecomastia, and other serious harms. A recent meta-analysis of eight randomized trials in men with non-metastatic high risk prostate cancer found that ADT was not associated with increased mortality from cardiac disease.28
What are the future research needs?
The past few years have provided important new information on prostate cancer prevention, detection, and treatment. Several key research endeavors are needed to close important knowledge gaps.
Firstly, we need to develop and evaluate novel research methods that can be used to obtain comparative effectiveness data in a timely and cost efficient manner but are adaptive to the incremental technological changes that occur over the lifetime of a study. Such studies may include designs that feature point of care enrolment and use of cohort multiple RCT methods.29 Recent findings show that RCTs are vital for accurately assessing the benefits and harms of prevention, detection, and treatment strategies.
Secondly, we need better methods for population screening and diagnostic strategies that avoid diagnosing men with clinically insignificant prostate cancer but still identify those with clinically important disease. Few, if any, biomarkers have been identified that could replace or complement PSA. One strategy that could be readily evaluated is the impact of using higher PSA thresholds (such as 6-10 ng/mL) to define abnormality, trigger prostate biopsy, and recommend early treatment. The current strategy of using transrectal biopsy or histologic outcomes from surgery as a means to validate new biomarkers is probably flawed because of the inherent spectrum and selection biases that result. Biomarkers require validation against a reference standard that can be applied to all men at risk. The target condition for detection should focus on clinically significant cancers.30 31 Imaging research that allows the targeting (for biopsy or treatment) of clinically significant lesions has shown promise and requires additional study.32
Thirdly, active surveillance protocols and communication about the benefits of observation and active surveillance need to be improved to be acceptable to physicians and patients. The use of a clinical examination that is inaccurate (digital rectal examination), a blood test that lacks specificity (PSA), and a histologic verification test (transrectal biopsy) that is temporally unstable and can cause considerable harm may contribute to this lack of acceptability. Results of the ongoing ProtecT study are urgently needed to assess the trade-offs between active surveillance versus surgery or radiotherapy, especially in men with higher PSA concentrations or higher risk disease. Research that uses imaging and tissue biomarkers as surveillance tools and prognostic markers is needed.
Fourthly, comparativeness effectiveness research, especially assessing minimally invasive treatments, using novel RCT designs to enhance recruitment is needed before implementation. Despite considerable overdetection and overtreatment, evidence indicates that interventions can alter the natural course of prostate cancer and may have clinically important benefits in some men. Men most likely to benefit include those with a long life expectancy (age <65 years, excellent health status, and few comorbidities) and those with tumors likely to cause serious health problems (palpable prostate cancer or PSA values greater than 10 ng/mL). Even in these men, however, the harms of current treatments are considerable and the absolute benefit modest. The role of minimally invasive treatments, such as cryosurgery and high intensity focused ultrasound, has recently been identified by the National Institute for Health and Clinical Excellence as a priority for NHS evaluation in clinical trials or as part of cohort registry studies.33 34 35 Early proof-of-concept studies have shown that tissue preserving focal therapy—in which the treatment is directed at the tumor rather than the whole organ—has a low rate of side effects and may be sufficiently effective.36
Fifthly, physicians need sufficient information, educational resources, and communication skills to confidently and accurately provide prevention, detection, and treatment recommendations. Although some information exists, health service research is needed to develop efficient and effective tools to communicate scientific findings showing that observation and not PSA screening is the preferred treatment approach for most men diagnosed as having prostate cancer. These tools will help primary care providers in their role as a patient’s healthcare resource regarding prostate cancer.
What is the prognosis for men with clinically localized prostate cancer?
The long term prognosis for most men with clinically localized prostate cancer is excellent, even with no early treatment. This is particularly true for men with prostate cancer that is detected by PSA screening and not palpable (most men diagnosed currently); in men with PSA values of 10 ng/mL or less; or those with “low risk disease” defined by PSA levels (≤10 ng/mL), tumor stage (T1 or T2a), and histologic grade (Gleason score <6). Prostate cancer mortality over at least 15 years in these men treated with observation is 5% or less. Nonetheless, prostate cancer remains a leading cause of cancer related morbidity and mortality. Men with PSA values greater than 10 ng/mL or with high risk disease (PSA >20 ng/mL; tumor stage >T2b; Gleason score 8-10) have a worse prognosis. Their 15 year risk of prostate cancer mortality when managed expectantly is 20% or more. In this group surgery seems to reduce overall and disease mortality and bone metastases. Patients, their families, physicians, and the public seek answers to reduce the physical, social, and financial costs of this disease. We need better markers of prognosis, determinants of treatment effectiveness, and methods to effectively communicate and implement prognostic information. We need safer and more effective strategies and more rational use of existing options.
What should we tell patients who request screening?
Physicians can improve the health of their male patients by recommending against PSA screening for prostate cancer. However, PSA screening is common, and some men will continue to request and some physicians will continue to offer such screening. A decision to start or continue PSA screening should reflect an explicit understanding of the possible benefits and harms and respect for patient preferences. The box gives examples of screening messages that can be delivered in primary care to patients who ask about PSA screening. Figure 3 provides information about the potential benefits and harms of testing and treatment. Community, employer based, and clinician ordered PSA testing in the absence of well informed decision making should be discontinued. Physicians should use higher thresholds to define abnormalities that trigger a diagnostic prostate biopsy in men who have undergone a PSA test, thereby reducing overdiagnosis and overtreatment.
Prostate cancer screening messages for men
Major evidence based guidelines recommend against the prostate specific antigen (PSA) blood test for prostate cancer screening because:
The test is unlikely to prevent you from dying of prostate cancer over 10-15 years or help you live longer
Elevated PSA values are common and lead to additional tests that have harms
PSA testing finds many cancers that will not cause health problems
Once we find cancer it is hard not to treat it
Treatments have harms that occur early, can be serious, and may persist, but have very little, if any, benefit
By choosing not to have the PSA test you can live a similar length of life, have little to no difference in your risk of dying from prostate cancer, and avoid the harms associated with tests, procedures, and treatments
Compared with early intervention with surgery or radiotherapy, observation can help most men with early stage prostate cancer detected by PSA testing live a similar length of life and avoid death from prostate cancer, as well as prevent treatment related harms. Physicians should encourage patient participation in RCTs examining new prevention, screening, and treatment approaches.
Additional educational resources for healthcare professionals and patients
These websites provide evidence based guidelines and recommendations from the United States and United Kingdom on screening and treatment for clinically localized prostate cancer. These resources are mainly developed by and for primary care providers and patients seen in primary care settings. Additional society guidelines and recommendations from other countries also exist. Each listed resource contains links to informational tools for both clinicians and patients to help in making well informed decisions about screening and treatment for prostate cancer
Cancer Research UK. www.cancerresearch.org
UK National Screening Committee. www.screening.nhs.uk
US Preventive Services Task Force. www.uspreventiveservicestaskforce.org/prostatecancerscreening.htmAmerican
Cancer Society. www.cancer.org/healthy/informationforhealthcareprofessionals/prostatemdcliniciansinformationsource/index
Foundation for Informed Medical Decision Making.
http://informedmedicaldecisions.org/imdf_decision_aid/deciding-if-the-psa-test-is-right-for-you/
Unanswered questions
The role of new methods in conducting randomized controlled trials where recruitment has proved difficult
The role of new screening strategies such as the impact of different thresholds of prostate specific antigen (PSA) abnormality and risk calculators on the trade-off between benefits and harms
Discovery, development, and validation of tissue and imaging (novel ultrasound, functional magnetic resonance imaging) biomarkers to detect clinically important prostate cancer using accurate reference standards that minimize spectrum and selection bias
The role of novel tissue and imaging biomarkers in men with localized prostate cancer undergoing active surveillance
The clinical and cost effectiveness of minimally invasive whole gland and focal treatments for men with intermediate to high risk disease.
Health services research to integrate findings from PSA screening and treatment trials into primary care
A patient’s perspective
I was diagnosed with prostate cancer on my 60th birthday, with a Gleason score of 3+4, not the most welcome of birthday presents. I had been urinating more frequently, so my general practitioner suggested doing a prostate specific antigen (PSA) test. The result was 10 ng/mL, so my local urologist arranged a biopsy. When it came back positive, he fully explained the meaning of the results, the potential prognosis, and treatment options. My wife accompanied me, but we could not remember much. Mention of the word “cancer” blanked out everything else. Fortunately, the consultant jotted down the main points on several sheets of paper. We were told that there were three treatment options—surgery, radiotherapy, and hormone treatment—watchful waiting not being considered appropriate. The main reason I chose surgery was that if it failed it could be followed by radiotherapy, which in turn could be followed by hormone treatment. Our choice was also colored by an emotional response. I had this “thing” growing inside me, and I wanted it got rid of and cut out quickly.
Although the postoperative PSA concentration fell to near zero, some adverse features were seen on histologic analysis. We decided not to wait for the PSA to rise (indicating failure), but to have pre-emptive radiotherapy to the pelvic and prostate bed area three months after surgery.
Three years later my PSA is below 0.1 ng/mL; in the short term the treatments seemed successful. The intervening period has given me time to reflect on what has happened. I found the surgery less stressful than the radiotherapy. Although the radical prostatectomy was a major operation, recovery seemed to follow an uphill path, with symptoms such as incontinence getting better over time. Radiotherapy seemed much more insidious, at first seemingly benign, only later revealing the true extent of the side effects. Bowel movements took some time to return to near normal, and incontinence was somewhat worse than it had been two months after surgery.
Although we were fully informed at every consultation about the potential side effects, we did not fully take this on board. When I was diagnosed, we focused on the effectiveness of the treatment against the cancer. The potential side effects seemed a minor matter. Three years later I am not so sure. Incontinence is still a problem as I use pads. I find myself acutely aware of the nearest toilet, becoming anxious in new places. With modern technology, erectile dysfunction is manageable, but sex is not what it once was.
One unanticipated consequence is the effect of the six monthly PSA tests on me. When first told about this regimen, I was happy, thinking that if anything developed it could be caught at an early stage. While accepting this logic, I dread the approach of each test, wondering if it will herald the re-emergence of the cancer. However, although I get depressed, I do realize that this is something that has to be done.
Has this affected the quality of my life? To some extent it has, but I am still here, and I am grateful for that. Given the situation when diagnosed, I am not sure that we would have done anything differently.
Contributors: TJW conceived the idea for the article and wrote the first draft. HUA and TJW both contributed to further drafts. TJW is guarantor.
Funding: No special funding received.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: HUA is co-principal investigator in several investigator led diagnostic and focal therapy trials in prostate cancer supported by the MRC (UK), National Institute for Health Research Health Technology Assessment programme, Pelican Cancer Foundation charity, and Prostate Cancer UK charity; HUA receives funding from USHIFU and Advanced Medical Diagnostics for clinical trials; HUA has received consultancy payments in the past from Steba Biotech and Oncura/GE Healthcare and payment for conference travel from USHIFU; TJW is a volunteer member of the US Preventive Services Task Force and chairman of the Prostate cancer Intervention Versus Observation Trial (PIVOT).
Provenance and peer review: Commissioned; externally peer reviewed.
The PROMIS trial is funded by the NIHR Health Technology Assessment programme (project number 09/22/67) and will be published in full in Health Technology Assessment. The views and opinions expressed therein and in any media associated with this study are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS, or the Department of Health.
Patient consent obtained.
Cite this as: BMJ 2013;346:f325
Related links
bmj.com
bmj.com/archive
Previous articles in this series
References
- 1.Cancer Research UK. Prostate cancer statistics. 2012. www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/.
- 2.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986-2005. J Natl Cancer Inst 2009;101:1325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilt TJ, Thompson IM. Clinically localised prostate cancer. BMJ 2006;333:1102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst 2005;97:1132-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X, Albertsen PC, Andriole GL, Roobol MJ, Schroder FH, Vickers AJ. Risk-based prostate cancer screening. Eur Urol 2012;61:652-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossuyt PM, Irwig L, Craig J, Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ 2006;332:1089-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilt TJ, Macdonald R, Hagerty K, Schellhammer P, Tacklind J, Somerfield MR, et al. 5-alpha-Reductase inhibitors for prostate cancer chemoprevention: an updated Cochrane systematic review. BJU Int 2010;106:1444-51. [DOI] [PubMed] [Google Scholar]
- 8.Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MPA, Horneber M. Selenium for preventing cancer. Cochrane Database Syst Rev 2011;5: CD005195. [DOI] [PMC free article] [PubMed]
- 9.Burford DC, Kirby M, Austoker J. Prostate cancer risk management programme information for primary care; PSA testing in asymptomatic men. Evidence document. 2010. www.cancerscreening.nhs.uk/prostate/pcrmp02.pdf
- 10.Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120-34. [DOI] [PubMed] [Google Scholar]
- 11.Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, et al. Screening for prostate cancer: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2011;155:762-71. [DOI] [PubMed] [Google Scholar]
- 12.Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol 2009;27:4321-6. [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, et al. National Institutes of Health state-of-the-science conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med 2012;156:591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed HU, Kirkham A, Arya M, Illing R, Freeman A, Allen C, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol 2009;6:197-206. [DOI] [PubMed] [Google Scholar]
- 15.Medical Research Council Clinical Trials Unit. PROMIS—prostate MRI imaging study: evaluation of multi-parametric magnetic imaging in the diagnosis and characterisation of prostate cancer. www.ctu.mrc.ac.uk/promis.aspx.
- 16.Heijnsdijk EA, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012;367:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McVey GP, McPhail S, Fowler S, McIntosh G, Gillatt D, Parker CC. Initial management of low-risk localized prostate cancer in the UK: analysis of the British Association of Urological Surgeons Cancer Registry. BJU Int 2010;106:1161-4. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol 2004;22:2141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iversen P, Madsen PO, Corle DK. Radical prostatectomy versus expectant treatment for early carcinoma of the prostate. Twenty-three year follow-up of a prospective randomized study. Scand J Urol Nephrol Suppl 1995;172:65-72. [PubMed] [Google Scholar]
- 20.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. [DOI] [PubMed] [Google Scholar]
- 21.Fleshner NE, Lucia MS, Egerdie B, Aaron L, Eure G, Nandy I, et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet 2012;379:1103-11. [DOI] [PubMed] [Google Scholar]
- 22.Widmark A, Tomic R, Modig H, Thellenberg Karlsson C, Lundbeck F, Hoyer M, et al. Prospective randomized trial comparing external beam radiotherapy versus watchful waiting in early prostate cancer (T1b-T2, pN0, grade 1-2 M0). 53rd Annual ASTRO Meeting, 2-6 October 2011, Florida.
- 23.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Kwast TH. The trade-off between sensitivity and specificity of clinical protocols for identification of insignificant prostate cancer. Eur Urol 2012;62:469-71. [DOI] [PubMed] [Google Scholar]
- 25.Dahabreh IJ, Chung M, Balk EM, Yu WW, Mathew P, Lau J, et al. Active surveillance in men with localized prostate cancer: a systematic review. Ann Intern Med 2012;156:582-90. [DOI] [PubMed] [Google Scholar]
- 26.Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess 2003;7:1-88. [DOI] [PubMed] [Google Scholar]
- 27.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250-61. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 2011;306:2359-66. [DOI] [PubMed] [Google Scholar]
- 29.Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. BMJ 2010;340:c1066. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed HU, Hu Y, Carter T, Arumainayagam N, Lecornet E, Freeman A, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol 2011;186:458-64. [DOI] [PubMed] [Google Scholar]
- 31.Lord SJ, Staub LP, Bossuyt PM, Irwig LM. Target practice: choosing target conditions for test accuracy studies that are relevant to clinical practice. BMJ 2011;343:d4684. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477-94. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Clinical Excellence. Prostate cancer. CG58. 2008. http://guidance.nice.org.uk/CG58.
- 34.National Institute for Health and Clinical Excellence. Focal therapy using cryoablation for localised stage prostate cancer. IPG423. 2012. www.nice.org.uk/nicemedia/live/13512/58915/58915.pdf.
- 35.National Institute for Health and Clinical Excellence. Focal therapy using high-intensity focused ultrasound (HIFU) for localised prostate cancer. IPG424. 2012. http://publications.nice.org.uk/focal-therapy-using-high-intensity-focused-ultrasound-for-localised-prostate-cancer-ipg424.
- 36.Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012;13:622-32. [DOI] [PMC free article] [PubMed] [Google Scholar]