Abstract
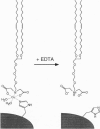
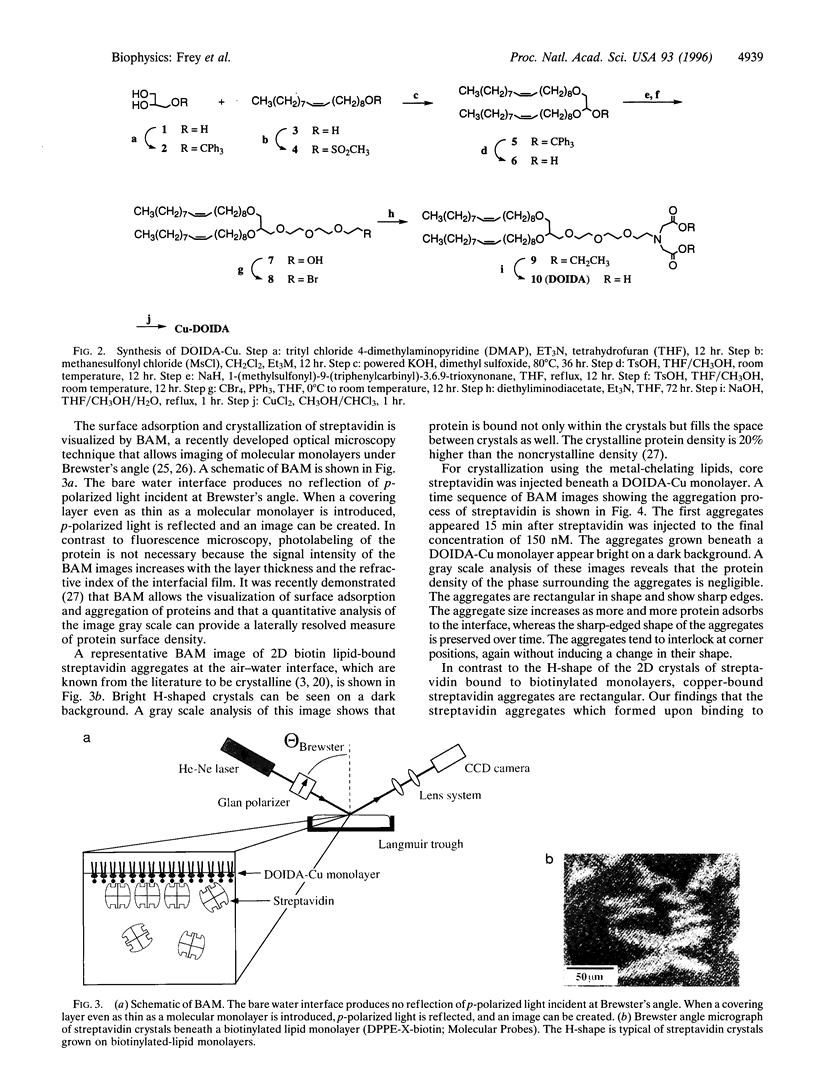
A powerful and potentially general approach to the targeting and crystallization of proteins on lipid interfaces through coordination of surface histidine residues to lipid-chelated divalent metal ions is presented. This approach, which should be applicable to the crystallization of a wide range of naturally occurring or engineered proteins, is illustrated here by the crystallization of streptavidin on a monolayer of an iminodiacetate-Cu(II) lipid spread at the air-water interface. This method allows control of the protein orientation at interfaces, which is significant for the facile production of highly ordered protein arrays and for electron density mapping in structural analysis of two-dimensional crystals. Binding of native streptavidin to the iminodiacetate-Cu lipids occurs via His-87, located on the protein surface near the biotin binding pocket. The two-dimensional streptavidin crystals show a previously undescribed microscopic shape that differs from that of crystals formed beneath biotinylated lipids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold F. H. Metal-affinity separations: a new dimension in protein processing. Biotechnology (N Y) 1991 Feb;9(2):151–156. doi: 10.1038/nbt0291-151. [DOI] [PubMed] [Google Scholar]
- Blankenburg R., Meller P., Ringsdorf H., Salesse C. Interaction between biotin lipids and streptavidin in monolayers: formation of oriented two-dimensional protein domains induced by surface recognition. Biochemistry. 1989 Oct 3;28(20):8214–8221. doi: 10.1021/bi00446a037. [DOI] [PubMed] [Google Scholar]
- Chilkoti A., Schwartz B. L., Smith R. D., Long C. J., Stayton P. S. Engineered chimeric streptavidin tetramers as novel tools for bioseparations and drug delivery. Biotechnology (N Y) 1995 Nov;13(11):1198–1204. doi: 10.1038/nbt1195-1198. [DOI] [PubMed] [Google Scholar]
- Chilkoti A., Tan P. H., Stayton P. S. Site-directed mutagenesis studies of the high-affinity streptavidin-biotin complex: contributions of tryptophan residues 79, 108, and 120. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1754–1758. doi: 10.1073/pnas.92.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst S. A., Ahlers M., Meller P. H., Kubalek E. W., Blankenburg R., Ribi H. O., Ringsdorf H., Kornberg R. D. Two-dimensional crystals of streptavidin on biotinylated lipid layers and their interactions with biotinylated macromolecules. Biophys J. 1991 Feb;59(2):387–396. doi: 10.1016/S0006-3495(91)82232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst S. A., Ribi H. O., Pierce D. W., Kornberg R. D. Two-dimensional crystals of Escherichia coli RNA polymerase holoenzyme on positively charged lipid layers. J Mol Biol. 1988 Sep 5;203(1):269–273. doi: 10.1016/0022-2836(88)90107-6. [DOI] [PubMed] [Google Scholar]
- Dietrich C., Schmitt L., Tampé R. Molecular organization of histidine-tagged biomolecules at self-assembled lipid interfaces using a novel class of chelator lipids. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9014–9018. doi: 10.1073/pnas.92.20.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno T., Sasabe H. Two-dimensional crystallization of streptavidin by nonspecific binding to a surface film: study with a scanning electron microscope. Biophys J. 1993 Oct;65(4):1714–1717. doi: 10.1016/S0006-3495(93)81225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming S. A., Bochkarev A., Darst S. A., Kornberg R. D., Ala P., Yang D. S., Edwards A. M. The mechanism of protein crystal growth from lipid layers. J Mol Biol. 1995 Feb 17;246(2):308–316. doi: 10.1006/jmbi.1994.0086. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. D., Todd R. J., Arnold F. H. Multipoint binding in metal-affinity chromatography II. Effect of pH and imidazole on chromatographic retention of engineered histidine-containing cytochromes c. J Chromatogr A. 1996 Feb 23;725(2):225–235. doi: 10.1016/0021-9673(95)00992-2. [DOI] [PubMed] [Google Scholar]
- Kubalek E. W., Le Grice S. F., Brown P. O. Two-dimensional crystallization of histidine-tagged, HIV-1 reverse transcriptase promoted by a novel nickel-chelating lipid. J Struct Biol. 1994 Sep-Oct;113(2):117–123. doi: 10.1006/jsbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- Lebeau L., Olland S., Oudet P., Mioskowski C. Rational design and synthesis of phospholipids for the two-dimensional crystallization of DNA gyrase, a key element in chromosome organization. Chem Phys Lipids. 1992 Sep;62(2):93–103. doi: 10.1016/0009-3084(92)90087-6. [DOI] [PubMed] [Google Scholar]
- Merritt E. A., Murphy M. E. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr D Biol Crystallogr. 1994 Nov 1;50(Pt 6):869–873. doi: 10.1107/S0907444994006396. [DOI] [PubMed] [Google Scholar]
- Pähler A., Hendrickson W. A., Kolks M. A., Argaraña C. E., Cantor C. R. Characterization and crystallization of core streptavidin. J Biol Chem. 1987 Oct 15;262(29):13933–13937. [PubMed] [Google Scholar]
- Todd R. J., Johnson R. D., Arnold F. H. Multiple-site binding interactions in metal-affinity chromatography. I. Equilibrium binding of engineered histidine-containing cytochromes c. J Chromatogr A. 1994 Feb 18;662(1):13–26. doi: 10.1016/0021-9673(94)85291-X. [DOI] [PubMed] [Google Scholar]
- Uzgiris E. E., Kornberg R. D. Two-dimensional crystallization technique for imaging macromolecules, with application to antigen--antibody--complement complexes. Nature. 1983 Jan 13;301(5896):125–129. doi: 10.1038/301125a0. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Ohlendorf D. H., Wendoloski J. J., Salemme F. R. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989 Jan 6;243(4887):85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]