Abstract
Objective
25-hydroxyvitamin D [25(OH)D] levels after recovery from tuberculosis (TB) may reflect pre-morbid levels and therefore provide insight into pathogenesis. We assessed 25(OH)D levels after recovery from TB disease, and compared to levels in persons without TB disease.
Methods
Case-control study. Cases were persons who had recovered from culture-confirmed Mycobacterium tuberculosis disease. Controls were persons without TB disease. Total 25(OH)D was measured from stored plasma specimens using liquid chromatography-mass spectrometry.
Results
29 persons with prior TB disease and 36 controls were included. Median 25(OH)D levels were 24.7 ng/mL (IQR, 18.3–34.1) in prior TB disease, and 33.6 ng/mL (IQR, 26.2–42.4) in controls (Mann-Whitney; P=0.01). Multivariable linear regression analysis showed that black race (adjusted mean difference [β]=−8.3 ng/mL; 95% CI −14.5, −2.2; P<0.01), enrollment in winter (β=−10.4 ng/mL; 95% CI −17.0, −3.8; P<0.01) and prior TB disease (β=−5.8 ng/mL; 95% CI −11.4, −0.3; P=0.05) were associated with lower 25(OH)D levels.
Conclusions
Persons who had recovered from TB disease had lower 25(OH)D levels compared to controls without TB disease, after adjusting for important confounders. Larger, longitudinal studies are needed to further characterize the possible role of low 25(OH)D in the pathogenesis of TB disease and TB recurrence after recovery.
Keywords: 25-hydroxyvitamin D, vitamin D, tuberculosis, Mycobacterium tuberculosis
Introduction
Previous clinical, epidemiologic and in vitro data have suggested that low levels of vitamin D may predispose to tuberculosis (TB).1,2 Clinical observational studies have found low circulating 25-hydroxyvitamin D [25(OH)D] levels at the time of TB diagnosis and during treatment.3–7 Epidemiologic studies have shown a higher incidence of TB disease in populations with diminished 25(OH)D levels such as persons of black race, and a peak of TB notifications in spring and early summer attributed to low 25(OH)D levels during winter.2,8,9 In vitro, the biologically active metabolite of vitamin D, 1,25-dihydroxyvitamin D, has been shown to modulate cytokine responses to Mycobacterium tuberculosis antigens and to enhance the mycobactericidal activity of macrophages infected with M. tuberculosis.10–12
Given that 25(OH)D levels could be affected by active TB disease itself or ongoing anti-TB treatment,2 we assessed 25(OH)D levels after recovery from TB disease as a possible marker of pre-morbid 25(OH)D levels, and compared them to levels in controls without TB disease.
Patients and methods
The population for this project was drawn from a case-control study conducted in Tennessee between 2008 and 2009 which recruited persons with prior extrapulmonary tuberculosis (EPTB), prior pulmonary tuberculosis (PTB), latent tuberculosis infection (LTBI), and uninfected contacts.13 Inclusion criteria for the study were as follows: ≥18 years at the time of TB diagnosis or enrollment; HIV seronegative; for EPTB and PTB, culture-confirmed M. tuberculosis disease and either completion or near completion (within one month) of anti-TB therapy. EPTB was defined as M. tuberculosis disease of any site other than the pulmonary parenchyma. Persons with both pulmonary and extrapulmonary involvement were classified as EPTB. PTB was defined as pulmonary disease with no extrapulmonary involvement. LTBI was defined as having a tuberculin skin test (TST) induration of ≥10 mm. Persons with LTBI could have received treatment, or not for LTBI. Uninfected contacts had a negative TST and had been exposed to culture-positive PTB patients. Exclusion criteria were as follows: serum creatinine >2 mg/dl; use of corticosteroids or other immunosuppressants at the time of diagnosis or enrollment; malignancy; diabetes mellitus; and pleural TB.
For our analysis, subjects were classified into two main study groups: (1) prior TB disease which included all persons with prior EPTB or PTB, and (2) non-TB disease which included all persons with LTBI and uninfected contacts, who served as controls. Persons who were still receiving anti-TB therapy at time of enrollment were excluded from our analysis because of the potential effect of anti-TB therapy on 25(OH)D levels.14
For each person, 250 microliters of stored plasma obtained from blood that had been drawn at the time of study enrollment was sent frozen in a microcentrifuge tube to Heartland Assays, Inc. (Ames, Iowa) for 25(OH)D analysis. Measurement of total 25(OH)D [25(OH)D2 and 25(OH)D3 ] was conducted using liquid chromatography-mass spectrometry. An amendment to the study protocol was approved by the Vanderbilt University Institutional Review Board to utilize the stored plasma specimens for this project.
Study data were collected and managed using a secure electronic data capture tool (REDCap).15 Data were analyzed using Stata software (version 12.0; StataCorp, Texas). 25(OH)D levels were compared between study groups by using the Mann-Whitney test. Multivariable linear regression was used to estimate the association between 25(OH)D levels and TB disease after adjusting for potential confounding factors. A secondary multivariable linear regression model log-transformed 25(OH)D levels, yielding similar results (data not shown). Because of the limited sample size, for multivariable analyses, only factors known to be potential predictors of the outcome that had a P ≤0.2 in the univariable analyses were included in multivariable regression models. Significance was established with a P ≤0.05.
Results
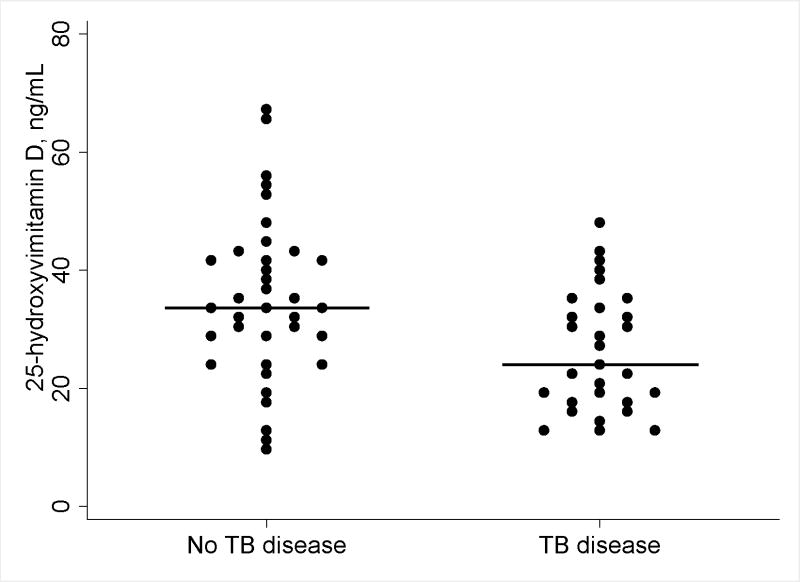
Twenty-nine persons with prior TB disease and 36 controls without TB disease were included in this analysis. The demographic and clinical characteristics of the study groups are shown in Table 1. TB disease was associated with lower 25(OH)D levels compared to controls without TB disease [median 25(OH)D, 24.7 ng/mL vs. 33.6 ng/mL; Mann-Whitney test, P=0.01] (Figure 1). Other factors associated with significantly lower 25(OH)D included black race [median 25(OH)D, 16.8 ng/mL vs. 33.4 ng/mL; P<0.01] and enrollment in winter [median 25(OH)D, 18.7 vs. 33.4; P<0.01] (Table 2).
Table 1.
Clinical and Demographic Characteristics of Study Groups
| Characteristicsa | Prior TB disease (n=29) | No TB disease (n=36) | Pb |
|---|---|---|---|
| Age in yearsc | 48 (35–59) | 44 (37–54) | 0.58 |
| Male sex | 16 (55) | 13 (36) | 0.12 |
| Black race | 11 (38) | 7 (19) | 0.10 |
| Hispanic | 4 (14) | 1 (3) | 0.10 |
| Non US-born | 13 (45) | 2 (6) | <0.01 |
| Enrollment in winter | 8 (28) | 6 (17) | 0.29 |
| 25(OH)D ng/mLc | 24.7 (18.3–34.1) | 33.6 (26.2–42.4) | 0.01 |
Data are presented as numbers (%) of individuals except indicated.
Obtained by using Mann-Whitney or Pearson’s chi-square test.
Data are presented as median numbers (interquartile range).
Figure 1.
Median levels of total 25-hydroxyvitamin D [25(OH)D] by study group. Each dot represents the level of 25(OH)D for an individual patient. Bars represent medians.
Table 2.
Baseline Characteristics and Effect on Plasma 25-hydroxyvitamin D [25(OH)D] Levels in Univariable Analysis (n=65)
| Factor | Univariable analysis
|
|
|---|---|---|
| Mean effect on 25(OH)D (95% C.I.) | P | |
| Age in years | 0.09 (−0.10, 0.28) | 0.35 |
| Male sex | −1.80 (−8.24, 4.63) | 0.58 |
| Black race | −11.36 (−17.92, −4.79) | <0.01 |
| Hispanic | −7.09 (−18.99, 4.80) | 0.24 |
| Non-US birth | −7.13 (−14.53, 0.27) | 0.07 |
| Winter enrollment | −12.93 (−20.02, −5.85) | <0.01 |
| TB disease | −8.50 (−14.58, −2.41) | <0.01 |
In multivariable linear regression modeling, black race (adjusted mean difference [β]=−8.3 ng/mL; 95% CI −14.5, −2.2; P<0.01), enrollment in winter (β=−10.4 ng/mL; 95% CI −17.0, −3.8; P<0.01) and prior TB disease (β=−5.8 ng/mL; 95% CI −11.4, −0.3; P=0.05) were independently associated with lower 25(OH)D levels (Table 3).
Table 3.
Factors Associated with Plasma 25-hydroxyvitamin D [25(OH)D] in Multivariable Analyses (n=65)
| Factor | Multivariable regression model
|
|
|---|---|---|
| Mean effect on 25(OH)D (95% C.I.) | P | |
| Black race | −8.33 (−14.49, 2.17) | <0.01 |
| Winter enrollment | −10.39 (−17.01, −3.77) | <0.01 |
| TB disease | −5.82 (−11.32, −0.32) | 0.05 |
For this model, F=10.11, adjusted-R2=0.29, and P<0.01
The median number of months after completion of therapy in the TB disease group was 7 (IQR, 1 – 21). There was no association in the univariable or multivariable analyses between 25(OH)D levels and the number of months from completion of anti-TB therapy to when 25(OH)D levels were drawn (Pearson’s r = 0.26; P=0.17; and β=0.17, −0.6 to 0.4; P=0.15; respectively).
To assess if 25(OH)D levels differed by site of TB disease among cases, we compared 25(OH)D levels between patients with prior PTB (n=18) and patients with prior EPTB (n=11) and found no significant difference [median 25(OH)D, 26.1 ng/mL (IQR, 19.2 – 32.1) in PTB and 22.1 ng/mL (IQR, 15.6 – 40.6) in EPTB; P=0.81]. We also compared 25(OH)D levels between persons with LTBI (n=16) and uninfected contacts (n=20) in the control group and found no significant difference [median 25(OH)D, 36.1 ng/mL (IQR, 29.5 – 43.9) in LTBI, 32.5 ng/mL (IQR, 23.1 – 40.5) in uninfected contacts; P=0.31].
Discussion
We found lower 25(OH)D levels among persons who recovered from TB disease compared to controls without TB disease, after adjusting for important confounding factors. To our knowledge, this is the first report of 25(OH)D levels in persons who have recovered from TB disease.
Prior cross-sectional studies have reported low levels of 25(OH)D at the time of TB diagnosis and during treatment.2 25(OH)D levels after recovery from TB disease may reflect pre-morbid levels. If true, it would suggest that low 25(OH)D is a risk factor for TB rather than a consequence of the disease process or anti-TB treatment. Furthermore, low levels of 25(OH)D after recovery from TB disease may be a predisposing factor for developing recurrent TB, as 25(OH)D insufficiency affects cellular immune responses and therefore may facilitate relapse or reinfection.16,17 If 25(OH)D levels remain low, they may contribute to the higher risk of TB disease seen among persons who have recovered from prior TB compared to the general population, even among those who completed fully active anti-TB regimens.18 We were unable to assess rates of recurrent TB in our population because study participants were not followed up after their study visit; however, our results suggest that the role of 25(OH)D in recurrent TB should be further investigated in larger, longitudinal studies. Black race and enrollment in winter were associated with lower 25(OH)D levels in the univariable and multivariable analyses.
Black race predisposes to both higher rates of TB disease and 25(OH)D insufficiency;8,19 however, little is known about the effect of race on the association between 25(OH)D and TB disease. Prior studies comparing 25(OH)D levels between patients with untreated TB disease and healthy controls have included very homogeneous populations and the effect of race was not analyzed.1 Interestingly, a post-hoc analysis of 95 subjects with active PTB enrolled in a multicenter TB-treatment trial showed lower 25(OH)D levels among blacks; however, a control group without TB disease was not included.20
Prior studies have not consistently adjusted for the effect of seasonal variation on 25(OH)D concentrations.1 Notably, TB notifications follow a similar annual seasonal pattern with peaks in spring and early summer.9,21 Although this may be in part due to increased M. tuberculosis transmission during indoor winter crowding, low 25(OH)D levels during winter may lead to reactivation of LTBI and subsequent recognition of TB disease during spring and early summer.2 Given this reciprocal seasonal variation in 25(OH)D levels and TB notifications, it may be difficult to separate the effects of seasonality when 25(OH)D levels are obtained at time of TB diagnosis. Our analysis of 25(OH)D after recovery from TB disease may have lessened this potential problem.
Previous EPTB has been associated with subtle immune defects compared to previous PTB, most notably lower CD4+ lymphocyte counts, lower cytokine production, and increased frequency of regulatory T cells.13,22,23 However, it is unclear whether 25(OH)D levels are different between PTB and EPTB. Only two prior case-control studies reported 25(OH)D levels in patients with EPTB. One such study was conducted in India and found that 25(OH)D levels were similarly low in persons with active PTB and EPTB (mean, 10.5 ng/mL and 11 ng/mL, respectively). These 25(OH)D levels appeared to be overall lower than those observed in our study perhaps due to differences in dietary vitamin D intake, method for 25(OH)D quantitation, or 25(OH)D measurement during active disease.24 In the second study, Wilkinson, et al analyzed 25(OH)D levels among Gujarati Asians with untreated TB living in the UK, an ethnic group with high TB rates. Although these investigators used a somewhat different classification of TB disease site (localized disease defined as TB confined to one anatomical site, and severe disease defined as pulmonary or miliary TB), they found no differences in 25(OH)D levels between groups.25 Taken together with our findings, these data suggest that low 25(OH)D levels are present in persons with active and resolved EPTB and PTB; and that these levels appear to be similar regardless of the disease site.
We used liquid chromatography-mass spectrometry for measuring 25(OH)D levels. This technique has the highest accuracy for measuring 25(OH)D among available assays and is currently considered the gold standard.26 In addition, studies have shown that type of specimen (plasma versus serum), length of storage, and multiple freeze-thaw cycles do not affect 25(OH)D concentrations.27 Therefore, the results of 25(OH)D analysis presented here likely reflect the true vitamin D status of the study patients at time of enrollment.
Our study had several limitations. First, our sample size was small and therefore might be underpowered to detect some associations. The difference in 25(OH)D levels seen between study groups in the multivariable analysis warrants further investigation in larger studies. Second, the retrospective design of our study did not allow us to collect information about dietary intake, sunlight exposure, or other factors that influence 25(OH)D status. Also, 25(OH)D concentrations may be affected by anti-TB therapy containing rifampin and isoniazid,14 although clinical studies have shown no significant effect of TB treatment in 25(OH)D levels.28 In addition, the cytokine profiles associated with immune activation in TB disease may lower 25(OH)D levels.29,30 We excluded from our analysis the 6 persons who were enrolled during their last month of anti-TB therapy therefore eliminating the potential effects of ongoing TB disease or treatment on 25(OH)D levels. Moreover, there was no association between 25(OH)D levels and the number of months since completion of anti-TB therapy in univariable or multivariable analysis.
In conclusion, we found lower 25(OH)D levels among persons who recovered from TB disease compared to controls without TB disease, after adjusting for important confounding factors. Larger, longitudinal studies are needed to further characterize the possible role of 25(OH)D in the pathogenesis of TB disease and recurrent TB.
Acknowledgments
Financial support
This research was supported in part by the Vanderbilt CTSA grant UL1 TR000445 from NCRR/NIH (MAH), K24A1065298 (TRS), and K23AI091692-01 (CTF).
Part of the results was presented at the 2013 American Thoracic Society (ATS) International Conference in Philadelphia, PA, USA, May 17 – 22. MAH was a recipient of an ATS Minority Trainee Development Scholarship (MTDS) based on this research.
Footnotes
Potential conflicts of interest
All authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008 Feb;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 2.Ralph AR, Lucas RM, Norval M. Vitamin D and solar ultraviolet radiation in the risk and treatment of tuberculosis. Lancet Infect Dis. 2013 Jan;13(1):77–88. doi: 10.1016/S1473-3099(12)70275-X. [DOI] [PubMed] [Google Scholar]
- 3.Davies PD, Brown RC, Woodhead JS. Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax. 1985 Mar;40(3):187–190. doi: 10.1136/thx.40.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan TY, Poon P, Pang J, et al. A study of calcium and vitamin D metabolism in Chinese patients with pulmonary tuberculosis. J Trop Med Hyg. 1994 Feb;97(1):26–30. [PubMed] [Google Scholar]
- 5.Wejse C, Olesen R, Rabna P, et al. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr. 2007 Nov;86(5):1376–1383. doi: 10.1093/ajcn/86.5.1376. [DOI] [PubMed] [Google Scholar]
- 6.Sita-Lumsden A, Lapthorn G, Swaminathan R, Milburn HJ. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax. 2007 Nov;62(11):1003–1007. doi: 10.1136/thx.2006.070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibney KB, MacGregor L, Leder K, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008 Feb 1;46(3):443–446. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 8.Nahid P, Horne DJ, Jarlsberg LG, et al. Racial differences in tuberculosis infection in United States communities: the coronary artery risk development in young adults study. Clin Infect Dis. 2011 Aug 1;53(3):291–294. doi: 10.1093/cid/cir378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martineau AR, Nhamoyebonde S, Oni T, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci U S A. 2011 Nov 22;108(47):19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006 Mar 24;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 11.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8(5):e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007 Nov;40(2):128–134. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Fiske CT, de Almeida AS, Shintani AK, Kalams SA, Sterling TR. Abnormal immune responses in persons with previous extrapulmonary tuberculosis in an in vitro model that simulates in vivo infection with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2012 Aug;19(8):1142–1149. doi: 10.1128/CVI.00221-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodie MJ, Boobis AR, Hillyard CJ, et al. Effect of rifampicin and isoniazid on vitamin D metabolism. Clin Pharmacol Ther. 1982 Oct;32(4):525–530. doi: 10.1038/clpt.1982.197. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crofts JP, Andrews NJ, Barker RD, Delpech V, Abubakar I. Risk factors for recurrent tuberculosis in England and Wales, 1998–2005. Thorax. 2010 Apr;65(4):310–314. doi: 10.1136/thx.2009.124677. [DOI] [PubMed] [Google Scholar]
- 17.Jolobe O. Potential risk factors for recurrence of pulmonary tuberculosis. Thorax. 2011 Aug;66(8):731. doi: 10.1136/thx.2010.145730. author reply 731. [DOI] [PubMed] [Google Scholar]
- 18.Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis. 2007 Aug;11(8):828–837. [PubMed] [Google Scholar]
- 19.Peiris AN, Bailey BA, Peiris P, Copeland RJ, Manning T. Race and vitamin D status and monitoring in male veterans. J Natl Med Assoc. 2011 Jun;103(6):492–497. doi: 10.1016/s0027-9684(15)30363-1. [DOI] [PubMed] [Google Scholar]
- 20.Yamshchikov AV, Kurbatova EV, Kumari M, et al. Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr. 2010 Sep;92(3):603–611. doi: 10.3945/ajcn.2010.29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parrinello CM, Crossa A, Harris TG. Seasonality of tuberculosis in New York City, 1990–2007. Int J Tuberc Lung Dis. 2012 Jan;16(1):32–37. doi: 10.5588/ijtld.11.0145. [DOI] [PubMed] [Google Scholar]
- 22.de Almeida AS, Fiske CT, Sterling TR, Kalams SA. Increased frequency of regulatory T cells and T lymphocyte activation in persons with previously treated extrapulmonary tuberculosis. Clin Vaccine Immunol. 2012 Jan;19(1):45–52. doi: 10.1128/CVI.05263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antas PR, Ding L, Hackman J, et al. Decreased CD4+ lymphocytes and innate immune responses in adults with previous extrapulmonary tuberculosis. J Allergy Clin Immunol. 2006 Apr;117(4):916–923. doi: 10.1016/j.jaci.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002 Apr;50:554–558. [PubMed] [Google Scholar]
- 25.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000 Feb 19;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 26.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008 Apr;87(4):1087S–1091S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 27.Gallicchio L, Helzlsouer KJ, Chow WH, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: Design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010 Jul 1;172(1):10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011 Jan 15;377(9761):242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conesa-Botella A, Meintjes G, Coussens AK, et al. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012 Oct;55(7):1004–1011. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanham G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996 Jan;103(1):30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]