Abstract
Background
An outbreak of haemolytic uraemic syndrome (HUS) due to Shiga toxin-secreting Escherichia coli (STEC) O104:H4 from contaminated fenugreek sprouts occurred in June 2011 near Bordeaux, France. In the context of this outbreak, all patients were treated with the monoclonal anti-C5 antibody, eculizumab.
Methods
The diagnosis of HUS was made based on haemolytic anaemia, low platelet count and acute kidney injury. Data were obtained from initial gastrointestinal symptoms to the end of follow-up 10 weeks after the start of eculizumab.
Results
Among 24 cases of STEC gastroenteritis, HUS developed in nine patients (eight adults and one child), 6 (median; range 3–12) days after digestive symptoms begun. The median (range) highest or lowest biological values were platelet count 26 (range 14–93) G/L; haemoglobin 6.6 (range 5–10.7) g/dL; LDH 1520 (range 510–2568) IU/L; creatinine 152 (range 48–797) µmol/L. All patients had extra-renal complications (liver 9, pancreas 5, brain 3 and heart 3). Two patients were dialysed, and one was ventilated. After failure of plasma exchange to increase platelets in the first three patients, eculizumab was administered in all nine patients, 0–4 days after HUS diagnosis (median 1 day). One patient with very severe neurological HUS received immunoadsorption. Outcome was favourable in all patients, with rapid normalization of haemoglobin, platelets, LDH levels, renal function and neurological improvement. There were no deaths and no serious adverse events related to eculizumab.
Conclusions
Early treatment of O104:H4 STEC-HUS by eculizumab was associated with a rapid and efficient recovery. Controlled prospective evaluation of eculizumab in STEC-HUS is warranted.
Keywords: complement inhibition, eculizumab, Escherichia coli, haemolytic uraemic syndrome, thrombotic microangiopathy
INTRODUCTION
Thrombotic microangiopathies (TMAs) are microvascular occlusive diseases with platelets aggregation, leading to peripheral thrombocytopenia and mechanical destruction of red blood cells [1, 2]. The common form of haemolytic uraemic syndrome (HUS) occurs in the setting of gastroenteritis caused mostly in children by Shiga toxin-producing Escherichia coli (STEC) of the O157:H7 serotype [2]. There are no established guidelines for STEC-HUS treatment; in addition to the best supportive care (BSC), the effect of therapeutic plasma exchange (TPE) is not proven in adults [3–5], and in children, a randomized study has not shown efficacy [6], the use of TPE is usually limited to HUS with neurological involvement, with uncertain efficacy [5–7].
During May and June 2011, an outbreak of STEC-HUS due to E. coli O104:H4 hit North Germany [2, 8–13]. Previously, E. coli O104:H4-related STEC-HUS had been reported in a sporadic case in South Korea [14]. Various treatments have been used during the German outbreak [15], including TPE [16, 17], immunoadsorption [18] and eculizumab [2, 17, 19, 20], a humanized monoclonal antibody that inhibits C5 terminal complement common pathway that has been approved in atypical HUS [21]. In early June 2011, French health authorities (former Agence Française de Sécurité Sanitaire des Aliments et des Produits de Santé—AFSSAPS) and TMA national reference centre (CNR-MAT) informed physicians to be aware of the possibility to use eculizumab in STEC-HUS whenever an extra-renal organ was involved or when renal improvement did not occur under TPE [22], since Lapeyraque et al. [23] had reported positive outcomes in children with severe neurological STEC-HUS.
In June 2011, an outbreak of HUS occurred in the town of Bègles near Bordeaux, France, due to E. coli O104:H4 [24, 25], with characteristics close to the German strain [26, 27]. The mode of contamination was epidemiologically proved as fenugreek sprouts served in a community meal with 169 participants [25]. Among 24 patients with O104:H4 STEC infection, 7 presented with HUS after the contaminating meal, and 2 later had household contamination [24]. All STEC-HUS patients were admitted to the University Hospital of Bordeaux. They all received early treatment with eculizumab, either alone or in combination with TPE or immunoadsorption, for reasons detailed further. Here, we report the clinical presentation and outcome of patients with STEC-HUS treated by eculizumab.
PATIENTS AND PROCEDURES
Patients
All patients presenting with STEC-HUS linked to E. coli of the O104:H4 serotype who were admitted from 21 to 31 June 2011 to the University Hospital (CHU) of Bordeaux, France, were included in this study.
Most patients who presented with abdominal pain and diarrhoea with or without blood after a contaminating meal from 8 June in the town of Bègles in the urban area of Bordeaux, France [25, 28], were under the care of general physicians, or hospitalized in the nearby Military Hospital, Villenave-d'Ornon, France. In accordance with local and national health authorities, a warning was sent to all physicians to transfer patients with signs of HUS to the CHU of Bordeaux, and to avoid treatment with antibiotics [2]. STEC-HUS was suspected based on the presence of diarrhoea (with or without blood) or abdominal pain, evidence of haemolysis as well as evidence of renal complications, including increased serum creatinine levels or proteinuria and haematuria on dipstick analysis.
Diagnosis of STEC-HUS and organ involvement
The diagnosis of HUS was focused on the association between thrombocytopenia (platelets <150 G/L), mechanical haemolysis (anaemia, increase in Lactated Dehydrogenase (LDH) serum levels, undetectable serum haptoglobin and schizocytes when present) and acute kidney injury (AKI; proteinuria and haematuria with or without renal failure) [13, 25, 29].
AKI was graded according to the Acute Kidney Injury Network [30]. Estimated glomerular filtration rate (eGFR) was evaluated by the MDRD simplified formula in adults and by Schwartz's formula in the child, after 10 weeks of follow-up (i.e. 2 weeks after last eculizumab administration). Baseline serum creatinine values were pre-STEC-HUS determinations if available or values at the time of diarrhoea.
STEC-HUS-related main organ involvement was defined by the following criteria:
neurological involvement: coma, seizures, psychiatric or other neurological signs,
heart: serum troponin C levels above normal, abnormal findings on ECG,
liver: serum transaminase or gamma-glutamyl transpeptidase levels above normal,
pancreas: serum lipase levels above normal and
skin: vasculitic purpura not related to thrombocytopoenia.
Biological confirmation of STEC-HUS
Blood samples were drawn to rule out other causes of TMA or haemolysis, including ADAMTS-13 activity, complete coagulation panel, lupus-like anti-coagulant, anti-nuclear antibodies, Coombs' test, HIV and viral hepatitis B and C serologies.
Bacteriological analyses consisted of stool sample or rectal swab for stool culture and Shiga toxin PCR, and serum sample for STEC serology. All samples were sent to the Institut Pasteur, Paris, or to the laboratory of microbiology in Hôpital Robert Debré, Paris, which are national reference laboratories for STEC bacteria (Centre National de Référence des Escherichia coli et Shigelles).
Follow-up
Clinical and biological data were obtained from initial gastrointestinal symptoms to the end of the follow-up at Week 10 after initiation of eculizumab.
Treatment of STEC-HUS
Plasma exchange
TPE with 60 mL/kg/body weight plasma volume was performed daily by plasma filtration (Prismaflex® system, Hospal) and substitution with solvent detergent-processed frozen plasma. Heparin or citrate was used as an anti-coagulant for the extracorporeal circuit.
Eculizumab
Eculizumab was administrated according to the following protocol: for adults, eculizumab 900 mg once per week for 4 weeks, 1200 mg Weeks 5, 7 and 9. For children from 10 to 20 kg of body weight, eculizumab 600 mg at first infusion (Week 1) then 300 mg at Weeks 2, 4, 6 and 8.
All patients received a quadrivalent meningococcal (A, C, Y and W-135) vaccine (Mencevax®, GlaxoSmithKline) prior to receiving first eculizumab infusion. In addition, patients were treated by oral azithromycin or intravenous spiramycin for 14 days and subsequently with phenoxymethylpenicillin lasting 2 weeks after last eculizumab infusion
Immunoadsorption
Best supportive care
Haemodialysis was used in patients with severe AKI. Anti-hypertensive therapy was based on inhibitors of the renin–angiotensin system. As part of anaemia therapy, iron and folate supplementation were initiated if necessary.
Ethics
The first case of STEC-HUS was admitted to the University Hospital of Bordeaux on 21 June 2011. We obtained approval from the Regional Ethics Committee on 29 June, for a study aimed to gain knowledge on pathophysiology, prognosis and treatment of STEC-HUS. This study was approved by the AFSSAPS on 13 July and was registered in ClinicalTrials NCT01406288. All patients, or their representatives, signed informed consent to participate in the study.
Statistical analysis
All data are presented as median and range. Non-parametric tests, Kruskall–Wallis and Wilcoxon's tests were used as appropriate with the Statistica® 10 (Tulsa, OK) software. A P-value of <0.05 was considered significant.
RESULTS
Patients
Nine patients (seven women and two men) aged 4–64 years (median 41.3 years) developed STEC-HUS, and two of them were contaminated through household transmission [24]. Digestive symptoms occurred after a median of 9 (range 5–12) days after the contaminating meal with bloody diarrhoea and/or severe abdominal pain in all patients. The interval between gastro-intestinal symptoms and STEC-HUS was 3–12 days (median 6 days). Four patients had received various antibiotics for diarrhoea. Escherichia coli O104:H4 possessing the Shiga toxin 2 (Stx2) virulence gene in faeces was confirmed for all patients [26].
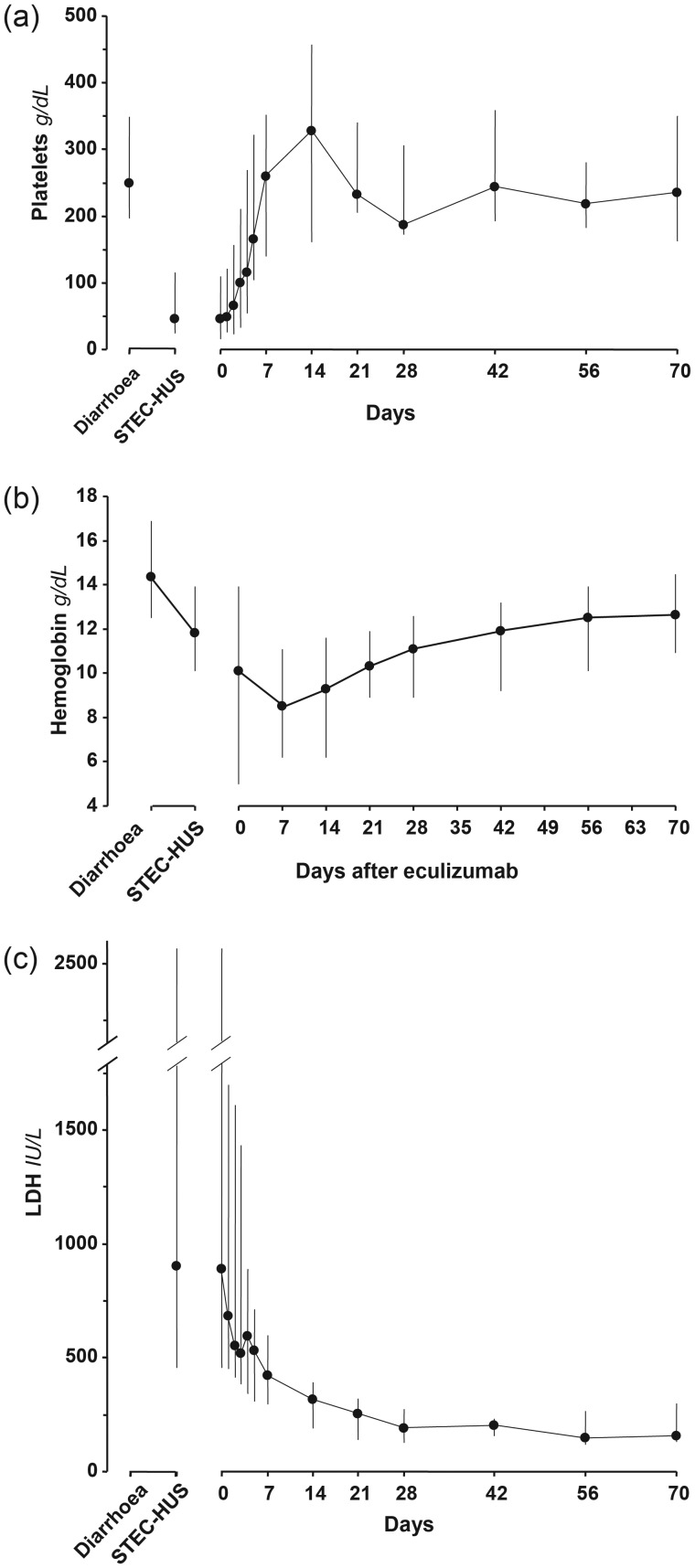
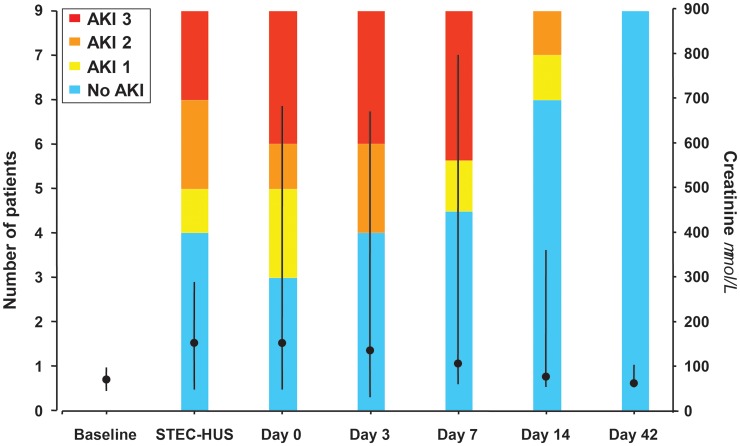
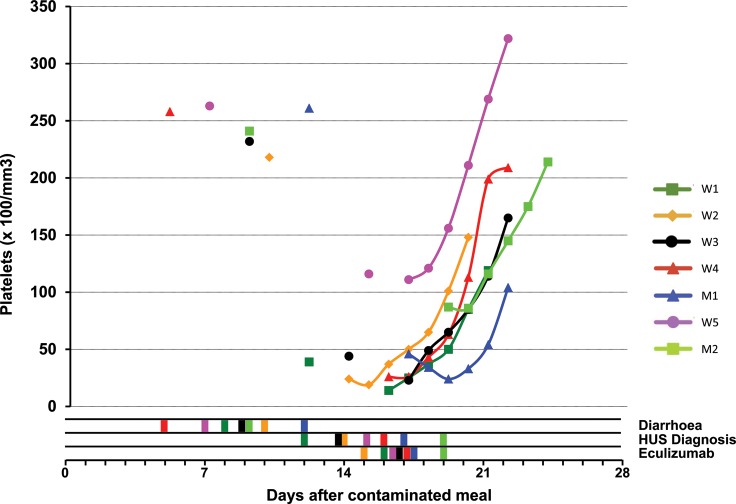
At the time of diarrhoea, no patient had thrombocytopoenia or renal failure, nor anaemia or haemolysis, when evaluated. At the diagnosis of STEC-HUS, the median platelet count was 46 (range 24–116 G/L, Figure 1a). All nine patients exhibited renal impairment (proteinuria—median 2.8 g/g creatinine) and five of them had overt AKI (Figure 2). Two patients required haemodialysis. The median haemoglobin level was 11.8 (range 10.1–13.9) g/dL compared with 14.4 (range 12.5–16.9) g/dL at the time of diarrhoea (P < 0.02, Figure 1b). Median LDH was increased 4-fold above normal (1.6–9-fold, Figure 1c), haptoglobin was undetectable in all patients and leucocyte count was 10.6 (range 5.87–24.9) G/L. During the course of HUS, the lowest biological values were platelet count 26 (range 14–93) G/L and haemoglobin 6.6 (range 5–10.7) g/dL; and the highest biological values were LDH 1520 (range 510–2568) IU/mL and creatinine 158 (range 48–366 µmol/L, excluding dialyzed patients). Detailed data on individual platelet counts in patients contaminated during the collective meal are presented in Figure 3.
FIGURE 1:
Haematological parameters of patients with O104:H4 E. coli HUS at the onset of diarrhoea, diagnosis of STEC-HUS and after initiation of eculizumab treatment. Day 0 = first dose of eculizumab. Data are expressed as median and range. The median interval between diarrhoea and onset of STEC-HUS was 6 (range 3–12) days. (a) Platelet count reached a nadir at diagnosis of STEC-HUS, and increased rapidly and significantly by Day 3 of eculizumab treatment (P = 0.02, Wilcoxon's test). (b) Haemoglobin levels continued to fall after diagnosis of STEC-HUS until Day 7 of eculizumab treatment, and increased significantly after 42 days of treatment (P < 0.05). (c) LDH levels were highest at diagnosis of STEC-HUS, and decreased rapidly and significantly by Day 4 of eculizumab treatment (P < 0.02). Upper limit of normal was 248 IU/L in our hospitallaboratory.
FIGURE 2:
Renal function evaluation of patients with O104:H4 E. coli HUS at the onset of diarrhoea, diagnosis of STEC-HUS and after initiation of eculizumab treatment. Coloured bars: grading of AKI according to the Acute Kidney Injury Network [30]. Closed circles and lines: evolution of median and range serum creatinine concentrations. At Day 42 after initiation of eculizumab treatment, serum creatinine had returned to baseline values.
FIGURE 3:
Individual evolution of platelet count for all seven patients who have been contaminated during the collective meal of 8 June 2011: times of symptoms of diarrhoea, diagnosis of HUS and first injection of eculizumab are displayed for each patient by colour bars in the three lines below the figure. Day 0 is 8 June 2011.
Extra-renal complications were observed in all patients (detailed in Table 1):
All patients had hepatic cytolysis (transaminases 1.5–12 times normal), and one patient exhibited cholestasis without bile ducts dilatation.
Five patients had pancreatic injury with elevated lipase without computed tomography scan abnormalities.
Three patients had myocardial injury with elevated troponin without electrical or echocardiographic abnormalities.
Three patients had neurological symptoms. One patient had severe neurological symptoms, i.e. disorders of consciousness and acute respiratory distress. Brain magnetic resonance imaging (MRI) showed diffuse hypersignals in the cerebral cortex and basal ganglia, and a small subtentorial meningioma [31]. The second patient had binocular diplopia and diffuse pyramidal irritation. The third patient had tremour of the upper arms and mild static and kinetic cerebellar syndrome. MRI was normal in these two last cases.
Table 1.
Characteristics of the patients in the order of HUS diagnosis
| Patient # | Age, years | Sex | Contamination | Lowest platelet count, 1000/mm3 | Lowest Hb level, g/dL | Highest LDH IU/L ULN: 248 IU/L | Highest creatinine, µmol/L | Neuro | Heart troponin (× N) | Liver transaminases (×N) | Pancreas lipase (×N) | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 41 | Female | Collective | 14 | 6.6 | 2451 | HD | 67 | 3 | |||
| W2 | 64 | Female | Collective | 14 | 6.8 | 2568 | HD | a | 23 | 2 | 4 | Lung |
| W3 | 49 | Female | Collective | 23 | 5.0 | 1520 | 150 | 10 | 7 | |||
| W4 | 31 | Female | Collective | 26 | 6.1 | 1981 | 366 | b | 8 | 2 | 3 | |
| M1 | 34 | Male | Collective | 24 | 6.3 | 1611 | 255 | c | 4 | Skin | ||
| W5 | 46 | Female | Collective | 92 | 6.2 | 613 | 68 | 12 | ||||
| M2 | 41 | Male | Collective | 86 | 10.7 | 607 | 152 | 2 | ||||
| W6 | 4 | Female | Household | 93 | 9.7 | 533 | 48 | 2 | ||||
| W7 | 36 | Female | Household | 86 | 8.5 | 510 | 69 | 1.5 |
Enzyme serum levels are presented as × times the upper limit of normal (ULN).
Neurological signs: a: encephalitis, coma, seizures; b: psychiatric, binocular diplopia, pyramidal syndrome; c: static and kinetic cerebellar syndrome.
Hb, haemoglobin; HD, haemodialysis.
Treatments
The first patient who was admitted for HUS had no diarrhoea, but abdominal pain. Shiga toxin on a rectal swab was demonstrated later by PCR; since the type of TMA (thrombotic thrombocytopenic purpura or HUS) was uncertain, she was treated by TPE on the day of admission [4]. Two other patients were admitted the day after, with diarrhoea and HUS, with extra-renal involvement, which led us to treat them with plasma exchange as first-line therapy, and later by eculizumab, as detailed further. One patient received three TPE, and one received two TPE. Another patient received only one TPE after which she had to be intubated and transferred to the intensive care unit [31]. There was no improvement of thrombocytopoenia following initiation of treatment in these three patients; we therefore decided to treat them with eculizumab because of the severity of illness, as explained above. One of these patients also received two immunoadsorption sessions with interruption of eculizumab, as reported [31]. All subsequent patients were treated with eculizumab as first-line therapy without TPE. Overall, the median interval between STEC-HUS diagnosis and treatment by eculizumab was 1 (range 0–4) days. Details on treatments used for each patient are provided in Table 2.
Table 2.
Time course of diarrhoea or digestive symptoms, HUS and eculizumab use
| Patient # | Days between diarrhoea and HUS diagnosis | Days between diarrhoea and eculizumab treatment | Days between HUS diagnosis and eculizumab treatment | Plasma exchange number of sessions | Immunoadsorption |
|---|---|---|---|---|---|
| W1 | 4 | 8 | 4 | 3 | No |
| W2 | 4 | 5 | 1 | 1 | Yes |
| W3 | 5 | 8 | 3 | 2 | No |
| W4 | 11 | 12 | 1 | 0 | No |
| M1 | 5 | 5 | 0 | 0 | No |
| W5 | 8 | 10 | 2 | 0 | No |
| M2 | 10 | 10 | 0 | 0 | No |
| W6 | 6 | 6 | 0 | 0 | No |
| W7 | 12 | 12 | 0 | 0 | No |
Haemolysis activity decreased upon treatment by eculizumab: platelet count increased from baseline by a median of 47% at Day 2. The 129% increase was significant at Day 3 (P < 10–6, Figure 1a); at Day 5, platelet count had increased by >100% (median 386%, range 126–679%, P < 0.0001). After 1 week of treatment, all patients had normal platelet counts (>150 G/L). Haemoglobin levels continued to decrease during the first week, from 10.1 (range 6.0–13.9) g/dL at Day 0 to 8.5 (range 6.2–11.1 g/dL; Figure 1b). Haemoglobin levels increased during the second week (9.3, range 6.2–11.6 g/dL) and tended to normalize thereafter. A decrease in LDH levels was observed the day after the infusion, and it became significant after 4 days (P < 0.02). After 1 week, LDH decreased by 41% (range 7–82%, P < 0.0002, Figure 1c). LDH was still elevated 1.5 times normal at 1 week and remained higher than normal at 2 weeks. At the end of follow-up (10 weeks), haemoglobin, platelets and LDH levels were normal in all patients, respectively, 12.5 (range 10.9–14.5 g/dL), 235 (range 163–350 G/L) and 155 (range 132–208 U/L).
Recovery from AKI was slower (Figure 2); no patient had serious renal sequelae. At the end of follow-up, median eGFR was 91.9 (range 67.9–131.7 mL/min/1.73 m2). GFR was higher than 90 mL/min/1.73 m2 in six of nine patients, and higher than 60 mL/min/1.73 m2 in all patients. Three patients had moderate hypertension, which was already present before STEC-HUS in one. The other two patients are those who had to be dialysed. One patient had macroalbuminuria (0.6 g/g creatinine). No deaths were observed; there were no extra-renal sequelae except one patient with moderate praxic and mnesic difficulties [31].
No serious adverse events related to eculizumab treatment were observed. Headaches of variable intensity were observed in the 48 h following eculizumab infusions in seven patients and nausea or vomiting in three patients.
DISCUSSION
This cohort of nine patients with HUS due to O104:H4 STEC represents all cases of an outbreak due to contaminated fenugreek sprouts served in a community meal, with direct or indirect exposure [24, 25, 28]. Eculizumab was used as specific treatment in all HUS patients. There was a rapid control of TMA, with a significant increase in platelet count within 3 days and normalization by 7 days in all patients. All patients had a normal eGFR at 10-week follow-up.
In this outbreak, the STEC strain was the same as during the large HUS outbreak in Germany [26, 27]. In this cohort, 9 of 24 (38%) patients presented with HUS, 855 of 2987 (22%) in the German cohort [32]. The clinical presentation was very similar, with systemic organ complications in all patients with variable severity. A whole-genome sequencing of multiple isolates from the outbreaks of E. coli O104:H4 in France and Germany has shown that the isolates are all closely related, with extremely limited diversity in the German outbreak isolates, while there was greater diversity among the isolates from the French outbreak [27].
Since the first three patients presented with severe renal insufficiency or extra-renal involvement, first-line treatment was TPE, but there was no impact on disease activity. Four main reasons prompted us to use eculizumab as a first-line treatment as soon as STEC-HUS was diagnosed: first, the disease was very aggressive in the first patients admitted, extra-renal complications were present to some degree in all patients; secondly, we found no improvement with TPE in the first 3 patients, although treatment had been started the day of admission because of the severity of the disease; thirdly, at the time of identification of the O104:H4 STEC strain in our patients, >30 deaths had been reported in the ongoing German outbreak [11], the epidemic could, therefore, have devastating effects and we had no idea of the number of patients who could have been contaminated; fourthly, as stated above, French health authorities had issued a recommendation to use eculizumab in severe forms of STEC-HUS [22]. In these patients who were treated early with eculizumab after STEC-HUS diagnosis, the platelet count increased in all within 3 days (see Figure 3). There was an improvement in renal function within 12 days and recovery of extra-renal organ complications within the first week except for one patient who received immunoadsorption [31].
We observed that patients with the most severe complications, such as anuria, severe central neurological complications or high troponin levels, were among the first four admitted to the hospital. Subsequent patients were identified better and earlier, since they were hospitalized because of bloody diarrhoea and followed prospectively. These patients were treated with eculizumab as the first signs of TMA were identified. This strategy of eculizumab treatment before severe organ function complications developed may have prevented progression to severe systemic complications in the remaining five patients of the cohort. An alternative explanation could be that STEC-HUS was less aggressive in patients who developed the disease later after STEC inoculation. In any case, these patients would have received BSC only according to current recommendations.
This outbreak involved a small number of patients with STEC-HUS (nine patients). No randomization to assess the effect of eculizumab versus BSC only or with the addition of TPE was ethically possible because of the severity and unpredictable evolution during the acute phase of O104:H4 infection. However, this cohort is very homogeneous in terms of the time of onset of contamination, STEC strain and therapy, since in most patients (6 of 9; 66%), eculizumab was the only STEC-HUS-specific therapy. Only three patients received TPE, and one patient immunoadsorption [18, 31]. The association between the start of eculizumab therapy and the recovery of the platelet count may be coincidental: the German experience in patients on BSC [12, 17] is that disease activity is worse on Days 6–8 after diarrhoea, the observed improvement might be the natural course. No firm conclusions can be drawn on eculizumab-specific efficacy on STEC-HUS.
Menne et al. [17] and Kielstein et al. [12] have reported the experience of the German outbreak in adults: patients were treated with BSC, PE or TPE and eculizumab. The same treatment regimen has been used for children, seven of whom were treated with eculizumab alone, and the data on these patients will be published separately [13]. Menne et al. and Kielstein et al. have not found any clear benefit from eculizumab, nor from TPE and steroids, while aggressive treatment by antibiotics seemed to be protective [17, 33, 34]. As underlined by the authors, eculizumab was given to the sickest patients when those with less severe forms of HUS were treated with TPE, while the mildest forms received supportive care only [17]. Patients treated with eculizumab were compared with a group of patients with HUS of similar severity in the study by Menne et al. [17]; a matched cohort analysis using a propensity score-based algorithm was performed in the study by Kielstein et al. [12]. As stated by Menne et al., a bias by indication cannot be ruled out. Furthermore, all patients who received eculizumab had received TPE before, and 40% of them received TPE after eculizumab. As other immunoglobulins, eculizumab is cleared from the circulation by TPE [35], which could have blunted its effect, as well as the delivery of complement components by fresh frozen plasma and additional complement activation during the TPE procedure itself [12]. In the present report, the use of TPE was very limited, and no patient received TPE after eculizumab: this study could be helpful to have better knowledge of the effects of eculizumab when given early in the course of the disease, without a bias by indication.
Because of eculizumab use, all patients received azithromycin or intravenous spiramycin for 14 days as prophylaxis against meningococcal infection: Nitschke et al. [34] have shown that azithromycin given for 3 days was associated with decolonization of stools from STEC. An effect of antibiotics cannot be ruled out in the positive outcome of these patients.
The rationale for terminal complement blockade by eculizumab in STEC-HUS is based on the results of debated pathophysiological studies that have shown that the complement cascade is activated in this form of TMA [2, 36–40]. In plasma samples from 12 HUS patients, Ståhl et al. [38] found microparticles derived from platelets and monocytes bearing C3 and C9, with decreased levels at recovery. Both Stx and O157 STEC lipopolysaccharide (LPS) induced the release of C3- and C9-bearing microparticles from platelets and monocytes [38]. In children with STEC-HUS, Thurman et al. [41] have shown activation of the alternative pathway of complement temporally related to the onset of disease, with resolution within 1 month. In in vitro studies, purified Stx2 markedly activated complement via the alternative pathway and was found to bind to factor H [39]. In a murine model of HUS obtained by co-injection of Stx2 and LPS, Morigi et al. [36] have shown the central role of complement, and the protective effect of complement inhibition.
Danish authors have reported their positive experience with the use of TPE at an early stage in five adults with STEC-HUS complicated by AKI and central nervous dysfunction during the German outbreak [16]. In the context of an epidemic, eculizumab is simpler to use than PE, which may require large volumes of plasma and highly specialized staff. Both therapies may induce adverse events, such as meningococcaemia for eculizumab, and for TPE allergic reactions, haemodynamic instability or bleeding associated with central catheters in the setting of thrombocytopoenia [42]. We believe that our experience supports the need for randomized clinical trials in STEC-HUS evaluating BSC, TPE and eculizumab as a stand-alone treatment [12, 15, 43].
Whether this is feasible in the context of an epidemic remains questionable, given the multiple difficulties to set up such a trial: pressure from politicians and the press, absence of knowledge about the magnitude and duration of the epidemic, mobilization of medical manpower to take care of patients [44] and short time-period to set up an approved protocol. We believe that a randomized trial might be more feasible for sporadic cases in children, since the disease is much more frequent than in adults, and the number of paediatric centres is limited. As detailed above, we have used eculizumab during this outbreak because of several reasons that may not be reproducible, our experience should be considered as indicative of the effects of eculizumab without TPE to set up future trials.
In conclusion, in this limited outbreak of O104:H4 E. coli and associated STEC-HUS, eculizumab was used as early as possible in the course of the disease. All patients recovered from STEC-HUS complications. No firm conclusions can be drawn on the role of eculizumab in this recovery.
CONFLICT OF INTEREST STATEMENT
Y.D. is an investigator for the trials of eculizumab in aHUS and has received speaker fees from Alexion. C.C. has received speaker fees from Alexion and is a consultant for Alexion. None of the other authors have conflicts of interest to declare. We declare that the results presented in this paper have not been published previously in whole or part, except in abstract format. Y.D. and B.V. contributed equally to the study.
ACKNOWLEDGEMENTS
The Centre Hospitalier Universitaire de Bordeaux supported this study. The authors thank patients for their approval to be included in the study, and all physicians and nurses who contributed to the care of the patients. We thank Alexion Pharmaceuticals for providing eculizumab (Soliris®).
REFERENCES
- 1.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Remuzzi G. A German outbreak of haemolytic uraemic syndrome. Lancet. 2011;378:1057–1058. doi: 10.1016/S0140-6736(11)61217-8. [DOI] [PubMed] [Google Scholar]
- 3.Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the apheresis applications committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 4.Clark WF. Thrombotic microangiopathy: current knowledge and outcomes with plasma exchange. Semin Dial. 2012;25:214–219. doi: 10.1111/j.1525-139X.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- 5.Bambauer R, Latza R, Schiel R. Therapeutic apheresis in the treatment of hemolytic uremic syndrome in view of pathophysiological aspects. Ther Apher Dial. 2011;15:10–19. doi: 10.1111/j.1744-9987.2010.00903.x. [DOI] [PubMed] [Google Scholar]
- 6.Loirat C, Sonsino E, Hinglais N, et al. Treatment of the childhood haemolytic uraemic syndrome with plasma. A multicentre randomized controlled trial. The French Society of Paediatric Nephrology. Pediatr Nephrol. 1988;2:279–285. doi: 10.1007/BF00858677. [DOI] [PubMed] [Google Scholar]
- 7.Michael M, Elliott EJ, Ridley GF, et al. Interventions for haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Cochrane Database Syst Rev. 2009;1:CD003595. doi: 10.1002/14651858.CD003595.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz U, Bernard H, Werber D. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med. 2011;365:1763–1770. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- 9.Askar M, Faber MS, Frank C, et al. Update on the ongoing outbreak of haemolytic uraemic syndrome due to Shiga toxin-producing Escherichia coli (STEC) serotype O104, Germany, May 2011. Euro Surveill. 2011;16:pii=19883. doi: 10.2807/ese.16.22.19883-en. [DOI] [PubMed] [Google Scholar]
- 10.Bielaszewska M, Mellmann A, Zhang W, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11:671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 11.Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 12.Kielstein JT, Beutel G, Fleig S, et al. Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: an analysis of the German STEC-HUS registry. Nephrol Dial Transplant. 2012;27:3807–3815. doi: 10.1093/ndt/gfs394. [DOI] [PubMed] [Google Scholar]
- 13.Loos S, Ahlenstiel T, Kranz B, et al. An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: presentation and short-term outcome in children. Clin Infect Dis. 2012;55:753–759. doi: 10.1093/cid/cis531. [DOI] [PubMed] [Google Scholar]
- 14.Bae WK, Lee YK, Cho MS, et al. A case of hemolytic uremic syndrome caused by Escherichia coli O104:H4. Yonsei Med J. 2006;47:437–439. doi: 10.3349/ymj.2006.47.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggenenti P, Remuzzi G. Thrombotic microangiopathy: E. coli O104:H4 German outbreak: a missed opportunity. Nat Rev Nephrol. 2012;8:558–560. doi: 10.1038/nrneph.2012.194. [DOI] [PubMed] [Google Scholar]
- 16.Colic E, Dieperink H, Titlestad K, et al. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet. 2011;378:1089–1093. doi: 10.1016/S0140-6736(11)61145-8. [DOI] [PubMed] [Google Scholar]
- 17.Menne J, Nitschke M, Stingele R, et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ. 2012;345:e4565. doi: 10.1136/bmj.e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greinacher A, Friesecke S, Abel P, et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: a prospective trial. Lancet. 2011;378:1166–1173. doi: 10.1016/S0140-6736(11)61253-1. [DOI] [PubMed] [Google Scholar]
- 19.Kunzendorf U, Karch H, Werber D, et al. Recent outbreak of hemolytic uremic syndrome in Germany. Kidney Int. 2011;80:900–902. doi: 10.1038/ki.2011.323. [DOI] [PubMed] [Google Scholar]
- 20.Dolgin E. As E. coli continues to claim lives, new approaches offer hope. Nat Med. 2011;17:755. doi: 10.1038/nm0711-755. [DOI] [PubMed] [Google Scholar]
- 21.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 22.AFSSAPS. 2011 Agence française de sécurité sanitaire des produits de santé. Point d'information de l'Afssaps sur l'utilisation de Soliris dans le SHU post diarrhéique à shiga toxine produite par E. coli. June 3Available fromhttp://www.afssaps.fr/Infos-de-securite/Points-d-information/Utilisation-de-Soliris-dans-le-SHU-post-diarrheique-a-shiga-toxine-produite-par-E.coli-Point-d-information. 3 June 2011, date last accessed. [Google Scholar]
- 23.Lapeyraque A-L, Malina M, Frémeaux-Bacchi V, et al. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–2563. doi: 10.1056/NEJMc1100859. [DOI] [PubMed] [Google Scholar]
- 24.Aldabe B, Delmas Y, Gault G, et al. Household transmission of haemolytic uraemic syndrome associated with Escherichia coli O104:H4, south-western France, June 2011. Euro Surveill. 2011;16:pii=19934. [PubMed] [Google Scholar]
- 25.King LA, Nogareda F, Weill F-X, et al. Outbreak of Shiga toxin-producing Escherichia coli O104:H4 associated with organic fenugreek sprouts, France, June 2011. Clin Infect Dis. 2012;54:1588–1594. doi: 10.1093/cid/cis255. [DOI] [PubMed] [Google Scholar]
- 26.Mariani-Kurkdjian P, Bingen E, Gault G, et al. Escherichia coli O104:H4 south-west France, June 2011. Lancet Infect Dis. 2011;11:732–733. doi: 10.1016/S1473-3099(11)70266-3. [DOI] [PubMed] [Google Scholar]
- 27.Grad YH, Lipsitch M, Feldgarden M, et al. Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. Proc Natl Acad Sci USA. 2012;109:3065–3070. doi: 10.1073/pnas.1121491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gault G, Weill F, Mariani-Kurkdjian P, et al. Outbreak of haemolytic uraemic syndrome and bloody diarrhoea due to Escherichia coli O104:H4, south-west France, June 2011. Euro Surveill. 2011;16:pii=19905. doi: 10.2807/ese.16.26.19905-en. [DOI] [PubMed] [Google Scholar]
- 29.Wong CS, Jelacic S, Habeeb RL, et al. The risk of the hemolytic–uremic syndrome after antibiotic treatment of Escherichia coli O157: H7 infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Combe C, Bui HN, de Precigout V, et al. Immunoadsorption in patients with haemolytic uraemic syndrome. Lancet. 2012;379:517–518. doi: 10.1016/S0140-6736(12)60228-1. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 32.Robert Koch Institute. Report: final presentation and evaluation of epidemiological findings in the EHEC O104:H4 outbreak, Germany 2011. 2012 http://www.rki.de/EN/Home/EHEC_final_report.html . [Google Scholar]
- 33.Artunc F. Treating Shiga toxin induced haemolytic uraemic syndrome. BMJ. 2012;345:e4598. doi: 10.1136/bmj.e4598. [DOI] [PubMed] [Google Scholar]
- 34.Nitschke M, Sayk F, Härtel C, et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA. 2012;307:1046–1052. doi: 10.1001/jama.2012.264. [DOI] [PubMed] [Google Scholar]
- 35.EMA: European Medicines Agency. Soliris: EPAR—Product Information. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000791/WC500054208.pdf. 27 July 2012, date last accessed.
- 36.Morigi M, Galbusera M, Gastoldi S, et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol. 2011;187:172–180. doi: 10.4049/jimmunol.1100491. [DOI] [PubMed] [Google Scholar]
- 37.Orth D, Würzner R. Complement in typical hemolytic uremic syndrome. Semin Thromb Hemost. 2010;36:620–624. doi: 10.1055/s-0030-1262883. [DOI] [PubMed] [Google Scholar]
- 38.Ståhl A-L, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117:5503–5513. doi: 10.1182/blood-2010-09-309161. [DOI] [PubMed] [Google Scholar]
- 39.Orth D, Khan AB, Naim A, et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J Immunol. 2009;182:6394–6400. doi: 10.4049/jimmunol.0900151. [DOI] [PubMed] [Google Scholar]
- 40.Noris M, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 41.Thurman JM, Marians R, Emlen W, et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:1920–1924. doi: 10.2215/CJN.02730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelat SG. Practical considerations for planning a therapeutic apheresis procedure. Am J Med. 2010;123:777–784. doi: 10.1016/j.amjmed.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Karpman D. Management of Shiga toxin-associated Escherichia coli-induced haemolytic uraemic syndrome: randomized clinical trials are needed. Nephrol Dial Transplant. 2012;27:3669–3674. doi: 10.1093/ndt/gfs456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harendza S. ‘HUS diary’ of a German nephrologist during the current EHEC outbreak in Europe. Kidney Int. 2011;80:687–689. doi: 10.1038/ki.2011.238. [DOI] [PubMed] [Google Scholar]