Abstract
Aims
To examine a syndrome of chronic manganism that occurs in drug addicts in Eastern Europe who use intravenous methcathinone (ephedrone) contaminated with potassium permanganate. The basal ganglia, especially the globus pallidus and the putamen, are damaged irreversibly in many cases. Routine neuropsychological assessment has revealed no cognitive deficits despite widespread abnormalities on brain imaging studies and severe extrapyramidal motor handicap on clinical examination.
Design
Case control study.
Setting
Ephedrone patients and patients with opioid dependence were recruited from Lviv, Ukraine.
Participants
We tested 15 patients with ephedrone induced toxicity, 13 opiate dependent patients, who were receiving opioid replacement therapy and 18 matched healthy volunteers.
Measurements
The ‘beads task’, an information gathering task to assess reflection impulsivity was used and feedback learning, working memory and risk taking were also assessed.
Findings
Opiate dependent patients differed from controls on three out of four tasks, whereas ephedrone patients differed from controls on only one task. More specifically both patient groups were more impulsive and made more irrational choices on the beads task than controls (p<0.001). However, ephedrone patients had no deficits in working memory (p>0.1) or risk taking (p>0.1) compared with controls. Opioid dependent patients had significantly worse working memory (p<0.001) and were significantly more risk prone than controls (p=0.002).
Conclusions
Ephedrone patients may have similar deficits in information gathering and decision making to opiate dependent patients, with preservation of working memory and risk taking. This may reflect specific damage to anterior cingulate- basal ganglia loops.
Introduction
Methcathinone also known as ephedrone and mephedrone, is one of several homemade synthetic cathinones with amphetamine like stimulant activity. Ephedrone users inject themselves several times a day in binges over several days. In eastern Europe, it is generally manufactured on a small scale using commercially available nasal decongestants including phenylpropranolamine (PPA) and pseudoephedrine, potassium permanganate, used as an oxidant and disinfectant(1) and vinegar. During this reaction, as a side product, manganese ions are formed, which then accumulate in the brain and cause dystonia, postural instability, a quiet slurred pallidal speech, dopaminergic unresponsive bradykinesia and later a typical “cock gait”(2). There have been no post mortem examinations so far but magnetic resonance imaging (MRI) of the brain revealed that the disorder affects mainly the globus pallidus, the substantia nigra and to a lesser degree the subthalamic nucleus, the putamen and the caudate nucleus(3). Dopamine transporter (DAT) scans confirm an intact nigrostriatal pathway (2). Although the white matter appears to be normal on T1-weighted MRI scans, diffusion tensor imaging studies showed extensive white matter changes particularly in the frontal and premotor areas and widespread damage to cortico-pallidal connections(4). Despite these extensive abnormalities on brain imaging, only mild deficits in executive function have been reported(3–7). Individual case reports have pointed towards a tendency towards impulsivity(8) but this has never been studied systematically. However, drug addiction is associated with executive, memory and decision making dysfunction(9). Opiate and amphetamine dependent patients have difficulties in planning, learning and memory(10) which persist during opiate replacement therapy(11). Opiate dependent patients also make more risky decisions which may reflect abnormal patterns of orbitofrontal cortex activation(12).
We have compared patients with ephedrone induced extrapyramidal symptoms to substance abusers without neurological deficits who were taking opioid replacement therapy and healthy volunteers on working memory (WM), feedback learning, risk taking and the beads task. The beads task explores the amount of information participants gather before making a decision sometimes referred as “reflection impulsivity”(13–15). Jumping to conclusions has previously been reported in patients with schizophrenia(16–18), patients with Parkinson’s disease (PD) with and without impulsive compulsive behaviours(19) and substance abusers (17). We combined the WM and the beads tasks because it has been suggested that jumping to conclusions might be a specific strategy to reduce WM load(20). We also used emotionally salient and neutral distractors in the WM task, given the negative effects of task irrelevant information on WM performance(21). We sought to distinguish between reflection impulsivity and risky choice which are two components of cognitive impulsivity(22). We also assessed reward and punishment learning separately as it is unclear whether potential abnormalities on tasks measuring reflection impulsivity or risk preference are driven by impaired negative or enhanced positive feedback learning.
In this study we were interested in assessing differences between former addicts with ephedrone toxicity and current drug dependent patients. Clinical impression has suggested that most patients with ephedrone induced basal ganglia damage lose their craving for illicit substances and cease abusing drugs. It is unclear whether their physical disability or damage from the accumbens-pallidum circuitry is responsible for this change in behaviour. Studies in rodents have shown that the globus pallidus plays a key role in the reinforcing effects of illicit drugs(9) and its damage might therefore abolish craving.
We hypothesized that both patient groups are likely to have orbitofrontal cortex dysfunction, considering its important role in drug preoccupation and impulsivity(23), given the wide spread abnormalities on neuroimaging studies in ephedrone (4) and drug abusers (24). Further we predicted that ephedrone patients would perform similarly to opiate dependent patients in tasks measuring reflection impulsivity, since jumping to conclusions is known not to recover even after prolonged abstinence in substance abusers(14). On other tasks, such as risk taking and feedback learning we speculated that ephedrone patients would perform better than opiate dependent patients given differences in drug craving and shorter duration of illicit drug abuse. We also expected that both patient groups would be worse on the WM task, especially when salient distractors were presented but would on the other hand remember distractors significantly better than healthy controls.
Methods
All participants provided written informed consent. The protocol was approved by the UCLH Trust or Ukrainian local ethics committee. All participants scored more than 26/30 on the Mini Mental State Examination (MMSE)(25) and were tested once usually mid-mornings. Participants received a modest reward (between £10–15) depending on their performance.
Patients
Ephedrone patients were recruited from a small database of attendees at the University Clinic Lviv and almost all patients agreed to take part several days prior to testing.
Opiate dependent patients who were interested in participating were chosen randomly amongst patients attending the psychiatric outpatient clinics in Lviv.
Ephedrone
Fifteen patients with ephedrone induced extrapyramidal symptoms were recruited from the department of Neurology of Lviv Regional Clinical Hospital, Ukraine. All patients had moderate to severe extrapyramidal symptoms, moderate to severe impairment in postural stability, dystonia and had decrement in finger tapping with some axial rigidity, induced by ephedrone. Fourteen patients developed extrapyramidal symptoms after intravenous methcathinone abuse, 1 patient after recurrent oral intake.
A detailed neurologic examination was performed by a movement disorder specialist on the day of testing. No patient had a resting tremor or was treated with dopamine replacement therapy. One patient was wheelchair bound. Seven out of 15 patients developed a characteristic “cock-gait “and had a pallidal speech, similar to patients with progressive supranuclear palsy. No patient was taking any illicit drugs within the last 2 years. Five ephedrone patients consumed other illicit drugs for a short time in the past, but were never dependent on these drugs.
Manganese levels have been measured in pubic the hair samples in 9 out of 15 confirming the diagnosis of manganese toxicity. For further details see (5).
Opiate dependent patients
Thirteen male patients with a recent history of illicit drug abuse, meeting DSM-IV-TR criteria for opioid dependence(26) were recruited from the Replacement Therapy Unit of Lviv, regional Clinical Narcological Dispensary and the department of Lviv Regional Clinical Narcological Dispensary, Ukraine. All patients had clinically normal cognitive function, and were on opioid replacement therapy with buprenorphine. Neurological examination was normal in all patients. Twelve out of 13 patients had a long standing history of intravenous opioid abuse (Table 1). All tests were performed prior to their dose of buprenorphine. Only those patients who were able to tolerate a delay of their buprenorphine dose were included. Patients who suffered from clinically evident withdrawal symptoms were excluded. None of these patients reported taking any illicit substances at the time of testing.
Table 1.
Demographic characteristics with details about past history of substance abuse in patients. All values are mean ± SD.
| Controls | Ephedrone | Opiate dependent patients |
t value, χ2 and F-value |
p-value | |
|---|---|---|---|---|---|
| Participants(no.) | 18 | 15 | 13 | ||
| Age (yrs) | 32.3±5.5 | 34.0±7.2 | 32.0±7.1 | 0.34 | 0.71 |
| Gender (male) | 18 | 13 | 12 | 2.4 | 0.3 |
| Education | 13.8±2.8 | 12.2±1.4 | 12.0±1.9 | 5.1 | 0.01* |
| Drug abuse (yrs) | 12.0±5.1 | ||||
| Replacement therapy (yrs) | 1.4±1.3 | ||||
| Ephedrone abuse (yrs) | 1.5±1.2 | ||||
| Ephedrone stopped (yrs) | 6.2±2.6 | ||||
| Parkinsonism (yrs) | 7.0±2.4 | ||||
| Substance abuse (patients) | 15 | 13 | |||
| i.v. opioid a | 4 | 12 | |||
| i.v.heroin | 2 | 4 | |||
| Cannabis | 3 | 3 | |||
| Cocaine | 1 | 1 | |||
| Morphine | 0 | 1 | |||
| Ephedrone (i.v/oral) | 14/1 | 0 | |||
Significant differences are labeled with “*”. Controls, ephedrone, opiate dependent patients.
Controls
Results were compared with 18 age matched healthy male volunteers. A similar sample size using identical tasks was used previously in PD patients (27, 28).
For demographic characteristics see Table 1.
Working memory task
WM was assessed using a task which uses abstract geometric figures, and emotional distractors during the memory interval. This allowed us to assess the effects of distractibility, and particular emotionally valenced distractibility on WM. Twenty four trials of the WM task were performed on a laptop computer, after an explanatory demonstration. Participants were asked to memorize either 2 or 3 geometric figures which were shown for 3 seconds, followed by a delay of 2 seconds. Then another geometric figure was presented and participants were asked whether this figure was within the set that they had to remember before. During the delay, one or two positive, neutral or negative distractors obtained from the validated International Affective Picture System were shown (29). Salient and neutral pictures contained mainly human characters. Positive pictures contained food or sexual motives, negative pictures included scenes of violence.
After the 24 trials participants were shown a series of distractor images and were asked whether they thought they have seen these images before. In half of the 24 series (12/24) distractors were previously shown.
Beads task
We used the beads task to assess the amount of information subjects gathered before committing to a decision. This is often called reflection impulsivity (30) and several studies have shown that subjects with substance dependence commit to decisions without gathering much information (14, 27).The beads task was administered on a laptop computer and was explained on a series of slides on the screen. Participants were told that a sequence of beads would be drawn from one of two cups, one containing predominantly green and the other predominantly blue beads. All bead draws for each sequence were drawn from the same cup. For each sequence of draws, participants were first shown a bead, either green or blue. After seeing the bead, they could choose to draw another bead from the cup, or they could choose to guess the cup that was being drawn from. This was repeated until they guessed a cup or they drew 10 beads, after which they had to guess which cup was being drawn from. We recorded the number of beads drawn before the participant guessed a cup and whether the choice represented a rational (e.g. if more blue beads were drawn the participant guessed blue) or irrational (i.e. guessing the less likely cup colour) choice. For further details see (27). Participants had to play 4 blocks with 2 blocks containing an 80:20 ratio of beads within each cup and 2 blocks a 60:40 ratio. Each block had 3 trials. They were told that they would win 10 units if they guess the correct cup. Incorrect choices by participants resulted in either a loss of nothing (in 2 blocks) or a loss of 10 units (in 2 blocks) and participants were informed of the loss condition before each trial. Participants knew that they could draw up to 10 beads before making a decision. They were, however “charged” 0.2 units for each additional draw, so additional draws reduced the amount they would win, although by only a small amount.
Risk task
A gambling task was used to probe the risk preference/aversion of the subjects (31). Studies have shown that addicts make decisions in the Iowa Gambling task that suggest that they are risk prone (32). However, the Iowa Gambling task includes both elements of risk and feedback learning. Therefore we analyzed each element separately. In each trial subjects were given a choice between two gambling options which were presented on the left and right of the screen. Each option had either a single sure outcome, or two possible outcomes. For example, if the subjects had a 20% chance of winning 10 units and an 80% chance of winning 5 units, the pie would be split 80/20. The sure options were displayed as solid circles, representing the 100% outcome. Immediate feedback was given after selecting the preferred gamble. For details see (28).
Feedback learning task
The ability of participants to integrate positive and negative feedback within a learning context was assessed using an instrumental learning task. The task had four blocks of 24 trials (33). In each trial participants were shown two stimuli and they had to select one of them. After choosing a stimulus they were informed of the outcome. Each block contained a fixed probability of winning or losing associated with each stimulus, and one stimulus was more often rewarded, or less often punished than the other. Participants were asked to select the stimulus that they thought was more likely to win in 2 “winning blocks” or less likely to lose in 2 “losing blocks”. In “winning blocks” participants could either win 0.5 units or win nothing, in the other 2 “losing blocks” subjects should avoid a loss or could lose 0.5 units. Feedback was given immediately. Winning probabilities for the two stimuli were 70%/30%.
Data Analysis
Statistical analysis was performed using SPSS, version 18. For the demographic variables, age, gender, years of education were used as dependent variables and groups (ephedrone, substance abusers and controls) were modeled as a between subject factor. We used ANOVA, t-test or χ2 test where appropriate. Years of education was modeled as a cofactor for all other analyses but did not change any results.
WM task
We used a generalized linear model (SPSS) with a binary logistic distribution. As a dependent variable we used score (correct response =1 or incorrect response =0). Distractor (positive, neutral or negative), number of memoranda (2 or 3 geometric figures), choice (yes, no) and actual shown figure (yes, no) were modeled as fixed factors. Groups (ephedrone, substance abusers and controls) were modeled as a between subject factor and subject was a random factor nested under group.
Beads task
We performed analyses using a generalized linear model (SPSS). As a dependent variable we used either the number of draws before making a decision or opposite colour choice, the number of times participants made an irrational decision (eg. blue bead shown, green cup selected). As these are both count variables we used a Poisson model which had a loglinear link function. For the first analysis beads ratio (80:20 or 60:40) and loss condition (loss, no loss) were modeled as fixed factors. Groups (ephedrone, controls, substance abusers) were modeled as a between factor and subject was a random factor nested under group.
Risk task and feedback learning task
Data analysis for the risk and learning tasks was carried out by fitting parametric decision making models to the behavior of each individual subject, and comparing the distributions of parameter fits from the model between groups in a within subject design. For further details see(28).
Results
Demographics
There was no age difference between the 3 groups (F(2,44)=0.34, p=0.7), but there was a significant difference in years of education (F(2,42)=5.1, p=0.01). Post hoc analysis showed that controls had significantly more years of education than ephedrone patients (p=0.033) and substance abusers (p=0.022).
WM task
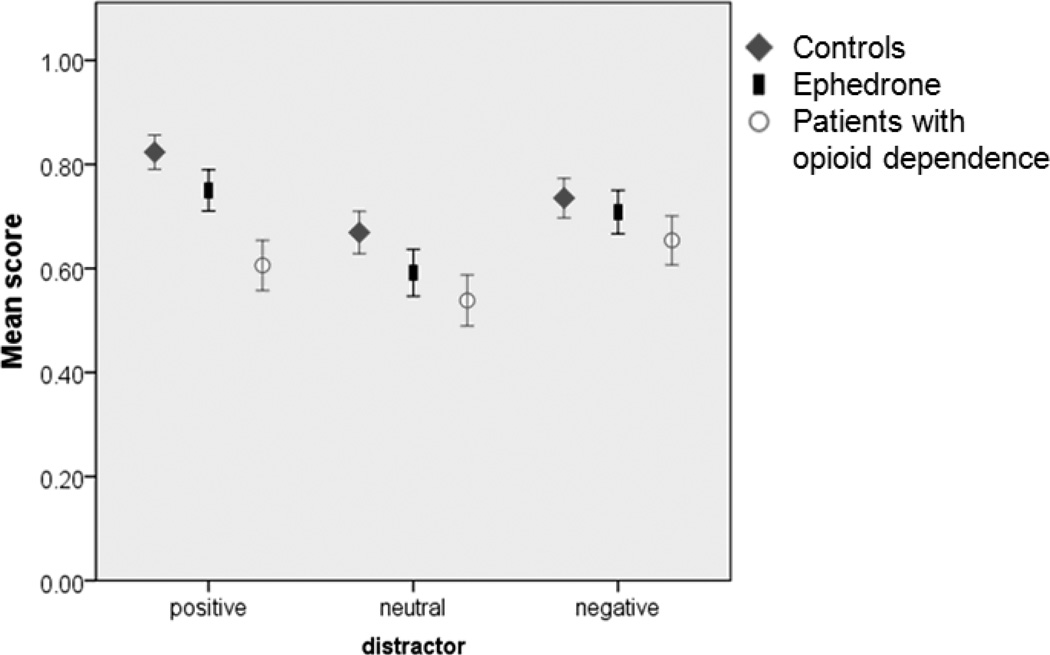
There was a significant effect of group (Wald χ2 =16.0, p<0.001), a significant effect of distractor type (Wald χ2 =17.8, p<0.001) (Fig. 1) and a significant distractor by number of memoranda interaction (Wald χ2=10.0, p=0.007). Pairwise analysis between the groups showed that opiate dependent patients performed significantly worse than ephedrone patients and controls (see Table 2). We also analyzed how often the distractors could be remembered at the end of the experiment, but found no group differences (Wald χ2=4.3, p=0.5).
Figure 1.
WM performance with positive, neutral and negative distractors. Error bars are +/− 1 SE.
Table 2.
Summary of pair-wise comparisons between groups. Both patient groups performed significantly worse on the beads task (“draws”, “opposite colour”) than controls. Patients with opioid dependence had worse WM than both other groups and were more risk prone than controls.
| Group (χ2,F-value and p-value) |
Opiate dependent patients |
Controls |
|---|---|---|
| Ephedrone | ||
| WM | χ2 =6.2, p=0.013 | χ2 =2.3, p=0.12 |
| Draws | χ2 =3.2, p=0.076 | χ2 =45.3, p<0.001 |
| Opposite colour | χ2=0.54, p=0.46 | χ2=30.1, p<0.001 |
| Risk taking | F1,29=2.03, p=0.496 | F1,31=4.67, p=0.116 |
| Opioid dependent patients | ||
| WM | χ2=15.4, p<0.001 | |
| Draws | χ2=30.0, p<0.001 | |
| Opposite color | χ2=34.1, p<0.001 | |
| Risk taking | F1, 30=14.75, p=0.002 | |
Significant p-values are highlighted in bold.
Beads task
We first examined the number of draws each participant made in the different conditions (Fig. 2a). We found a significant main effects of group (Wald χ2 =73.0, p<0.001), beads ratio (Wald χ2 =4.5, p=0.033), a significant group by loss condition interaction (Wald χ2 =6.5, p=0.037) and a significant group by ratio interaction (Wald χ2=9.5, p=0.009).
Figure 2.
Beads task. A: Average number of draws per condition by group. B: Number of times participants chose the opposite colour. Error bars are +/− 1 SE
Pairwise comparisons between the 3 groups showed a significant group by loss interaction between the ephedrone group and opiate addicts (Wald χ2=5.5, p=0.019) and a significant group by ratio interaction between the ephedrone and the control group (Wald χ2=9.3, p=0.02) (Table 2).
Opposite color choice
We subsequently examined the number times participants chose the opposite color, or the less probable cup, given the beads that had been drawn (Fig. 2b) and found a significant main effect of group (Wald χ2 = 34.6, p<0.001) (Table 2).
Risk task
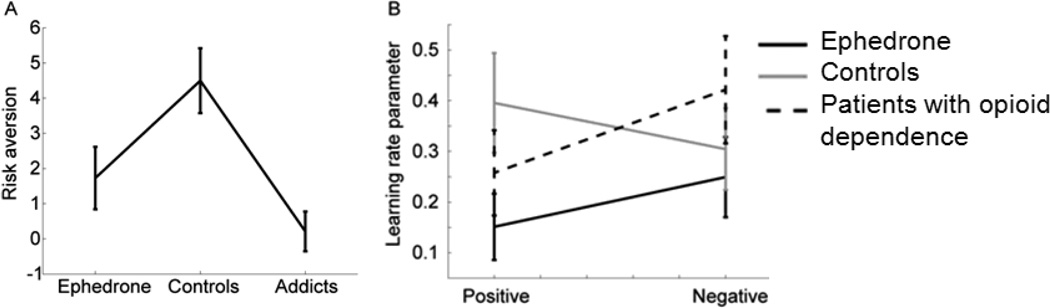
There were group difference in preference for risky gambles (F2, 45=7.06, p=0.002). Opiate dependent patients were more risk prone than controls, but there were no other significant differences (Fig. 3a, Table 2).
Figure 3.
A: Plot showing risk preference across the groups. B: Learning behaviour across the 3 groups.
Learning task
Performance on the learning task was analyzed by fitting separate learning rate parameters to the positive and negative feedback conditions (34). All groups learned equally well (F2, 43=1.78, p=0.173) and that there was no difference in how groups responded to either learning to win or learning to avoid losing, measured as a group by feedback type interaction (F2, 43=1.07, p=0.345). There was also no difference in how positive vs. negative feedback (Fig. 3b) were integrated (F1,43= 0.6, p=0.438).
Discussion
This is the first study to systematically analyze WM, feedback learning, risk taking and information gathering in patients with ephedrone toxicity. Results were compared with patients with opioid dependence and healthy volunteers.
There was no difference in WM performance between controls and ephedrone patients even when salient distractors were shown. Both these groups performed significantly better than opiate dependent patients. Previous studies have demonstrated poor attention in substance abusers when required to ignore salient stimuli during WM tasks(35). Our results are also consistent with previous studies that have shown impaired WM performance in opiate dependent patients (36). All patients on opioid replacement therapy were tested prior to their daily buprenorphine dose and therefore conceivably might have had subtle withdrawal symptoms and low brain dopamine levels(9) Thus impaired WM performance in this group might be explained by the inverted “U-shape” hypothesis, suggesting that too low or excessive dopamine levels both can impair cognitive function(37). It is however also possible that subclinical anxiety due to withdrawal might have contributed to poor WM performance. The normal WM performance in the ephedrone group is in keeping with other studies showing normal scores on MMSE and frontal assessment battery (FAB) scores(7, 38). Interestingly controls and ephedrone patients performed better when salient distractors were presented. Emotional stimuli can enhance cognitive functions (e.g. precise recall of a moment during an emotional event) but can also worsen WM capacity, particularly when they need to be ignored(21). Thus our hypothesis that WM performance would decline with salient distractors proved incorrect. One possible explanation is, that during high cognitive load the impact of salient distractors is reduced whilst activity in the dorsolateral prefrontal cortex increases(39). An easier version of the task might have led to stronger effects of distractors on WM performance.
We also found a relative improvement of WM performance with positive distractors. Implicit exposure to positive images might induce striatal dopamine release and might indirectly boost WM performance, given the role of striatal dopamine in WM(40). However there was no similar effect in opiate dependent patients. It is possible that in this group, due to changes of the amygdala during addiction(9), salient photos might be stimulating to a lesser extent and therefore fail to lead to an enhancing memory effect. Chronic buprenorphine has been also shown to reduce the salience of the drug-associated cues(41) and might have reduced attention to salient cues.
Decision making on the beads task is processed via a circuit involving the anterior cingulate, the parietal cortex, the insula and the ventral striatum(15). Controls who gathered more information had more parietal cortex activation(15). The anterior cingulate is necessary for optimal decision making and to integrate risks(42). Thus damaged connections from the anterior cingulate to the striatum and globus pallidus-cortical circuits in substance abusers and ephedrone patients(4, 5, 43), albeit due to different mechanisms, could explain the impaired performance on the beads task.
In our study controls drew significantly more beads than patients. Both patient groups also made more irrational decisions and chose the less likely cup more often than controls. Although group differences between ephedrone patients and substance abusers only reached trend levels, ephedrone patients gathered more evidence in the no loss conditions than patients with opioid dependence.
Various deficits in decision making have been reported in substance abusers(44). Impaired decision making has also been found in patients with ventromedial prefrontal cortex lesions(45). ‘Delusional thinking’, defined as a belief based on incorrect inference(26), been reported in treated PD patients with impulsive compulsive behaviours(46, 47), who also chose the opposite cup significantly more often than controls(27). It has been also positively correlated with fewer draws on the beads task in delusional patients with and without schizophrenia(17). Our results are also in line with other studies showing a positive correlation of jumping to conclusion behavior and prefrontal cortex dysfunction during task performance(48). Thus lesions within the anterior cingulate circuit in the ephedrone patients(4) might explain poor performance on the beads task, whilst the dorsolateral prefrontal loop, necessary for WM, may be relatively intact. This discrepancy between impairment in “reflection impulsivity” but intact WM function is consistent with other studies suggesting a dissociation of WM and decision making processing within the prefrontal cortex(49). Increased reward seeking behaviour with a reduced sensitivity to negative feedback or more likely insensitivity to unpredictable future consequences are possible explanations(49). However, the feedback learning task where reward and punishment learning was separately assessed, did not reveal any group differences.
We also examined risk taking behaviour across groups and found that only opiate dependent patients made more risky decisions than controls, whilst group differences between ephedrone and controls only reached trend levels.
One limitation in our study is that we did not assess a full battery of standard neuropsychological tasks and only two tasks assessing cognitive impulsivity were performed. In summary we have demonstrated ‘reflection impulsivity’ in patients with brain damage due to ephedrone toxicity but intact WM and feedback learning. An impaired ability to evaluate risk may contribute to risk taking behaviours including risky injecting practices. Additional neuropsychological studies taking into consideration gender differences are needed and comparisons with patients with chronic manganese toxicity from other causes(50, 51) who have been reported to suffer from compulsive behaviour and emotional lability(52) would be of considerable interest.
Acknowledgement
The authors wish to thank the patients and families who participated in the study. This research was supported in part by the Intramural Research Program of the NIH, NIMH.
Footnotes
Declaration of interest: SOS: Honoraria: Britannia Pharmaceuticals otherwise none. AJL: Consultancies:Genus;Honoraria: Novartis, Teva, Meda, Boehringer Ingelheim, GSK, Ipsen, Lundbeck, Allergan, Orion; Grants: PSP Association, Weston Trust – The Reta Lila Howard Foundation; otherwise none. BBA: Grants:Wellcome, and the Intramural research program of the NIH;otherwise none. Other authors: none.
References
- 1.Chintalova-Dallas R, Case P, Kitsenko N, Lazzarini Z. Boltushka: a homemade amphetamine-type stimulant and HIV risk in Odessa, Ukraine. Int J Drug Policy. 2009 Jul;20(4):347–351. doi: 10.1016/j.drugpo.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanotsky Y, Lesyk R, Fedoryshyn L, Komnatska I, Matviyenko Y, Fahn S. Manganic encephalopathy due to "ephedrone" abuse. Mov Disord. 2007 Jul 15;22(9):1337–1343. doi: 10.1002/mds.21378. [DOI] [PubMed] [Google Scholar]
- 3.Sikk K, Haldre S, Aquilonius SM, Taba P. Manganese-Induced Parkinsonism due to Ephedrone Abuse. Parkinsons Dis. 2011;2011:865319. doi: 10.4061/2011/865319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stepens A, Stagg CJ, Platkajis A, Boudrias MH, Johansen-Berg H, Donaghy M. White matter abnormalities in methcathinone abusers with an extrapyramidal syndrome. Brain. 2010 Dec;133(Pt 12):3676–3684. doi: 10.1093/brain/awq281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selikhova M, Fedoryshyn L, Matviyenko Y, Komnatska I, Kyrylchuk M, Krolicki L, et al. Parkinsonism and dystonia caused by the illicit use of ephedrone--a longitudinal study. Mov Disord. 2008 Nov 15;23(15):2224–2231. doi: 10.1002/mds.22290. [DOI] [PubMed] [Google Scholar]
- 6.Sikk K, Haldre S, Aquilonius SM, Taba P. Manganese-Induced Parkinsonism due to Ephedrone Abuse. Parkinsons Dis. 2011:865319. doi: 10.4061/2011/865319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepens A, Logina I, Liguts V, Aldins P, Eksteina I, Platkajis A, et al. A Parkinsonian syndrome in methcathinone users and the role of manganese. N Engl J Med. 2008 Mar 6;358(10):1009–1017. doi: 10.1056/NEJMoa072488. [DOI] [PubMed] [Google Scholar]
- 8.Yildirim EA, Essizoglu A, Koksal A, Dogu B, Baybas S, Gokalp P. [Chronic manganese intoxication due to methcathinone (ephedron) abuse: a case report] Turk Psikiyatri Derg. 2009 Fall;20(3):294–298. [PubMed] [Google Scholar]
- 9.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010 Jan;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006 May;31(5):1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prosser J, Cohen LJ, Steinfeld M, Eisenberg D, London ED, Galynker II. Neuropsychological functioning in opiate-dependent subjects receiving and following methadone maintenance treatment. Drug Alcohol Depend. 2006 Oct 1;84(3):240–247. doi: 10.1016/j.drugalcdep.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ersche KD, Fletcher PC, Roiser JP, Fryer TD, London M, Robbins TW, et al. Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacology (Berl) 2006 Oct;188(3):364–373. doi: 10.1007/s00213-006-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999 Oct;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 14.Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006 Sep 1;60(5):515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011 Nov 30;31(48):17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Averbeck BB, Evans S, Chouhan V, Bristow E, Shergill SS. Probabilistic learning and inference in schizophrenia. Schizophr Res. 2011 Apr;127(1–3):115–122. doi: 10.1016/j.schres.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine C, Gardner M, Craigie J, Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007 Jan;12(1):46–77. doi: 10.1080/13546800600750597. [DOI] [PubMed] [Google Scholar]
- 18.Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999 Jun;38(Pt 2):113–154. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- 19.Djamshidian A, O’Sullivan S, Sanotsky Y, Sharman S, Matviyenko Y, Foltynie T, et al. Decision-making, impulsivity and addictions: Do Parkinson's disease patients jump to conclusions? Mov Disord. doi: 10.1002/mds.25105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley RE, John CH, Young AW, Over DE. The effect of self-referent material on the reasoning of people with delusions. Br J Clin Psychol. 1997 Nov;36(Pt 4):575–584. doi: 10.1111/j.2044-8260.1997.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 21.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006 Feb 15;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000 Mar;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 24.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000 Mar;10(3):334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: 2000. [Google Scholar]
- 27.Djamshidian A, O'Sullivan SS, Sanotsky Y, Sharman S, Matviyenko Y, Foltynie T, et al. Decision making, impulsivity, and addictions: Do Parkinson's disease patients jump to conclusions? Mov Disord. 2012 Aug;27(9):1137–1145. doi: 10.1002/mds.25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Djamshidian A, Jha A, O'Sullivan SS, Silveira-Moriyama L, Jacobson C, Brown P, et al. Risk and learning in impulsive and nonimpulsive patients with Parkinson's disease. Mov Disord. 2010 Oct 15;25(13):2203–2210. doi: 10.1002/mds.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL, USA: University of Florida; 2008. [Google Scholar]
- 30.Kagan J. Reflection--impulsivity: the generality and dynamics of conceptual tempo. J Abnorm Psychol. 1966 Feb;71(1):17–24. doi: 10.1037/h0022886. [DOI] [PubMed] [Google Scholar]
- 31.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006 Mar 2;49(5):765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003 Spring;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 33.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006 Aug 31;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010 Jan 14;65(1):135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009 Sep;93(3):270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Rapeli P, Kivisaari R, Autti T, Kahkonen S, Puuskari V, Jokela O, et al. Cognitive function during early abstinence from opioid dependence: a comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry. 2006;6:9. doi: 10.1186/1471-244X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41(11):1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 38.Sikk K, Taba P, Haldre S, Bergquist J, Nyholm D, Zjablov G, et al. Irreversible motor impairment in young addicts--ephedrone, manganism or both? Acta Neurol Scand. 2007 Jun;115(6):385–389. doi: 10.1111/j.1600-0404.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Dillen LF, Heslenfeld DJ, Koole SL. Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. Neuroimage. 2009 May 1;45(4):1212–1219. doi: 10.1016/j.neuroimage.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009 Feb;19(2):445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorge RE, Stewart J. The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology (Berl) 2006 Sep;188(1):28–41. doi: 10.1007/s00213-006-0485-1. [DOI] [PubMed] [Google Scholar]
- 42.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006 Jul;9(7):940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 43.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999 Apr;20(4):322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 44.Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007 Oct 26;318(5850):602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- 45.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007 Jan 24;27(4):951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolters E, van der Werf YD, van den Heuvel OA. Parkinson's disease-related disorders in the impulsive-compulsive spectrum. J Neurol. 2008 Sep;255(Suppl 5):48–56. doi: 10.1007/s00415-008-5010-5. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher DA, O'Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson's disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord. 2007 Sep 15;22(12):1757–1763. doi: 10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- 48.Lunt L, Bramham J, Morris R, Bullock P, Selway P, Xenitidis K, et al. Prefrontal cortex dysfunction and ‘Jumping to Conclusions’: Bias or deficit? Journal of Neuropsychology. 2011 doi: 10.1111/j.1748-6653.2011.02005.x. [DOI] [PubMed] [Google Scholar]
- 49.Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation Of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998 Jan 1;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuler P, Oyanguren H, Maturana V, Valenzuela A, Cruz E, Plaza V, et al. Manganese poisoning; environmental and medical study at a Chilean mine. Ind Med Surg. 1957 Apr;26(4):167–173. [PubMed] [Google Scholar]
- 51.Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, et al. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005 Jun 28;64(12):2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- 52.Cotzias GC. Manganese in health and disease. Physiol Rev. 1958 Jul;38(3):503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]