Abstract
Subcutaneous panniculitis-like T-cell lymphoma (SPTL-AB) and cutaneous gamma/delta T-cell lymphoma (CGD-TCL) are rare cutaneous T-cell lymphomas for which no standard treatment exists. We report our experience with bexarotene, an oral retinoid, in 15 adults and children with these disorders. In this series, we found a 77% overall response rate of bexarotene with limited toxicity for these disorders.
Introduction
Subcutaneous panniculitis-like T-cell lymphoma (SPTL-AB) and cutaneous gamma/delta T-cell lymphoma (CGD-TCL) are rare T-cell lymphomas with varying clinical courses. There is no standard treatment, although chemotherapy and hematopoietic stem cell transplantation are commonly used. We describe results using bexarotene for children and adults with these disorders.
Methods
We identified 15 patients (12 adults, 3 children) who were treated with bexarotene between 2000 and 2010 from the Memorial Sloan-Kettering Cancer Center lymphoma database, the Stanford Cancer Center Registry, and the National Cancer Institute (NCI) pediatric lymphoma database. There were 8 females and 7 males, with a median age of 45 years (range, 3 years to 85 years). All patients had stage IV disease. Two of 15 and 4 of 15 patients had documented CGD-TCL and SPTL-AB, respectively; others were presumed to have SPTL-AB. Bexarotene was administered at flat doses corresponding to 91 to 339 mg/m2/d. Two of 15 patients received concurrent denileukin diftitox. Two children received bexarotene as maintenance therapy and were not evaluable for response.
Results
Among those treated with bexarotene alone, the overall response rate (ORR) was 82% (6/11 complete response [CR], 3/11 partial response [PR]). One of the 2 patients treated with concomitant denileukin diftitox responded for an ORR of 10/13 (77%), including 54% CR and 23% PR. Median progression-free survival was 38.4 months; median duration of response was 26.3 months. Six patients developed hypothyroidism and 9 developed hyperlipidemia; one patient developed dose-limiting hypertriglyceridemia. One pediatric patient developed insulin-dependent diabetes mellitus.
Conclusions
In this retrospective series, bexarotene showed a high response rate in SPTL-AB and CGD-TCL. It was generally well-tolerated with durable responses; therefore, bexarotene represents a promising therapy for children and adults with these disorders.
Keywords: Bexarotene, Cutaneous gamma/delta T-cell lymphoma, Subcutaneous panniculitis-like T-cell lymphoma
Introduction
Subcutaneous panniculitis-like T-cell lymphoma (SPTL-AB), first described by Gonzalez et al, is a T-cell lymphoma with clinicopathologic features simulating a panniculitis. In its initial description, it was also associated with hemophagocytic syndrome (HPS) and an aggressive clinical course.1 The disease has been recognized as a distinct disorder in the World Health Organization (WHO) classification and was subdivided into two entities in the updated 2008 WHO classification based on the phenotype of the T-cell receptor α/β (SPTL-AB) or γ/δ (cutaneous gamma/delta T-cell lymphoma [CGD-TCL]) with different clinical courses, phenotypes, and prognoses.2–4 SPTL-AB and CGD-TCL are both cytotoxic, post-thymic T-cell neoplasms that are characterized by the presence of primarily subcutaneous infiltrates of small, medium-sized, or large pleomorphic T cells and can histologically mimic nonspecific panniculitis or lobar panniculitis.5 As discussed in a worldwide retrospective analysis, SPTL-AB and CGD-TCL are rare disorders respectively accounting for 0.9% and 0.1% of all T-cell lymphomas.5,6.
SPTL-AB and CGD-TCL are primarily disorders of adults with average ages of diagnosis in the mid to late 30s and late 50s, respectively. Only rare cases have been reported in children.7 Both SPTL-AB and CGD-TCL have a female predominance with a male to female ratio of 0.5.8 Patients generally present with solitary or multiple nodules and erythematous plaques that predominantly affect the lower extremities and trunk but can also occur on the face, neck, and back.9 Cutaneous findings may occur in the context of systemic symptoms such as fevers, malaise, anorexia, and weight loss. Spontaneous regression may occur in rare cases.8
The distinction was made between the two phenotypes because they correlate to distinct clinical courses with CGD-TCL usually correlating with an aggressive course and SPTL-AB having a much more variable course. Patients with CGD-TCL have a high rate of development of HPS (50%) and have a poorer prognosis (17% 5-year overall survival); whereas, those with SPTL-AB rarely develop HPS and have a better prognosis as mortality from HPS itself is high.8,10 In comparison to SPTL-AB, which is associated with only subcutaneous disease and nonulcerating lesions, CGD-CTL lesions tend to ulcerate in addition to their subcutaneous involvement.8 Extracutaneous involvement is uncommon, although there have been reports of bone marrow, lymph node, liver, spleen, lung, and peripheral blood involvement.8,11–17 Extracutaneous involvement occurs with a higher frequency in CGD-TCL.8,16
Because SPTL-AB and CGD-TCL are rare disorders, optimal treatment modalities remain undefined. For indolent or localized disease, systemic steroids and radiation have been used. In patients with more aggressive disease, combination chemotherapy regimens are frequently used, such as CHOP (cyclophosphamide, adriamycin, vincristine, prednisone), other anthracycline-based regimens, fludarabine-based regimens, and, rarely, high-dose chemotherapy followed by hematopoietic stem cell transplant (SCT) with moderate success.18 In a review of 156 patients with SPTL-AB and CGD-TCL, Go and Wester reported a 50% overall response rate (ORR) with steroids; however, the duration of response was less than 6 months for most patients. Only 20% of these patients maintained a sustained remission with prednisone, ranging from 18 to 160 months (median, 36.5 months). Additionally, it was reported that combination chemotherapy resulted in a 53% ORR with 17 of 20 responses occurring after use of CHOP.18 However, responses to combination chemotherapy were often short-lived, ranging from 2 months to 72 months.18 Additionally, Go and Wester reported that 13 patients who had recurrent or refractory disease were treated with high-dose chemotherapy followed by SCT (12 autologous, 1 allogeneic allogeneic). Ninety-two percent of these patients (n = 12/13), including the patient treated with allogenic SCT, achieved a complete remission (CR) with a median duration of >14 months at last follow-up examination.12,18
In a recent report of the limited experience in children (n = 18),7 most patients had been treated with CHOP or CHOP-like chemotherapy (n = 10), which was associated with five CRs, three deaths from disease or sepsis, one relapse within 2 months of therapy, and one unknown outcome (lost to follow-up). Two patients were treated with cyclosporine and steroids, of which one died of HPS and the other achieved a CR. Two patients were treated with steroids alone with one CR and one death from sepsis. Four pediatric patients have had SCT (3 allogeneic and 1 autologous) for SPTL-AB and CGD-TCL, all of whom have achieved CR.7
In addition to chemotherapy and SCT, there are limited data with biologic treatments for SPTL-AB and CGD-TCL. Case reports describe durable responses with denileukin diftitox in cases of SPTL-AB.19,20,21 Interferon-α is also reported to be active in refractory SPTL-AB.18
Retinoids are biologically active derivatives of vitamin A that are involved in the regulation of cell differentiation, proliferation, and apoptosis. Bexarotene (Targretin) is an oral retinoid that has been well-studied and approved for the treatment of mycosis fungoides and is used in the treatment of other cutaneous T-cell lymphomas22,23 and lymphomatoid papulosis.24 Bexarotene is a selective retinoid X receptor agonist that inhibits cell cycle progression, induces apoptosis and differentiation, prevents multidrug resistance, and inhibits angiogenesis and metastasis.25 It is believed that bexarotene’s primary action is through inducing apopotosis of T-cells.26
A recent study of the use of bexarotene in advanced cutaneous T-cell lymphoma (mycosis fungoides, N = 40; and Sézary syndrome, N = 26) showed a 44% ORR with 9% CR, and a median duration of response of 8 months (range, 1 month to ≥ 48 months).21 In this retrospective review, we report our experience treating 15 patients, including 3 children, with SPTL-AB and CGD-TCL with bexarotene.
Patients and Methods
The Memorial Sloan-Kettering Cancer Center lymphoma database, the Stanford University Cancer Center Registry, and the National Institutes of Heath (NIH) Cancer Center Registry were searched for all patients with SPTL-AB or CGD-TCL who were treated with bexarotene between 2000 and 2010. Data were collected regarding patients’ age at diagnosis, date of diagnosis, findings on histopathology, involved sites of the skin and other sites of involvement, prior therapies, response to therapy, duration of response, last follow-up, and date of death. Responses were evaluated and reported by the treating physician.
Bexarotene was initially started at 75 to 150 mg daily and was increased approximately every 2 weeks to a target dosage of 300 mg/m2/d, until clinical response or intolerance. Patients were evaluated as needed based on response and tolerance to treatment and thyroid function tests and lipid panels were obtained on a bimonthly to monthly basis. Most patients were administered triglyceride-lowering agents prophylactically for hypertriglyceridemia secondary to bexarotene and were administered levothyroxine when free T4 decreased to below normal levels or when patients developed symptoms consistent with hypothyroidism with low/normal free T4 levels. Those who required levothyroxine after initiating bexarotene were defined as having hypothyroidism secondary to bexarotene. Data are included from the initiation of treatment with bexarotene until last follow-up examination or July 2010. CR was defined as resolution of all skin lesions and/or radiographic evidence of disease on positron-emission tomography/computed tomography (PET/CT). Duration of best response was defined as time from initial documentation of maximal response to the time of documentation of progression. Progression-free survival (PFS) was defined as the time from initiation of treatment to the time of documentation of progression or last follow-up examination. Descriptive statistics were used for baseline patient characteristics. Kaplan-Meier analysis was performed using SPSS software (IBM SPSS Statistics, Version 19, Armonk, NY: 2010).
Results
Twelve adult patients, 5 females and 7 males, and 3 pediatric patients, all of whom were female, with SPTL-AB or CGD-TCL were identified (Table 1). The median age was 45 years (range, 3 years to 81 years). Routine analysis for beta F1 and gamma/delta expression was not available for all patients and some tissues were not readily accessible for re-review. Two were documented to have known gamma/delta T-cell phenotype (negative beta F1) consistent with CGD-TCL. Four had documented SPTL-AB; all others were presumed to have SPTL-AB. Based on Ann Arbor staging criteria, all patients had stage IV disease at time of diagnosis. Fourteen of 15 patients had only subcutaneous disease. One patient showed retroperitoneal lymphadenopathy, which demonstrated fluorodeoxyglucose avidity on PET/CT, but a biopsy was not performed. None of our patients had demonstrated HPS. Seven adult patients received bexarotene as their initial treatment; four adults were refractory to prednisone, one adult had relapsed after CHOP and failed interferon-α. Two of the three children were treated with bexarotene as maintenance therapy after achieving CR on CHOP; one child had a second local relapse after CHOP, then ifosfamide, carboplatin, etoposide, and SCT, and was treated with bexarotene thereafter. Two patients were treated with bexarotene in combination with denileukin diftitox. One patient had not been able to tolerate his initial dose of bexarotene and was categorized as a nonresponder. Bexarotene was generally administered at flat doses with median daily dose of 300 mg (range, 75 mg to 675 mg; 91 mg/m2 to 339 mg/m2). Median duration of therapy was 10.5 months (range, 2.9 months to 38.5 months). At the time of analysis, three patients were still receiving bexarotene.
Table 1.
Patient Data
| Gender | |
| Male | 7 pts |
| Female | 8 pts |
| Age | |
| Median Age (overall) | 45 y |
| Median Age (Adults) | 51 y |
| Range (Adults) | 23–59 y |
| Median Age (Children) | 4.9 y |
| Range (Children) | 3.6–14.8 y |
| Prior Therapies | |
| Steroids | 4 pts |
| CHOP/CHOP-like | 4 pts |
| Autologous Transplant | 1 pts |
| None | 6 pts |
| Disease Description | |
| History of HPS | 0 pts |
| Dose of Bexarotene | |
| Median Dose (Adults) | 300 mg |
| Range of Dose (Adults) | 75–675 mg |
| Median Dose (Children) | 130 mg/m2 |
| Range of Dose (Chidren) | 119–308 mg/m2 |
Abbreviations: CHOP = cyclophosphamide, adriamycin, vincristine, prednisone regimen; HPS = hemophagocytic syndrome.
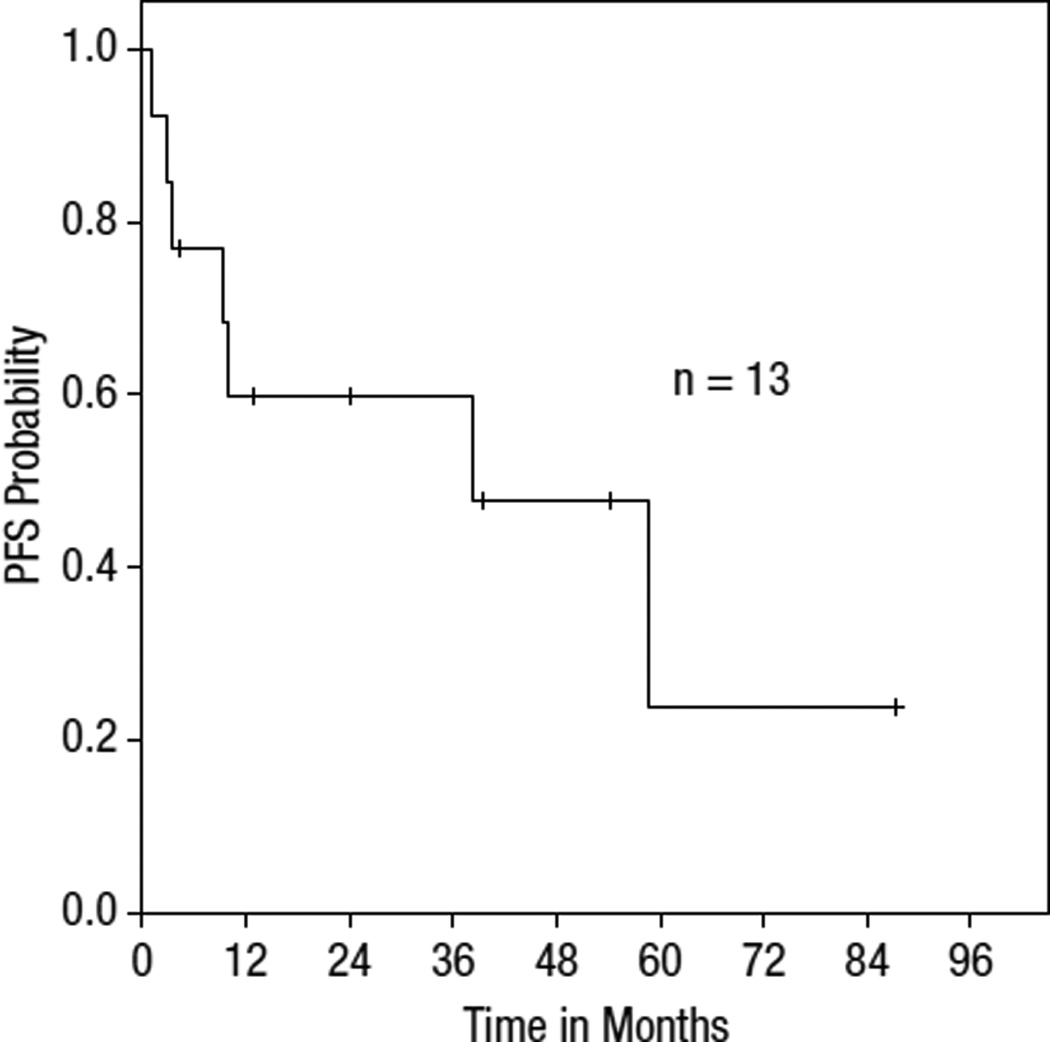
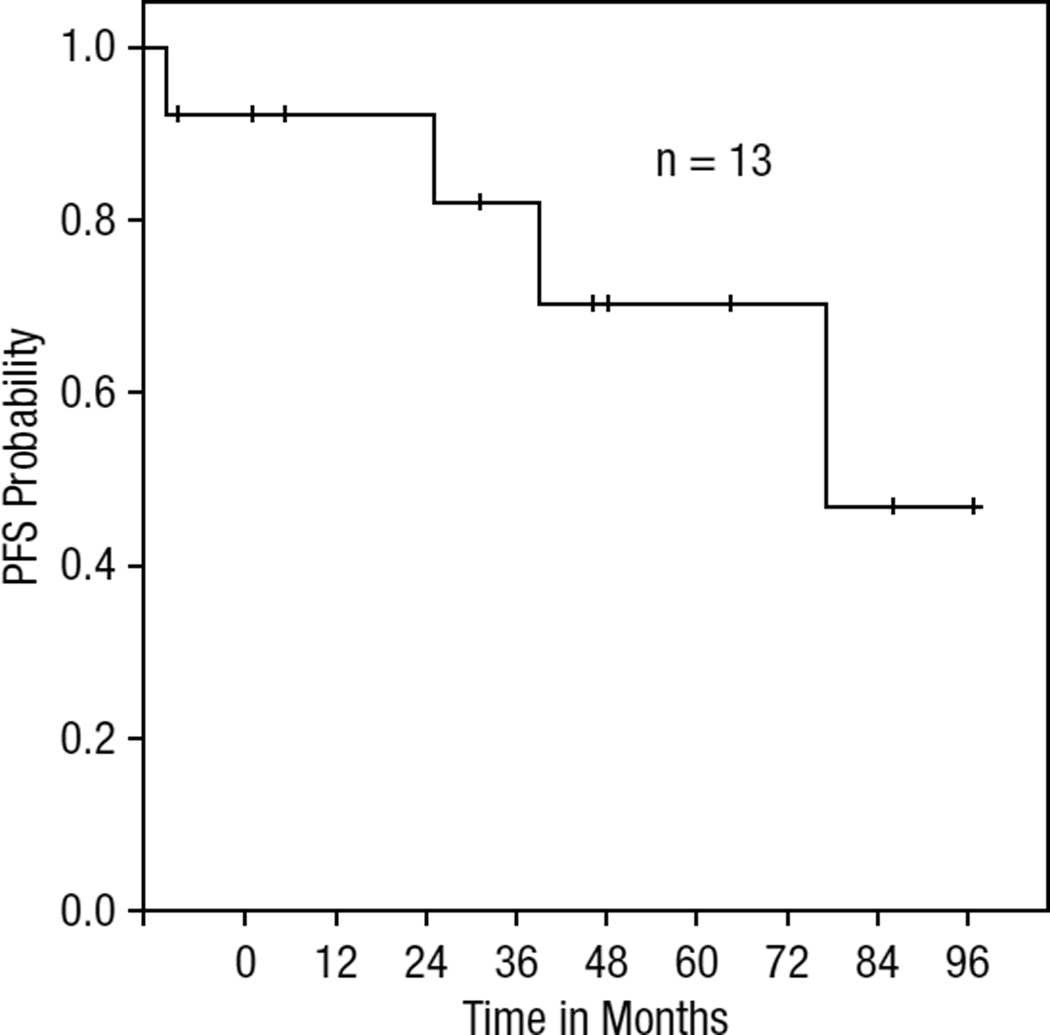
Responses were primarily based on the clinical assessment of the physician, and PET scans were used in two cases to document response. Among those treated with bexarotene alone, the ORR was 82% (9/11) with CR in 55% (6/11). The ORR for all evaluable patients in this series was 77% (n = 10/13) with 54% CR (n = 7/13) and 23% partial response (PR; n = 3/13). Two patients received bexarotene as maintenance therapy and were not evaluable for response and were not included in the survival curves. Of these 13, 2 patients were treated with bexarotene and denileukin diftitox concurrently; one patient achieved CR and the other progressed on therapy. Notably, all three pediatric patients achieved or maintained CR. The median duration of response in all patients was 26.3 months. Among complete responders, the median duration of response was 41.5 months (range, 4.1 months to 57.8 months). In contrast, partial responders had a median duration of response of 5.9 months (range: 1.3 to 10.5 months). The one child treated with bexarotene after relapse had a 41.5-month duration of response and remains in CR. The other two children also maintained CR with adjuvant treatment with bexarotene. Additionally, both patients with CGD-TCL responded (one PR, one CR), but with relapse at 1.3 months and 4.2 months, respectively. Among patients without documented CGD-TCL phenotype, the response rate was 73%. Based on Kaplan-Meier analysis, median PFS on bexarotene was 38.4 months for the entire cohort. Survival curves for PFS and overall survival are shown in Figures 1 and 2.
Figure 1.
Progression-Free Survival
Figure 2.
Overall Survival
Nine of the 15 patients achieved or maintained responses with doses less than 300 mg/m2. Two adults who achieved CR only received low doses of bexarotene, less than 225 mg, which corresponded to 91.5 mg/m2 and 98.6 mg/m2. Among children, the median dose used was 130 mg/m2 (range, 119 mg/m2 to 309 mg/m2).
At the time the data were collected, three patients remained on therapy, four had stopped in CR, and three had stopped after either relapse or progression of disease. One of the three who stopped after relapse had initially achieved CR on combination bexarotene with denileukin diftitox and remained on therapy until relapse. One additional patient discontinued bexarotene within 2 weeks secondary to poor performance status and was categorized as a nonresponder. Of the patients who were on therapy at the time data were collected, one had achieved PR and two had achieved CR. Of the four patients who stopped in CR, two patients had relapses at 3.3 months and 36.8 months after discontinuation of bexarotene. These two patients were on bexarotene for 38.4 months and 27.0 months, respectively. The other two patients remained in CR at 57.8 months and 91.7 months, respectively.
The most common toxicities on bexarotene were central hypothyroidism (n = 6) and hypertriglyceridemia (n = 9) with most patients receiving prophylactic lipid-lowering agents. In the pediatric cases, patients developed hyperlipidemia (n = 2), hypothyroidism (n = 1), and insulin-dependent diabetes mellitus (n = 1) after starting bexarotene. In all patients, toxicities occurred at a median of 4 weeks after the initiation of treatment. One adult patient required dose reduction secondary to severe hyperlipidemia, and one pediatric patient required discontinuation of bexarotene secondary to hypertriglyceridemia.
Another pediatric patient required dose reduction secondary to pill aversion. Additionally, one pediatric patient developed insulin-dependent diabetes mellitus while on bexarotene. There are no published reports of bexarotene causing insulin-dependent diabetes mellitus. The diabetes persisted after discontinuation of bexarotene.
Of the 15 patients studied, there were 3 pediatric cases with median age of 4.9 years (range, 3.5 years to 14.8 years). Two patients had localized skin-only disease and one patient had concomitant lymph node involvement. All 3 patients were female, 2 were documented to have alpha/beta disease, and the third’s phenotype could not be determined. In 2 of the 3 cases, bexarotene was used as maintenance therapy after achieving a CR on CHOP. The other patient relapsed after CHOP and autologous SCT and was treated with bexarotene subsequently; this patient achieved CR, which was maintained at 41.5 months of treatment. The median dose in children was 150 mg or 130.4 mg/m2/d.
Discussion
SPTL-AB and CGD-TCL are rare disorders that can have a variable clinical course for which standard treatment has not been established. Studies, primarily in the form of case reports and series, have shown varied responses to therapies and frequent difficulties in maintaining responses. Most of the literature regarding treatment of SPTL-AB and CGD-TCL involved chemotherapy. The largest series of patients with SPTL-AB was published by Go and Wester and included 156 patients; this series showed an approximately 53% response rate to combination chemotherapy.18 In the series by Ghobrial et al, the ORR to CHOP was 53%.12 Among patients who had progressive disease despite chemotherapy, median survival was only 7 months. Among 5 patients who underwent SCT (three autologous, two allogeneic), 3 achieved CR.12 Other case reports also describe successful outcomes with allogeneic SCT.27
In case studies, treatment with denileukin diftitox has also shown durable responses in SPTL-AB and CGD-TCL.19,21 Hathaway et al reported two cases of SPTL-AB showing response to denileukin diftitox with durations of response of 6 months and ≥ 18 months.19 McGinnis et al similarly reported one case of SPTL-AB with a 9-month duration of response to denileukin diftitox.21
Prior studies of bexarotene in mycosis fungoides have shown a dose response relationship with an ORR of approximately 45% at 300 mg/m2.21,28 Our series extends an experience previously presented in abstract form.29 In our series, bexarotene was associated with an 77% overall response in SPTL-AB and CGD-TCL, with 54% showing CR and 23% showing PR. In addition, several patients had durable responses of 56.6, 57.8, and 91.7 months. The median duration of response was 26.2 months and PFS was 38.4 months. Among those who achieved CR, the median duration of response was 36.4 months. Although most patients received standard doses of bexarotene, responses were also seen with low-dose bexarotene (eg, 91.5 mg/m2/d and 98.6 mg/m2/d).
The most common toxicities noted with bexarotene are similar to those observed in patients with other types of cutaneous T-cell lymphomas including hypothyroidism and hypertriglyceridemia.22,23,24,30 Most patients who developed toxicity to bexarotene were able to be treated with medical management alone. Of note, 1 pediatric patient developed insulin-dependent diabetes while on bexarotene which has not previously been reported and persisted after discontinuation of the drug.
Three patients in this series underwent SCT after bexarotene. Two received an allogeneic SCT and, of them, 1 remains in CR whereas the other died secondary to transplant-associated infectious complications. The third patient, who relapsed after achieving PR on bexarotene, later achieved CR with an autologous SCT. Two of the 3 patients who underwent SCT had the CGD-TCL subtype.
In the pediatric population, we present the first known use of bexarotene for SPTL-AB. All 3 children achieved or maintained a CR on bexarotene. One of these patients had relapsed after autologous bone marrow transplant; the other 2 patients were treated with bexarotene maintenance therapy after CR with CHOP-like chemotherapy. In patients who received bexarotene as maintenance after remission to chemotherapy, the effect of bexarotene alone cannot be ascertained.
In this series, we found significant and often durable responses with oral bexarotene in patients with presumed SPTL-AB. The 2 patients with documentation of the more aggressive CGD-TCL had achieved CR and PR on bexarotene with denileukin diftitox and bexarotene alone, respectively; however, both progressed in less than 4 months. Among the other patients, sustained CRs were seen even with low-dose bexarotene. In patients who achieved CR, bexarotene was continued for 3.2 to 38.4 months (median, 20.3 months) without cumulative toxicity and continued maintenance may be preferred for those with multiply relapsed or refractory disease as long as tolerance is acceptable. In those receiving bexarotene as initial therapy, 4 stopped treatment in CR; however, relapses were seen in 2 of the 4 patients at 4.1 to 56.6 months (median, 6.4 months). Two patients remained in CR off therapy at 57.8 months and 91.7 months, respectively.
Further studies must be conducted to evaluate the optimal dose and duration of therapy with bexarotene in these disorders. Considering that relapses were observed after discontinuation and treatment was well tolerated, alternate dosing strategies could include uniform treatment until progression, or dose reduction or tapering once response is obtained. Evaluation of response rate at retreatment is also not adequately evaluated in this series. Because this study was retrospective, not all cases had been phenotyped as having either SPTL-AB or CGD-TCL and further information regarding their status could not be ascertained. Certainly future prospective studies would need to stratify based on T-cell receptor phenotype as we now understand that these are distinct entities with different prognoses. Further studies are also needed to further elucidate how bexarotene compares to other treatments for SPTL-AB and CGD-TCL as well as to define its optimal use as a single agent or in combination. Additionally, our patients did not demonstrate HPS and had primarily SPTL-AB; therefore, it remains unclear whether bexarotene has efficacy in patients with more aggressive features such as HPS or CGD-TCL. However, this series shows that bexarotene may represent a less toxic alternative to chemotherapy that can result in durable disease control in both adults and children with SPTL-AB.
Clinical Practice Points.
Subcutaneous panniculitis-like T-cell lymphoma (SPTL-AB) and cutaneous gamma/delta T-cell lymphoma (CGD-TCL) are rare cutaneous T-cell lymphomas with variable clinical courses and for which there is no standard treatment. The current treatment modalities for these disorders consist primarily of chemotherapeutic regimens, however there have been reports on the activity of some biologic agents. Bexarotene is an oral retinoid used primarily in mycosis fungoides and has limited toxicity.
We report our experience of 15 patients (12 adults and 3 children) with STPL-AB and CGD-TCL who were treated with bexarotene alone (n = 13) or bexarotene and denileukin diftitox (n = 2). Among those treated with bexarotene alone, there was an 82% overall response rate (ORR). Among all patients, the ORR was 77%. The median progression-free survival was 38.4 months. All three pediatric cases achieved or maintained a complete response with bexarotene. The most common toxicities including hypertriglyceridemia and hypothyroidism are well described with bexarotene and only 1 patient required dose reduction that was secondary to hypertriglyceridemia.
In this retrospective series, bexarotene showed a high response rate in SPTL-AB, although we had less experience in documented CGD-TCL. It was generally well-tolerated and some responses were durable; therefore, bexarotene represents a potential treatment option in both children and adults with STPL-AB or CGD-TCL.
Acknowledgment
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Jocelyn Maragulia for her assistance in the statistical analyses for this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no conflicts of interest.
References
- 1.Gonzalez Cl, Medeiros LJ, Braziiel RM, et al. T-cell lymphoma involving subcutaneous tissue: a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17–27. doi: 10.1097/00000478-199101000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Stein H, et al., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Hematopoetic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 3.Jaffe ES, Harris NL, Stein H, et al. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cuteanous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 5.Gallardo F, Pujol RM. Subcutaneous panniculitic-like T-cell lymphoma and other primary cutaneous lymphomas with prominent subcutaneous tissue involvement. Dermatol Clin. 2008;26:529–540. doi: 10.1016/j.det.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-celly lymphoma, not otherwise specificed: a report of 340 cases from the International T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 7.Koh MJ, Sadarangani SP, Chan YC, et al. Aggressive subcutaneous panniculitis-like T-cell lymphoma with hemophagocytosis in two children. J Am Acad Dermatol. 2009;61:875–881. doi: 10.1016/j.jaad.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 8.Willemze R, Jansen PM, Cerroni L, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: and EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:838–845. doi: 10.1182/blood-2007-04-087288. [DOI] [PubMed] [Google Scholar]
- 9.Cassis TB, Fearneyhough PK, Callen JP. Subcutaneous panniculitis-like T-cell lymphoma with vacuolar interface dermatitis resembling lupus erythematous panniculitis. J Am Acad Dermatol. 2004;50:465–469. doi: 10.1016/s0190-9622(03)02784-1. [DOI] [PubMed] [Google Scholar]
- 10.Santucci M, Pimmpinelli N, Massi D, et al. Cytotoxic/natural killer cell cutaneous lymphomas. Report of EORTC Cutaneous Lymphoma Task Force Workshop. Cancer. 2003;97:610–627. doi: 10.1002/cncr.11107. [DOI] [PubMed] [Google Scholar]
- 11.Sahany KE, Macon WR, Choi JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22:881–893. doi: 10.1097/00000478-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Ghobrial I, Weenig R, Pittlekow MR, et al. Clinical outcome of patients with subcutaneous panniculitis-like T-cell lymphoma. Leuk Lymphoma. 2005;46:703–708. doi: 10.1080/10428190500051380. [DOI] [PubMed] [Google Scholar]
- 13.Savage KJ, Chhanabhai M, Gascoyne RD, et al. Characterizatin of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–1475. doi: 10.1093/annonc/mdh392. [DOI] [PubMed] [Google Scholar]
- 14.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407–3412. doi: 10.1182/blood-2002-05-1597. [DOI] [PubMed] [Google Scholar]
- 15.Takeshita M, Imayama S, Oshiro Y, et al. Clinicopathologic analysis of 22 cases of subcutaneous panniculitis-like CD56− or CD56+ lymphoma and review of 44 other reported cases. Am J Clin Pathol. 2004;121:408–416. doi: 10.1309/TYRG-T196-N2AP-LLR9. [DOI] [PubMed] [Google Scholar]
- 16.Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnositic criteria in the recent World Health Organization-European Organization for research and treatment of cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303–308. doi: 10.5858/133.2.303. [DOI] [PubMed] [Google Scholar]
- 17.Guizzardi M, Hendrickx IA, Mancini LL, et al. Cytotoxic gamma/delta subcutaneous panniculitis-like T-cell lymphoma: report of a case with pulmonary involvement unresponsive to therapy. J Eur Acad Dermatol Venereol. 2003;17:219–222. doi: 10.1046/j.1468-3083.2003.00600.x. [DOI] [PubMed] [Google Scholar]
- 18.Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma. A systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404–1413. doi: 10.1002/cncr.20502. [DOI] [PubMed] [Google Scholar]
- 19.Hathaway T, Subtil A, Kuo P, et al. Efficacy of denileukin diftitox in subcutaneous pannicultitis-like T-cell lymphoma. Lymphoma Myeloma. 2007;7:541–545. doi: 10.3816/clm.2007.n.040. [DOI] [PubMed] [Google Scholar]
- 20.Harris NL, McNeely WF, Shepard J, et al. Case 34–2001 — a 54-year-old woman with multiple sclerosis, prolonged fever, and skin Nodules. N Engl J Med. 2001;345:1409–1415. doi: 10.1056/NEJMcpc342001. [DOI] [PubMed] [Google Scholar]
- 21.McGinnis KS, Shapiro M, Junkins-Hopkins JM, et al. Denileukin diftitox for the treatment of panniculitic lymphoma. Arch Dermatol. 2002;138:740–742. doi: 10.1001/archderm.138.6.740. [DOI] [PubMed] [Google Scholar]
- 22.Abbott RA, Whittaker SJ, Morris SL, et al. Bexarotene therapy for mycosis fungoides and Sezary syndrome. Br J Dermatol. 2009;160:1299–1307. doi: 10.1111/j.1365-2133.2009.09037.x. [DOI] [PubMed] [Google Scholar]
- 23.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II–III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 24.Krathen RA, Ward S, Duvic M. Bexarotene is a new treatment option for lymphomatoid papulosis. Dermatology. 2003;206:142–147. doi: 10.1159/000068451. [DOI] [PubMed] [Google Scholar]
- 25.Qu L, Tang X. Bexarotene: a promising anticancer agent. Cancer Chemother Pharmacol. 2010;65:201–205. doi: 10.1007/s00280-009-1140-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Hazarika P, Ni X, et al. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer Res. 2002;8:1234–1240. [PubMed] [Google Scholar]
- 27.Ichii M, Hatanaka K, Imakita M, et al. Successful treatment of refractory subcutaneous panniculitis-like T-cell lymphoma with allogeneic peripheral blood bone marrow transplantation from HLA-mismatched sibling donor. Leuk Lymph. 2006;47:2250–2252. doi: 10.1080/10428190600783619. [DOI] [PubMed] [Google Scholar]
- 28.Talpur R, Ward S, Apisarnthanarax N, et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Derm. 2002;47:672–684. doi: 10.1067/mjd.2002.124607. [DOI] [PubMed] [Google Scholar]
- 29.Molina A, Advani R, Reddy S, et al. Bexarotene is highly active in the treatment of subcutaneous panniculitis-like T-cell lymphoma. Blood. 2005;106:3344. (abstract). [Google Scholar]
- 30.Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med. 1999;340:1075–1079. doi: 10.1056/NEJM199904083401404. [DOI] [PubMed] [Google Scholar]