In this issue of the journal, Agarwal et al. report the very first study to make a head-to-head comparison of a beta-blocker with an ACEi, the Hypertension in Haemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) trial, which was recently presented at the 2013 ASN Congress in Atlanta.
Keywords: hemodialysis, hypertension, randomized trial
Abstract
Background
The purpose of this study was to determine among maintenance hemodialysis patients with echocardiographic left ventricular hypertrophy and hypertension whether in comparison with a β-blocker-based antihypertensive therapy, an angiotensin converting enzyme-inhibitor-based antihypertensive therapy causes a greater regression of left ventricular hypertrophy.
Methods
Subjects were randomly assigned to either open-label lisinopril (n = 100) or atenolol (n = 100) each administered three times per week after dialysis. Monthly monitored home blood pressure (BP) was controlled to <140/90 mmHg with medications, dry weight adjustment and sodium restriction. The primary outcome was the change in left ventricular mass index (LVMI) from baseline to 12 months.
Results
At baseline, 44-h ambulatory BP was similar in the atenolol (151.5/87.1 mmHg) and lisinopril groups, and improved similarly over time in both groups. However, monthly measured home BP was consistently higher in the lisinopril group despite the need for both a greater number of antihypertensive agents and a greater reduction in dry weight. An independent data safety monitoring board recommended termination because of cardiovascular safety. Serious cardiovascular events in the atenolol group occurred in 16 subjects, who had 20 events, and in the lisinopril group in 28 subjects, who had 43 events {incidence rate ratio (IRR) 2.36 [95% confidence interval (95% CI) 1.36–4.23, P = 0.001]}. Combined serious adverse events of myocardial infarction, stroke and hospitalization for heart failure or cardiovascular death in the atenolol group occurred in 10 subjects, who had 11 events and in the lisinopril group in 17 subjects, who had 23 events (IRR 2.29, P = 0.021). Hospitalizations for heart failure were worse in the lisinopril group (IRR 3.13, P = 0.021). All-cause hospitalizations were higher in the lisinopril group [IRR 1.61 (95% CI 1.18–2.19, P = 0.002)]. LVMI improved with time; no difference between drugs was noted.
Conclusions
Among maintenance dialysis patients with hypertension and left ventricular hypertrophy, atenolol-based antihypertensive therapy may be superior to lisinopril-based therapy in preventing cardiovascular morbidity and all-cause hospitalizations. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases; ClinicalTrials.gov number: NCT00582114)
INTRODUCTION
Worldwide, ∼2 million people with end-stage renal disease (ESRD) undergo maintenance hemodialysis, 20–25% of which are in the USA. Among these patients, hypertension is common and is often poorly controlled [1], and when measured in the interdialytic period with ambulatory blood pressure (BP), monitoring is strongly associated with all-cause mortality [2–4]. An important cause of hypertension is volume excess but even after aggressive volume management, many patients receiving thrice-weekly dialysis remain hypertensive [4, 5]. In such patients, nonvolume mechanisms—such as activation of the renin angiotensin system or the sympathoadrenal system—are important to sustain hypertension [6]. Meta-analyses of randomized trials suggest that the risk of cardiovascular events can be cut by a third by using antihypertensive drug therapy among hemodialysis patients especially when they are hypertensive [7, 8]. However, it is not clear whether one class of antihypertensive agent is superior to others in improving cardiovascular outcomes.
Randomized trials among hypertensive people with left ventricular hypertrophy suggest that the inhibition of the renin–angiotensin system is superior to the β-blockade in producing a regression of left ventricular hypertrophy and preventing cardiovascular morbidity and mortality [9]. In this study, we hypothesized that among hypertensive patients on hemodialysis, targeted to a similar home BP goal measured monthly, the use of an angiotensin converting enzyme (ACE) inhibitor (lisinopril) will be more effective than a β-blocker (atenolol)-based antihypertensive therapy in causing regression of left ventricular hypertrophy.
MATERIALS AND METHODS
The Hypertension in Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) was a randomized, open-label, parallel group, active control, single-center trial that compared the safety and efficacy of ACE-inhibitor-based therapy with β-blocker-based treatment, each administered three times weekly after dialysis. The study was conducted between August 2005 and September 2013 at four dialysis units affiliated with Indiana University. Recruitment and data collection were performed by the principal investigator, coordinators and technicians. An independent data and safety monitoring board reviewed the safety data and the study progress on an annual basis. The study was approved by the Institutional Review Board of Indiana University and the Research and Development Committee of the Roudebush VA Medical Center, Indianapolis, and all subjects gave written informed consent. The principal investigator (R.A.) takes full responsibility to the fidelity of this report.
Participants
Patients 18 years or older who had end-stage renal disease treated with chronic hemodialysis dialyzed three times a week (TIW) for at least 3 months with hypertension and left-ventricular hypertrophy were subjects for this study. Patients were excluded if they had ongoing atrial fibrillation, body mass index of ≥40 kg/m2, history of missing one or more hemodialysis treatments in the previous month, known drug abuse, severe chronic obstructive airway disease, stroke or myocardial infarction within the previous 6 months or known contraindication to atenolol or lisinopril.
Study design
Randomization
Subjects were randomized in a 1:1 ratio to either atenolol or lisinopril using concealed opaque envelopes, using a random permuted block design. A permuted block design was chosen to avoid imbalance in assignment to the study drugs over time. Random sequence was generated by a statistician using a computer program and study technicians opened these envelopes after confirming eligibility with the principal investigator. Given that atenolol predictably slows heart rate, it would be easy to guess the assigned drug. Furthermore, a double-blind trial is more resource intensive and costly; therefore, an open-label trial design was chosen. Technicians performing echocardiograms were masked to the treatment assignment.
Diagnosis of hypertension
Subjects were asked to monitor their home BP following a mid-week dialysis for 4 days (as described in the Supplementary data). If patients were treated with antihypertensive medications, these medications were tapered and home BP obtained every week up to a maximum of 3 weeks. If home BP increased to ≥160/100 mmHg during this washout period, further tapering of antihypertensive medications was stopped and 44-h interdialytic ambulatory BP monitoring was performed. A diagnosis of hypertension was made if the 44-h interdialytic ambulatory BP monitoring was ≥135 mmHg systolic or ≥85 mmHg diastolic. After one subject had a stroke shortly after washout, the protocol was amended such that washout was required only if the treated home BP was <150/90 mmHg or the ambulatory BP remained normal after tapering antihypertensive medications.
To evaluate the comparative effectiveness of the two drug regimens in controlling hypertension, interdialytic ambulatory BP monitoring was performed at 3 months, 6 months and at the end of the study.
Diagnosis of left ventricular hypertrophy
Echocardiographic determination of chamber diameters is sensitive to volume changes. Accordingly, echocardiograms were performed immediately following dialysis (as detailed in the Supplementary data). Left ventricular hypertrophy was defined as echocardiographic left ventricular mass index (LVMI) of ≥104 g/m2 in women and ≥116 g/m2 in men [10]. We recognized that cardiac magnetic resonance imaging is less susceptible to volume fluxes and can more accurately detect change in left ventricular mass, but, at the time of initiation of the study, this technique was not available to us.
Drug dosing and titration
Target home BP was 140/90 mmHg or less. Initial study drug dose selection was based on baseline ambulatory BP. If 44- h interdialytic ambulatory BP was 135–154/85–94 mmHg, study drugs were started at the lower dose and titrated upwards using the following protocol. Subjects received atenolol 25 mg TIW or lisinopril 10 mg TIW, and the dose was doubled every 2–4 weeks up to a maximum dose of 100 mg TIW for atenolol and 40 mg TIW for lisinopril. If BP control was not possible felodipine or amlodipine 10 mg QD (once daily) was added, followed by other antihypertensive therapies in the following order: doxazosin, minoxidil and guanfacine. If ambulatory BP was ≥155 mmHg systolic or ≥95 mmHg diastolic patients, the maximum dose of the drug was used: for lisinopril 40 mg TIW after dialysis or for atenolol 100 mg TIW after dialysis.
However, following the protocol amendment, study medications were generally used in maximum doses at initiation along with amlodipine and other drugs. Off-study ACE inhibitors, angiotensin receptor blockers and β-blockers were forbidden. Dry weight was adjusted based on the clinical assessment of volume status. At least monthly titration of antihypertensive drugs was based on home BP measurements.
An interim history, concomitant medication list and adverse events related or unrelated to the study were monitored at each monthly visit. Tolerance to BP reduction was assessed by interdialytic symptoms during the course of the trial at monthly intervals. Study duration was 12 months.
Other measurements
Quality of life was measured by the kidney disease quality of life tool at the beginning and end of the trial. Postdialysis weights were monitored at least monthly.
Outcome
The primary outcome was between group differences in change from baseline (CFB) to 12 months in LVMI.
Statistical analysis
The study was powered to detect between treatment difference of 11 g/m2 in LVMI over 1 year using 83 hypertensive ESRD patients with left ventricular hypertrophy per group with a power of 80% at a 0.05 two-sided significance level. Assuming a 15% drop out, a total of 100 patients per group were recruited.
The primary outcome of the study was the average reduction in left ventricular mass indexed for body surface area from baseline to 1 year. The analysis was performed by intention to treat, if the patient received at least one dose of the randomized drug (which was the case for each subject) regardless of the availability of a postbaseline echocardiogram. A mixed model was used with LVMI as the outcome variable. Fixed effects were indicator variables for time, treatment and their interaction. Random effect was subject and statistical inference was made using the maximum likelihood estimator. No imputation was made for missing data.
Cardiovascular events were counted by subject and included the following: myocardial infarction, stroke, hospitalization for congestive heart failure, hospitalized angina, arrhythmias, cardiac arrest, coronary revascularization and heart valve replacement. Adverse events reported are those during the course of 12 months of participation in the trial. All serious adverse events were jointly adjudicated by R.A. and A.D.S. who were masked to the drug assignment at the time of adjudication. The duration of participation in the study per subject, which according to the trial design could be up to 12 months, was determined. The cardiovascular event rate was calculated by treatment group assignment. Incidence rate ratio (IRR) by treatment was then determined along with the 95% confidence intervals (95% CIs). As a post hoc analysis, we also determined the narrower definition of cardiovascular events per group that included myocardial infarction, stroke, congestive heart failure or cardiovascular death. At the request of the data safety and monitoring board, we also calculated the hospitalization rates by treatment and their confidence intervals.
All analyses were conducted using Stata version 11.2 (Stata Corp., College Station, TX, USA). The P values reported are two-sided and taken to be significant at <0.05.
The funding source had no input in the decision to submit the manuscript for publication. R.A. was responsible for the decision to submit the manuscript. All authors had full access to data at all times.
RESULTS
Between January 2005 and May 2013, we randomized 200 subjects undergoing maintenance dialysis with hypertension and echocardiographic left ventricular hypertrophy. The trial flow is shown in the Supplementary Figure S1. Of the 100 subjects randomized to the lisinopril group, 6 received a β-blocker at some point during the course of the trial. The reasons were cardiomyopathy, heart failure, myocardial infarction, coronary revascularization, patient preference and in error. In contrast, of the 100 subjects randomized to the atenolol group, only 4 received off-study ACE inhibitors or angiotensin receptor blockers during the course of the trial. The reasons were stroke, cardiomyopathy and in two subjects their preference.
The clinical characteristics of patients were balanced between groups and are provided in Table 1. The population was predominantly black (86%) with average age of 52.7 years and 34.5% of the participants were women. A lower socioeconomic status reflects an inner city population. Documented cardiovascular disease and hospitalization for heart failure was evenly balanced between groups, although the lisinopril group had more women, more coronary revascularization and a slightly greater urea reduction ratio. All subjects were on thrice weekly dialysis. Prescribed duration of dialysis averaged 4 h and delivered duration was slightly less.
Table 1.
Baseline characteristics of the study sample
| Clinical characteristic | Atenolol (n = 100) | Lisinopril (n = 100) | All subjects (n = 200) |
|---|---|---|---|
| Age (years) | 52.2 ± 11.7 | 53.1 ± 13.5 | 52.7 ± 12.6 |
| Male sex, n (%)* | 73 (73) | 58 (58) | 131 (65.5) |
| Blacks, n (%) | 86 (86) | 86 (86) | 172 (86) |
| Hispanic, n (%) | 1 (1) | 0 (0) | 1 (0.5) |
| Etiology of chronic kidney disease | |||
| Diabetes mellitus, n (%) | 29 (29) | 27 (27) | 56 (28) |
| Hypertension, n (%) | 54 (54) | 46 (46) | 100 (50) |
| Glomerulonephritis, n (%) | 4 (4) | 5 (5) | 9 (4.5) |
| Polycystic kidney disease, n (%) | 0 (0) | 1 (1) | 1 (0.5) |
| Other etiologies, n (%) | 13 (13) | 21 (21) | 34 (17) |
| Dialysis vintage (years) | 4.2 ± 4.4 | 3.9 ± 4.2 | 4.1 ± 4.3 |
| Anuric, n (%) | 68 (68) | 66 (66) | 134 (67) |
| Diabetes mellitus, n (%) | 43 (43) | 43 (43) | 86 (43) |
| Hospitalized heart failure, n (%) | 25 (25) | 37 (37) | 62 (31) |
| Coronary artery disease, n (%) | 22 (22) | 31 (31) | 53 (26.5) |
| Coronary revascularization, n (%)** | 4 (4) | 15 (15) | 19 (9.5) |
| Cerebrovascular disease, n (%) | 13 (13) | 20 (20) | 33 (16.5) |
| Peripheral vascular disease, n (%) | 10 (10) | 11 (11) | 21 (10.5) |
| Education (years) | 12 ± 2 | 12 ± 2 | 12 ± 2 |
| Marital status | |||
| Single, n (%) | 53 (53) | 56 (56) | 109 (54.5) |
| Married, n (%) | 23 (23) | 19 (19) | 42 (21) |
| Divorced/separated, n (%) | 18 (18) | 15 (15) | 33 (16.5) |
| Widower, n (%) | 6 (6) | 10 (10) | 16 (8) |
| Employed | |||
| Working, n (%) | 11 (11) | 7 (7) | 18 (9) |
| Not working, n (%) | 67 (67) | 70 (70) | 137 (68.5) |
| Retired, n (%) | 22 (22) | 23 (23) | 45 (22.5) |
| Income | |||
| <$25 000/year | 84 (84) | 80 (80) | 164 (82) |
| ≥$25 000/year | 10 (10) | 7 (7) | 17 (8.5) |
| Refused | 6 (6) | 13 (13) | 19 (9.5) |
| Smoking, n (%) | 43 (43) | 43 (43) | 86 (43) |
| Alcohol, n (%) | 27 (27) | 19 (19) | 46 (23) |
| Height (in) | 68.3 ± 4.1 | 67.6 ± 3.7 | 67.9 ± 3.9 |
| Weight (kg) | 85.1 ± 21.7 | 80.9 ± 24.3 | 83 ± 23.1 |
| Body mass index (kg/m2) | 28.4 ± 7 | 27.5 ± 8.3 | 27.9 ± 7.7 |
| Access type | |||
| Fistula | 59 (59) | 59 (59) | 118 (59) |
| Graft | 16 (16) | 14 (14) | 30 (15) |
| Catheter | 25 (25) | 27 (27) | 52 (26) |
| Blood flow rate (mL/min) | 394.3 ± 30.9 | 392.4 ± 36.4 | 393.4 ± 33.7 |
| Dialyzate flow rate (mL/min) | 779.6 ± 61.9 | 761.3 ± 82 | 770.4 ± 73 |
| Prescribed dialysis duration (min) | 239.4 ± 19 | 239.4 ± 25.9 | 239.4 ± 22.7 |
| Delivered dialysis duration (min) | 224.1 ± 34.7 | 219.8 ± 33.7 | 222 ± 34.2 |
| Urea reduction ratio (%)* | 74 ± 8 | 76 ± 8 | 75 ± 8 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 |
| Hemoglobin (g/dL) | 11.3 ± 1.2 | 11.3 ± 1.4 | 11.3 ± 1.3 |
| Creatinine (mg/dL) | 10.3 ± 3.5 | 10 ± 3.6 | 10.1 ± 3.6 |
*P < 0.05 and **P < 0.01 for comparison between treatment groups.
Prior to any washout, majority of the patients received antihypertensive drugs, with an average of 2.7 drugs in those who took these drugs (Table 2). β Blockers were prescribed to nearly three-quarters of the subjects and nearly as many were prescribed either an ACE inhibitor or an angiotensin receptor blocker. Other prescribed medications such as aspirin, statins and erythropoietin stimulating agents were also well balanced between groups. However, at baseline, the atenolol group had greater intravenous iron use and vitamin D use, but less clopidogrel use.
Table 2.
Nature and number of antihypertensive and other drugs
| Antihypertensive drug | Atenolol (n = 100) | Lisinopril (n = 100) | All subjects (n = 200) |
|---|---|---|---|
| Antihypertensive drugs (n) | 2.6 ± 1.5 | 2.7 ± 1.3 | 2.7 ± 1.4 |
| No antihypertensive drug use, n (%) | 5 (5) | 2 (2) | 7 (3.5) |
| Angiotensin converting enzyme inhibitors, n (%) | 57 (57) | 66 (66) | 123 (61.5) |
| Angiotensin receptor blockers, n (%) | 13 (13) | 7 (7) | 20 (10) |
| β-Blockers, n (%) | 80 (80) | 72 (72) | 152 (76) |
| α-Blockers, n (%) | 12 (12) | 8 (8) | 20 (10) |
| Centrally acting agents, n (%) | 29 (29) | 35 (35) | 64 (32) |
| Non dihydropyridine calcium-channel blockers, n (%) | 2 (2) | 6 (6) | 8 (4) |
| Dihydropyridine calcium-channel blockers, n (%) | 47 (47) | 52 (52) | 99 (49.5) |
| Vasodilators, n (%) | 22 (22) | 25 (25) | 47 (23.5) |
| Loop diuretics, n (%) | 3 (3) | 0 (0) | 3 (1.5) |
| Erythropoietin stimulating agents, n (%) | 71 (71) | 66 (66) | 137 (68.5) |
| Intravenous iron, n (%)*** | 44 (44) | 22 (22) | 66 (33) |
| Insulin, n (%) | 27 (27) | 30 (30) | 57 (28.5) |
| Oral hypoglycemic agents, n (%) | 4 (4) | 3 (3) | 7 (3.5) |
| Aspirin, n (%) | 49 (49) | 40 (40) | 89 (44.5) |
| Clopidogrel, n (%)** | 2 (2) | 13 (13) | 15 (7.5) |
| Warfarin, n (%) | 4 (4) | 7 (7) | 11 (5.5) |
| Vitamin D, n (%)* | 67 (67) | 53 (53) | 120 (60) |
| Cinacalcet, n (%) | 25 (25) | 19 (19) | 44 (22) |
| Statin, n (%) | 40 (40) | 44 (44) | 84 (42) |
*P < 0.05, **P < 0.01 and ***P < 0.001 for comparison between treatment groups.
BP control between groups and interventions toimprove BP
Figure 1 shows 44-h interdialytic ambulatory BP measurements at baseline and over time and self-measured home BP measurements. At baseline, 44-h ambulatory BP was 151.5/87.1 mmHg in the atenolol group. BP was similar in the lisinopril group, improved over time in both groups, and no statistical difference between drugs was noted (see figure legend for details).
FIGURE 1:
BP profiles at baseline and over time. BP obtained in the interdialytic period (Left panel) and self-measured by the patients at home (right panel) are shown. Ambulatory BP monitoring was performed in the interdialytic period over 44 h at baseline, 3, 6 and 12 months. Solid line shows the atenolol group and the dotted line the lisinopril group; vertical bars represent standard error of mean. The table at the bottom of each graph shows the number of patients in each drug [atenolol (n), lisinopril (n)]; the change from baseline (CFB) and between group comparisons of the changes (lisinopril–atenolol CFB). The declines in both systolic and diastolic blood pressure were numerically greater with atenolol but no statistical difference was present between drugs. Home BP monitoring was performed at baseline and at every month for the entire duration of the trial; the mean reduction in BP overall was reduced more with atenolol therapy (linear rate of change for atenolol was −1.5 mmHg systolic/month and that for lisinopril was 0.47 mmHg flatter (P = 0.037). The square root transformation of time had a between group difference of slope with a P value of 0.012. Both these analyses were post hoc).
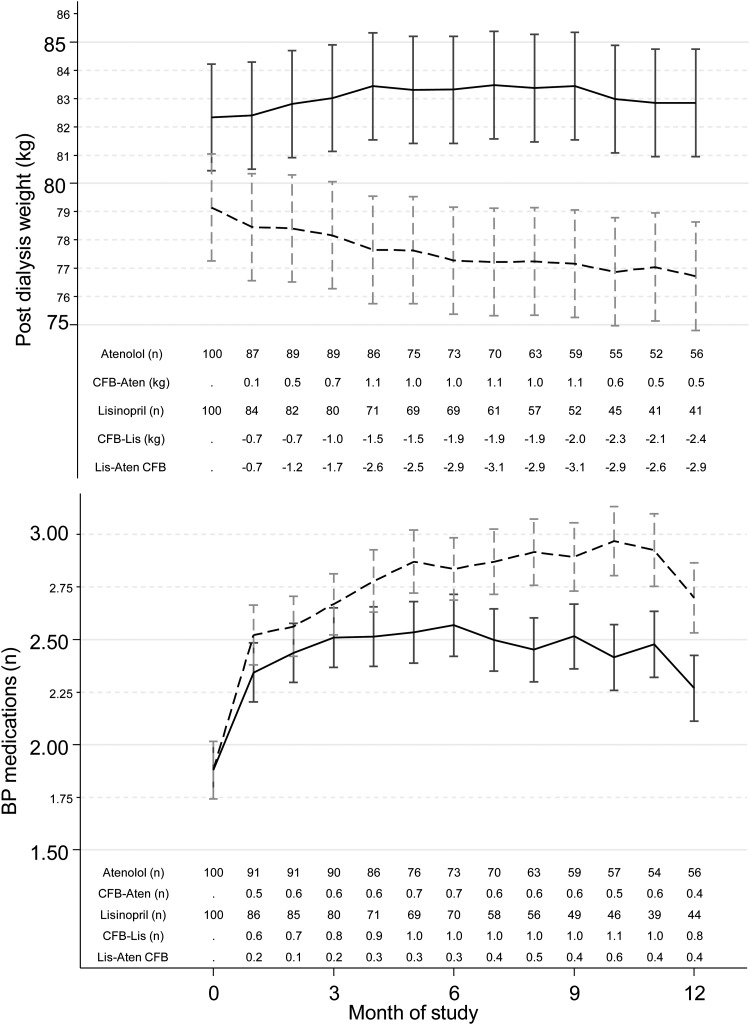
Figure 2 shows the postdialysis weight at baseline and over the course of the trial. On average, there was a 1.5-kg reduction in weight in the lisinopril group compared with the 0.9-kg increase in weight in the atenolol group. The difference between groups was clinically and statistically significant. Furthermore, there was a greater need for titration of antihypertensive medication in subjects randomized to the lisinopril group (see figure legend for details).
FIGURE 2:
Time course of change in postdialysis weight and antihypertensive drug number. Whereas atenolol group gained weight, on average the lisinopril group experienced a mean 1.5 kg reduction in weight. The between group changes in weight was statistically significant. Despite the reduction in weight the lisinopril group required greater number of antihypertensive drugs to achieve a similar degree of BP control. The notations in the table appearing below each graph are explained in the legend for Figure 1.
Early termination of the trial
We terminated the trial on the unanimous recommendation of the independent data safety monitoring board which found a clear signal for cardiovascular safety on an annual monitoring meeting after complete randomization. At their annual meeting, the committee also noted that the lisinopril group experienced an increase in the following: all-cause serious adverse events, all-cause hospitalization rates, hypertension and hyperkalemia. Given the totality of evidence, they recommended termination of the trial.
Serious adverse events and cardiovascular eventsthat led to trial termination
Table 3 shows the serious adverse events between groups over the course of the trial. Cumulatively, we had 81.2 patient-years (PY) of follow up in the atenolol group and 74.1 PY in the lisinopril group. Serious cardiovascular events in the atenolol group occurred in 16 subjects, who had 20 events (24.6/100 PY) and in the lisinopril group in 28 subjects, who had 43 events [58/100 PY; IRR 2.36 (95% CI 1.36–4.23, P = 0.001)]. In post hoc analyses, combined serious adverse events of myocardial infarction, stroke, hospitalization for heart failure or cardiovascular death in the atenolol group occurred in 10 subjects, who had 11 events (13.5/100 PY) and in the lisinopril group in 17 subjects, who had 23 events [31.0/100 PY; IRR 2.29 (P = 0.021)]. Hospitalizations for heart failure were worse in the lisinopril group (IRR 3.13, P = 0.021).
Table 3.
Serious adverse events reported following randomization
| Event type | Atenolol |
Lisinopril |
||||||
|---|---|---|---|---|---|---|---|---|
| Subjects (n) | Events (n) | Incidence rate(events/100patient-years) | Subjects (n) | Events (n) | Incidence rate(events/100patient-years) | IRR Lisinopril/atenolol (95% CI) | P | |
| Overall serious adverse events | 58 | 140 | 172.4 | 70 | 188 | 253.6 | 1.47 (1.18–1.84) | <0.001 |
| All-cause hospitalization rate | 37 | 73 | 89.9 | 59 | 107 | 144.3 | 1.61 (1.18–2.19) | 0.002 |
| Infections | 24 | 30 | 36.9 | 20 | 29 | 39.1 | 1.07 (0.62–1.85) | 0.78 |
| Access-related | 17 | 24 | 29.6 | 19 | 30 | 40.5 | 1.28 (0.73–2.30) | 0.36 |
| Central nervous system | 3 | 3 | 3.7 | 3 | 5 | 6.7 | 1.81 (0.35–11.63) | 0.44 |
| Cancer-related complications | 2 | 4 | 4.9 | 2 | 3 | 4 | 0.82 (0.12–4.85) | 0.81 |
| Cardiovascular events | 16 | 20 | 24.6 | 28 | 43 | 58 | 2.36 (1.36–4.23) | 0.001 |
| Combined MI, Stroke, CHF, CV-related Death | 10 | 11 | 13.5 | 17 | 23 | 31 | 2.29 (1.07–5.21) | 0.02 |
| Angina | 0 | 0 | 0 | 2 | 2 | 2.7 | NA | |
| Arrhythmia | 2 | 2 | 2.5 | 3 | 5 | 6.7 | 2.75 (0.45–28.88) | 0.24 |
| Cardiac arrest | 0 | 0 | 0 | 2 | 2 | 2.7 | NA | |
| Congestive heart failure | 5 | 5 | 6.2 | 10 | 15 | 20.2 | 3.13 (1.08–10.99) | 0.02 |
| Myocardial infarction | 2 | 2 | 2.5 | 3 | 3 | 4 | 1.61 (0.18–19.26) | 0.63 |
| Peripheral vascular disease | 1 | 1 | 1.2 | 5 | 6 | 8.1 | 6.35 (0.77–291.93) | 0.06 |
| Revascularization | 3 | 4 | 4.9 | 4 | 4 | 5.4 | 1.08 (0.20–5.82) | 0.91 |
| Stroke | 2 | 2 | 2.5 | 2 | 2 | 2.7 | 1.10 (0.08–15.11) | 0.93 |
| Valve replacement surgery | 1 | 1 | 1.2 | 1 | 1 | 1.3 | 1.10 (0.01–86.00) | 0.95 |
| Cardiovascular death | 2 | 2 | 2.5 | 3 | 3 | 4 | 1.61 (0.18–19.23) | 0.63 |
| Noncardiovascular death | 2 | 2 | 2.5 | 1 | 1 | 1.3 | 0.55 (0.01–10.58) | 0.68 |
| Fractures | 7 | 7 | 8.6 | 1 | 1 | 1.3 | 0.17 (0.00–1.29) | 0.06 |
| Parathyroidectomy | 3 | 3 | 3.7 | 1 | 1 | 1.3 | 0.37 (0.01–4.60) | 0.43 |
| Biliary-related | 1 | 1 | 1.2 | 2 | 2 | 2.7 | 2.16 (0.11–127.69) | 0.58 |
| Bowel-related | 3 | 3 | 3.7 | 5 | 5 | 6.7 | 1.83 (0.36–11.79) | 0.43 |
| Falls | 6 | 6 | 7.4 | 3 | 3 | 4 | 0.55 (0.09–2.57) | 0.42 |
| Gastrointestinal bleed | 2 | 4 | 4.9 | 5 | 7 | 9.4 | 1.90 (0.48–8.87) | 0.32 |
| Hypertensive crisis | 3 | 3 | 3.7 | 10 | 11 | 14.8 | 3.81 (1.01–21.25) | 0.03 |
| Hyperglycemia | 1 | 2 | 2.5 | 3 | 3 | 4 | 1.59 (0.18–19.02) | 0.64 |
| Hyperkalemia | 3 | 3 | 3.7 | 10 | 10 | 13.5 | 3.38 (0.87–19.14) | 0.05 |
| Hypoglycemia | 2 | 3 | 3.7 | 4 | 4 | 5.4 | 1.41 (0.24–9.63) | 0.67 |
| Hypotension with hospitalization | 6 | 8 | 9.9 | 5 | 5 | 6.7 | 0.69 (0.18–2.39) | 0.53 |
| Miscellaneousa | 12 | 14 | 17.2 | 18 | 24 | 32.4 | 1.84 (0.92–3.85) | 0.07 |
aMiscellaneous hospitalizations occurred for the following: noncardiac chest pain, outpatient/elective surgery, allergic reaction, vertigo, bradycardia, pseudogout, depression, suicidal ideation, gangrene, pulmonary embolism, syncope, abdominal pain, motor vehicle accident, bowel perforation, bilateral nephrectomy, dislocated shoulder, lethargy, asthma exacerbation, leg pain, deep vein thrombosis, anemia, nausea and vomiting.
All-cause hospitalizations in the atenolol group occurred in 37 subjects, who had 73 hospitalizations (89.9/100 PY), and in the lisinopril group in 59 subjects, who had 107 hospitalizations [144.3/100 PY; IRR 1.61 (95% CI 1.18–2.19, P = 0.002)]. There were more hypertensive events and hyperkalemia in the lisinopril group and more falls and fractures in the atenolol group.
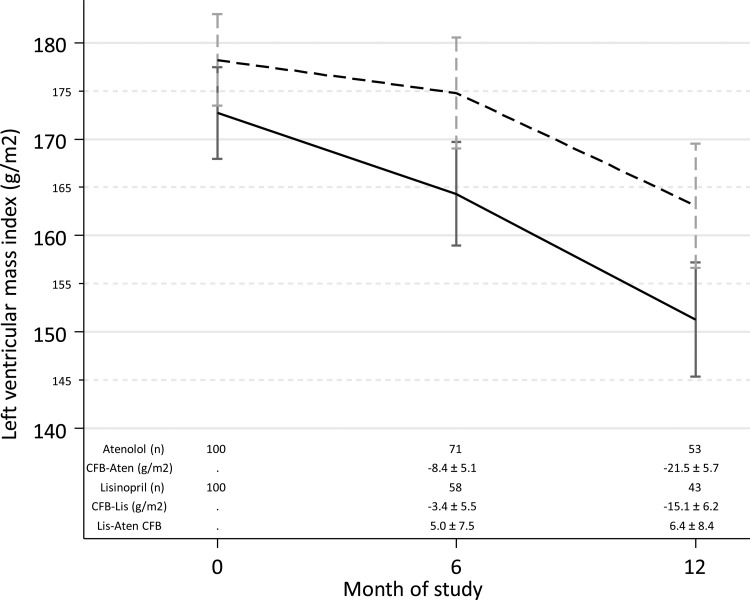
Results of LVMI
Figure 3 shows the LVMI in the two groups at baseline over time. LVMI improved with time (P < 0.05 for each within group comparison); no difference between drugs was noted. Given the early termination of the trial, the power to detect between group differences was limited. Midwall fractional shortening is an objective and accurate index of left ventricular systolic function. Left atrial diameter may reflect the volume state. Supplementary Table S3 shows no difference at baseline or over time between groups.
FIGURE 3:
Time course of change in echocardiographic LVMI. At 12 months, each group had an improvement in LVMI (P = 0.015 for lisinopril and P <0.001 for atenolol at 12 months). Between group changes were not significant. CFB, change from baseline. Lis, lisinopril dotted line, Aten, atenolol solid line. ‘±’ indicates standard error of the modeled means.
Other measurements
Supplementary Table S1 shows the kidney disease quality of life questionnaire administered at baseline and at the end of the trial. No differences were statistically significant between groups.
DISCUSSION
Among hypertensive patients with left ventricular hypertrophy on maintenance hemodialysis, in the HDPAL randomized, controlled trial treatment with either atenolol or lisinopril-based antihypertensive therapy produced statistically and clinically significant reductions in BP from baseline that was sustained over the 12-month course of the trial. Despite a greater reduction in dry weight, compared with atenolol, the administration of lisinopril was associated with an increased risk of hospitalizations for congestive heart failure. In addition, lisinopril therapy was associated with an increased risk of all-cause hospitalizations and cardiovascular morbidity. Specifically, lisinopril administration was also associated with an increased incidence of the combined risk for hospitalizations due to congestive heart failure, myocardial infarction, strokes and cardiovascular death. Furthermore, lisinopril-based therapy was also associated with an increased risk of hyperkalemia and emergent treatment for hypertensive crises.
Both antihypertensive drugs, atenolol [11] and lisinopril [12], have been individually shown to lower BP effectively when administered TIW after dialysis. Furthermore, using interdialytic ambulatory BP monitoring, BP lowering with monotherapy with either drug is sustained over the entire interdialytic interval [11, 12]. The HDPAL trial shows, for the first time, that atenolol-based antihypertensive therapy is superior in lowering BP compared with lisinopril-based therapy. Despite both groups being targeted to home BP of <140/90 mmHg at each monthly visit, the lisinopril-based group required a statistically greater number of antihypertensive drugs and a greater need for lowering dry weight to lower BP. Moreover, in the lisinopril group, BP lowering remained numerically less using ambulatory BP monitoring and statistically less when assessed by home BP monitoring. Atenolol-based therapy may have therefore conferred cardiovascular protection by improving BP control. If a superior decline in BP with atenolol is in the causal pathway for cardiovascular protection, this randomized trial provides evidence that BP lowering is not deleterious for dialysis patients. It provides support to the notion that time-dependent fall in BP among dialysis patients is not causally related to adverse cardiovascular events as reported by several observational cohorts [13, 14]. Together with meta-analyses of hypertension treatment trials in dialysis patients, our results urge clinicians to manage hypertension assiduously. However, our study cannot recommend goal BP levels in these individuals.
A prior small study from our group has shown that lisinopril reduces ambulatory BP by 22-mmHg systolic even when given TIW after dialysis [12]. Whether daily administration of lisinopril could have led to a greater reduction in BP is possible but unlikely. However, head-to-head comparison with atenolol given TIW or lisinopril given TIW clearly demonstrates superiority of atenolol in reducing BP in hemodialysis patients.
It is unlikely that cardiovascular events in the lisinopril group were because of abrupt withdrawal of β-blockers; β-blockers were gradually tapered during baseline if patients were on this class of therapy. However, majority of the subjects in our trial had prior cardiovascular disease. It is possible that withholding β-blocker therapy from such subjects could have precipitated cardiovascular events in the long term independently of BP. Even if this was the case, it would still support the continued use of β-blocker therapy among hypertensive patients on hemodialysis.
There were some signals of possible harm associated with atenolol-based therapy. For example atenolol-based antihypertensive therapy was associated with increased risk of falls and fractures. The latter may reflect a greater ability of atenolol to reduce BP or transient heart block and therefore incur more hypotensive events, dizziness and falls.
Two meta-analyses of small trials among maintenance dialysis patients suggest that antihypertensive therapy can improve cardiovascular events [7, 8]. Two studies are particularly relevant. Zannad et al. randomized maintenance hemodialysis patients with left ventricular hypertrophy with or without hypertension to either fosinopril or placebo to evaluate cardiovascular protection of the ACE inhibitor [15]. Nearly 40% of the cohort was normotensive. Compared with placebo, the hazard ratio for cardiovascular events with fosinopril was nonsignificantly improved by 7%. Results of this trial suggest that ACE inhibitors are likely not harmful among patients on hemodialysis with left ventricular hypertrophy. In comparison, among patients on maintenance hemodialysis who also had symptomatic heart failure and dilated cardiomyopathy (left ventricular ejection fraction <35% by echocardiography), and who all received either an ACE inhibitor or an angiotensin receptor blocker as baseline, Cice et al. randomly assigned 114 patients to either placebo or carvedilol [16]. Although hypertension or left ventricular hypertrophy were not criteria for recruitment in the trial, at 12 months there was substantial benefit of this drug on echocardiographic parameters. At 24-month follow-up, fewer deaths and improved cardiovascular outcomes were noted in the carvedilol group. Compared with the placebo group, carvedilol treatment was associated with 15/6 mmHg reduction in BP from baseline. Whether the benefit of carvedilol was due to reduction in BP or independent of it can be debated, nonetheless data suggest that among dialysis patients with symptomatic dilated cardiomyopathy treated with an ACE inhibitor or an angiotensin receptor blocker, compared with a placebo group, β-blocker-based therapy was superior.
Dysfunction of the sympathetic pathway is thought to be particularly important in the pathogenesis of hypertension among black people with hypertension [17, 18]. Plasma renin activity is often suppressed in these individuals, which suggests volume excess rather than activation of the renin–angiotensin system plays a greater role in the pathogenesis of hypertension among blacks [19, 20]. Among patients with kidney disease, sympathetic activation is seen among patients on long-term dialysis [21] and is associated with cardiovascular events [22]. A randomized trial comparing losartan with atenolol in hypertensive patients with left ventricular hypertrophy (not on dialysis) has earlier demonstrated that losartan was superior to atenolol therapy in preventing strokes, myocardial infarctions and cardiovascular death [9]. In a subgroup analysis of this trial, the reverse was found to be true among blacks. Our patients were predominantly black, and we had only 14% whites. Whether similar results will be seen among white people remains unclear.
There are several limitations and some strengths of the HDPAL trial. First, the trial had an open label design. This design was not likely to affect measurement of ambulatory BP or the achieved BP over the course of the trial, but may have affected the selection of additional antihypertensive therapy. Second, there were predominantly black patients in our study. Whether the results can be extrapolated to a predominantly white population cannot be answered by the present trial. Third, the HDPAL trial did not have a placebo group. Accordingly, it cannot recommend a goal BP that should be targeted among maintenance hemodialysis patients with hypertension. Among the trials conducted to evaluate role of antihypertensive drugs in hypertensive maintenance hemodialysis patients, the HDPAL trial is unique in requiring interdialytic ambulatory BP monitoring for the diagnosis of hypertension. Using home BP monitoring to guide antihypertensive therapy and other therapeutic interventions is also a notable strength of this trial.
In conclusion, among predominantly black hemodialysis patients with hypertension and left ventricular hypertrophy, an initial strategy using atenolol, β-blocker therapy, is superior to ACE-inhibitor-based therapy. Strict attention to dry weight and periodic home BP monitoring may further improve BP control and cause regression of left ventricular hypertrophy. However, we are unable to draw any conclusion regarding between group differences in the regression of left ventricular hypertrophy.
AUTHORS’ CONTRIBUTIONS
R.A.: literature search, figures, study design, data analysis, data interpretation, writing; A.D.S.: data collection, writing; M.K.P.: data collection, data analysis. T.N.A.: data collection; G.T.T.: data collection.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
Results of this trial were orally presented at the late-breaking clinical trials session of the American Society of Nephrology Annual Meeting, Atlanta, GA, USA on Nov 9, 2013. R.A. has consulted for several pharmaceutical companies that make antihypertensive drugs, but none related to this trial. Other authors have no conflict of interest. (See related article by Zoccali and Mallamaci. Cardiovascular protection by β-blockade in hypertensive haemodialysis patients: the hypertension in haemodialysis patients treated with atenolol or lisinopril (HDPAL) trial. Nephrol Dial Transplant 2014; 29: 483–485.)
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by the National Institutes of Health (grants 2R01-DK063020-10).
REFERENCES
- 1.Agarwal R, Nissenson AR, Batlle D, et al. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Benedetto FA, Tripepi G, et al. Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney Int. 1998;53:1078–1084. doi: 10.1111/j.1523-1755.1998.00853.x. [DOI] [PubMed] [Google Scholar]
- 3.Amar J, Vernier I, Rossignol E, et al. Influence of nycthemeral blood pressure pattern in treated hypertensive patients on hemodialysis. Kidney Int. 1997;51:1863–1866. doi: 10.1038/ki.1997.254. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension. 2010;56:512–517. doi: 10.1161/HYPERTENSIONAHA.110.154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal R, Alborzi P, Satyan S, et al. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankestijn PJ, Ligtenberg G. Volume-independent mechanisms of hypertension in hemodialysis patients: clinical implications. Semin Dial. 2004;17:265–269. doi: 10.1111/j.0894-0959.2004.17324.x. [DOI] [PubMed] [Google Scholar]
- 7.Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53:860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R. Supervised atenolol therapy in the management of hemodialysis hypertension. Kidney Int. 1999;55:1528–1535. doi: 10.1046/j.1523-1755.1999.00359.x. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R, Lewis RR, Davis JL, et al. Lisinopril therapy for hemodialysis hypertension: hemodynamic and endocrine responses. Am J Kidney Dis. 2001;38:1245–1250. doi: 10.1053/ajkd.2001.29221. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Lacson E, Jr, Lowrie EG, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45:811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 15.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 16.Cice G, Ferrara L, D'Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 17.Lang CC, Stein CM, Brown RM, et al. Attenuation of isoproterenol-mediated vasodilatation in blacks. N Engl J Med. 1995;333:155–160. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- 18.Stein CM, Lang CC, Singh I, et al. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 19.Weir MR, Chrysant SG, McCarron DA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. 1998;31:1088–1096. doi: 10.1161/01.hyp.31.5.1088. [DOI] [PubMed] [Google Scholar]
- 20.Chrysant SG, Danisa K, Kem DC, et al. Racial differences in pressure, volume and renin interrelationships in essential hypertension. Hypertension. 1979;1:136–141. doi: 10.1161/01.hyp.1.2.136. [DOI] [PubMed] [Google Scholar]
- 21.Converse RL, Jr, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Mallamaci F, Parlongo S, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.