Abstract
Anti-tumor necrosis factor agents are now considered to be a vital component of the treatment algorithm for pediatric inflammatory bowel disease. Despite the clear benefit of these agents and the re-alignment of treatment goals to achieve early mucosal healing, the decision to initiate therapy is often delayed due to uncertainties regarding risks and benefits. The purpose of this review is to summarize the currently available data regarding anti-tumor necrosis factor agents in pediatric inflammatory bowel disease. Specifically, we review their expected efficacy in both Crohn’s disease and ulcerative colitis, and the likelihood of side-effects associated with these agents. In addition, we address the barriers physicians face when communicating these data and help to identify how pediatric patients and their parents can be more involved in a shared decision making process. Through the creation of a new decision aid (Option Grid), we hope to allow for a more clear line of communication at the bedside when helping patients and parents make these difficult treatment decisions.
Keywords: Pediatric Inflammatory Bowel Disease, Crohn’s disease, ulcerative colitis, anti-TNF, risk, shared decision making, option grid
Introduction
Inflammatory Bowel Disease (IBD) is a chronic relapsing, remitting illness which presents during childhood or adolescence in up to 25% of patients. The global incidence of pediatric IBD also appears to be on the rise.1–3 The significant impact of this disease on growth, development and health related quality of life,4, 5 along with an enhanced understanding of disease mechanisms, has led to the re-alignment of treatment strategies towards early initiation of biologic therapy for better lifetime control.6–9
Despite the clear impact of biologics on disease outcomes, this “top-down approach” is often met with uncertainty by patients and parents. With parents often underestimating benefit and overestimating risk,10 the decision to initiate biologics can frequently be delayed until patients are felt to be “sick” enough to warrant the risks of therapy.11 In order for parents and patients to make informed, balanced and timely decisions, they must be able to understand not only the chance of responding to therapy but the risk for significant side effects and alternative treatment options as well. In order for physicians to effectively communicate these outcomes, after understanding the data, we need to develop tools that can be used with parents and patients to allow for a more open and clear discussion in the clinic.
This review will first discuss the safety, efficacy and durability of biologics in pediatric IBD. Next, we will further discuss the role of parents and children in medical decision making, factors impacting those decisions and methods through which to effectively communicate risks and benefits. Finally, through the creation of an “Option Grid” we will provide a tool to efficiently review expected outcomes with patients and parents at the bedside.
Efficacy of Biologics in Pediatric IBD
Crohn’s disease
Initial retrospective studies helped to establish the safety and efficacy of single dose infliximab (IFX) induction therapy for Crohn’s Disease, demonstrating significant improvements in symptoms at 4 and 12 week follow-up.12 Later prospective studies, using more objective measures of response (PCDAI – Pediatric Crohn’s Disease Activity Index), demonstrated remission in >50% of patients at some point of treatment.13, 14 Although these studies helped to solidify the role of IFX in pediatric Crohn’s disease, and identified 5–10mg/kg to be the most beneficial dosage, this single dose induction protocol was associated with a significant rate of relapse (42–78%). In an attempt to prolong response to therapy by using a 3 dose induction regimen at 0, 2 and 6 weeks, Cezard et al.15 achieved significantly higher rates of remission at 4 weeks (90%) and 12 weeks (76%). Relapse rates however remained high at 90%, with the majority (74%) relapsing within 6 months of initial treatment. Borrelli et al.16 showed similar outcomes 8 weeks after induction therapy, but in this study 8/18 parents allowed for continuation of therapy with maintenance IFX every 8 weeks. At 6 month follow-up, the mean PCDAI was significantly lower in the maintenance group as compared to those treated with 3 dose induction alone (10.5 ± 1.41 vs. 18.8 ± 4.54; p < 0.05).
The REACH trial, a randomized multi-center open label trial, evaluated the outcomes of induction therapy with IFX in 112 patients. They achieved clinical response and remission, as defined by the PCDAI score, in 88% and 59% of patients respectively at 10 weeks. When evaluating the sub-group of patients with fistulizing disease at baseline (n=22), 41% of patients attained partial or complete response 2 weeks after the initial infusion and 68% achieved complete response by week 54.17, 18 This study addressed the necessity of an Q8 week dosing interval by randomizing patients responding to IFX induction therapy to either Q8wk or Q12wk maintenance. The Q8wk group had an increased likelihood for maintaining response (63.5% vs. 33.3%, p = 0.002) and remission (55.8% vs. 23.5%, p < 0.001) at 1 year.18 When further comparing episodic or “on demand” treatment intervals to scheduled maintenance therapy, Ruemmele et al. again showed that scheduled Q8 therapy was the superior treatment protocol at one year follow-up,19 and Crombe et al. demonstrated it to be the superior treatment protocol as far as 3 years after inducing remission.20 During the open-label extension of the REACH trial, approximately 80% of patients continued to have minimal to no disease activity up to 3 years after initiation of IFX.21
Adalimumab (ADA) has been to shown to induce and maintain response in adult Crohn’s patients naïve, intolerant or no longer responsive to IFX.22, 23 Its use in pediatric patients has largely been off-label for refractory disease.24–29 The IMAgINE 1 study, a phase 3 multi-center randomized open-label induction double-blind maintenance trial, recently evaluated the efficacy of ADA in patients refractory to conventional therapy (PCDAI >30, 40% previously treated with IFX).29 They demonstrated that ADA was well tolerated and a response to induction was seen in 82% of patients, with >50% maintaining response at 6 and 12 month follow-up. Of the 36 patients with fistulas, 26 had improvement at 1 year with 11 having complete closure. This study demonstrated that IFX naïve patients had higher rates of response and remission to ADA than those previously exposed to IFX (only secondary non-responders were included), achieving rates comparable to those seen in the REACH trial which included only anti-TNF naïve patients.18 Although ADA is currently not FDA approved for pediatric Crohn’s disease, taken together these data suggest that outcomes may be comparable to that of IFX.
Ulcerative Colitis
Unlike pediatric Crohn’s Disease, data on the use of IFX in pediatric ulcerative colitis (UC) is limited largely to two prospective cohort studies and several small retrospective case series. Turner et al. described a cohort of 128 UC patients hospitalized for a severe flare, 33 of which underwent treatment with IFX for disease refractory to steroids.30 Short-term response (Pediatric Ulcerative Colitis Activity Index [PUCAI] <35) was seen in 76% of patients with 55% maintaining long term response and remaining colectomy free. Patients with new onset disease and those with a shorter duration of disease activity were more likely to respond to IFX than those with a longer disease history. In the largest pediatric UC study to date (n=332), Hyams et al. treated a mixed cohort of steroid refractory (34/52, 65%) and steroid dependent (18/52, 35%) patients with maintenance or episodic therapy and achieved short-term (3 month - Physician Global Assessment [PGA]) response in 36% of patients. The likelihood of remaining colectomy-free after IFX treatment was 75%, 72% and 62% at 6, 12 and 24 month follow-up, respectively.31
Biologics affect on growth and development
With nearly 25% of IBD patients presenting during childhood or adolescence, and the majority of this being around puberty, the impact of disease activity on growth and development is significant. The principal determinants of impairment are chronic nutritional deficiencies, secondary to malabsorption and reduced intake, along with chronic inflammation resulting in interruption of the IGF1-GH axis (IL-6, IL-1β, IGF-1, TNF).32 The greatest consequence appears to be in pediatric Crohn’s Disease, where nearly half of all patients have a reduction in growth velocity before diagnosis, compared to only 3–10% in UC.33, 34 There is now strong evidence to support the improvement of linear growth with IFX independent of steroid dose reduction or progression through puberty.35, 36
The REACH study demonstrated significant improvements in height z-scores (mean baseline z-score: −1.5) at 30 (mean improvement in z-score of 0.3; P < 0.001) and 54 week (mean improvement in z-score of 0.5; P < 0.001) follow-up.18 This improvement in linear growth was related to inhibition of TNF-alpha effects on osteoblasts as well as coupling of bone formation and resorption, and continued through the open-label extension with a median change from baseline of: 0.82, 1.01 and 1.56 at 1,2 and 3 years respectively.21, 37 The patients benefiting most from this improvement were those on steroids at enrollment and those with at least 1-year delay in bone age. These growth benefits also appear to be significantly more pronounced in children with severe CD treated either prior to the onset of puberty or in its early stages (Tanner I–III), reconfirming the role for early initiation of biologic therapy.35
In summary, IFX is an effective treatment option for both pediatric Crohn’s Disease and UC. In UC patients suffering from an acute severe flare, early IFX induction is recommended in patients with a PUCAI score of >45 on hospital day 3 or >65 on hospital day 5 to avoid the substantial morbidity and mortality associated with delayed treatment of this population.38 IFX should also be considered in children with persistently active or steroid-dependent UC, uncontrolled by 5-ASA and thiopurines.39 ADA is an effective alternative in CD patients intolerant or no longer responsive to IFX therapy. The role of ADA in pediatric UC still remains unclear and large multi-center trials are needed.
Safety in Pediatric IBD
Immunogenicity and Infusion Reactions
The formation of antibodies to infliximab (ATI) has been seen in 3–35% of IFX treated pediatric patients and is thought to impact clinical efficacy as well as increase the risk for both acute infusion reactions (AIR) and delayed type III hypersensitivity reactions (serum-sickness).18, 40–45 It should be mentioned that the REACH study, which only detected ATI in 3% of pediatric patients, had inconclusive results in 77% of patients due to presence of circulating infliximab, limiting the conclusions which can be drawn.18
A combination of both immune (type I hypersensitivity) and non-immune (rate-related) mediated reactions, symptoms of AIR commonly include shortness of breath, flushing, rash, headaches and tachycardia.46 Rarely, these patients suffer from anaphylaxis and shock. Pooling data from 32 cohort studies for pediatric IBD patients undergoing treatment with IFX, 149 of 1,550 patients (9.6%) receiving more than 13,940 infusions experienced a total of 184 AIR (1.3%) with nearly a quarter of these being severe in nature (41/184, 22%).12–16, 18–20, 30, 47–51,17, 21, 31, 52–73 When expressed based by the duration of IFX exposure, the overall incidence for infusions reactions was 147/1,000 patient years of exposure (PYE) with an incidence of 33/1,000 PYE for severe reactions. (Table 1) Forty-two patients required discontinuation of further treatment (2.7%) with no patient deaths secondary to AIR. Five patients developed symptoms consistent with a delayed hypersensitivity reaction (0.3%, 0.22 per 100 patient years of follow-up).
TABLE 1.
Pooled Rates for Adverse Events for IFX and ADA
| No. of Patients with Event (N = 1979) | Rate per 1000 Patient-years | |
|---|---|---|
| Severe adverse events/reactions | 151 | 57 |
| Discontinuation due to event | 132 | 50 |
| Deaths | 4 | 1.5 |
| Infections | 350 | 132 |
| Serious infections | 54 | 20 |
| Lymphoma | 1 | 0.38 |
| Hepatic | 18 | 12a |
| Hematological | 40 | 26a |
| Autoimmune | 5 | 3a |
| Cardiac | 5 | 3a |
| Infusion/local site reactions | 220 | 141a |
Calculated based on patient-years of exposure (total exposure during treatment = 1,559 patient-years) as opposed to PYF (total follow-up during and after therapy = 2,649 patient-years). This is specified because the events calculated based on patient-years of exposure occurred during active treatment, not later in the follow-up period.
Female gender, immunosuppressive use for less than 4 months and a prior infusion reaction have been found to be risk factors for AIR in children.45, 74, 75 Episodic treatment has been shown to increase the rate of severe delayed infusion reactions among adult patients, but one publication suggests that this does not appear to be the case in children.45 This may be partially explained by the fact that adult patients undergoing episodic treatment with lengthy intervals between infusions, are at a higher risk for development of ATI compared to children.41 Pre-medication with anti-histamines or corticosteroids does not appear to prevent the development of initial infusion reactions in children.76, 77 Although premedication was not used or commented on routinely throughout the studies, our review suggests that it does appear to possibly prevent repeat reactions in those patients who continue on treatment after an initial AIR and therefore should be considered for patients continuing on therapy.41
Pediatric data on the formation of antibodies to ADA (AAA) are limited to the IMAgINE 1 study which detected AAA in 3% of patients.29 Adult data have reported the formation of AAA in 3–17% of patients, but unlike IFX these antibodies only appear to impact clinical efficacy and not adverse reactions.78, 79
Autoimmunity
Of the 250 IFX treated patients where anti-nuclear antibody (ANA) testing was performed, 17% (n=43) developed positive ANAs, 4% (n=10) developed anti-double stranded antibodies and 2% (n=5) developed anti-tissue antibodies. These antibodies often disappear within 6 months of treatment discontinuation,15 making the significance of these asymptomatic antibodies unclear. Drug induced clinical lupus was rare and only occurred in 2 patients, with one of these having negative serology.51, 61 The patient with negative serology was successfully retreated with 2 more infusions and the rash thought to be associated with drug-induce lupus resolved over 6 months. The other was switched to ADA with no further autoimmune phenomenon reported. Other autoimmune disorders described include: vascular purpura (n=2) which resolved with discontinuation of IFX, Henoch-Schonlein purpura (n=1) and an ANCA associated vasculitis involving the fingers (n=1) which was felt to likely be related to the underlying IBD.20, 61, 65 To date, there are no reported cases of autoimmune disorders developing after treatment with ADA, but there are far fewer data as compared to IFX.
Infections
Pooled data for both IFX and ADA show an overall infection rate of 17.7% with 2.8% of treated patients suffering from serious infections.80 Expressed by the length of patient follow-up, the incidence of infections per 1,000 patient years of follow-up (PYF) was 132/1,000 PYF overall and 20/1,000 PYF for serious infections. Given the potential for under reporting in retrospective studies, data were re-analyzed limiting this evaluation to prospective studies (n=15 studies, 1,186 patients with 1,901 PYF). The incidence was 177/1,000 PYF for all infections (n=337) and 17/1,000 PYF for serious infections (n=33).
The majority of pediatric infections with IFX were upper respiratory tract in origin (41%) or non-severe non-specified illnesses (25%). Other common infections included: zoster/varicella (5%), pneumonia (3%), fevers with or without septicemia (3%), abscesses (5%) and fungal infections (2%).14, 15, 18–21, 30, 31, 48, 51–53, 55, 56, 58, 61, 63, 65, 67–69 There have been a few cases reported of other infections in patients taking IFX including: positive PPD without CXR findings (n=1), otitis media (n=1), oral herpes (n=1), molluscum contagiosum (n=1), pseudomonas cellulitis at a gastrotomy site (n=1), osteomyelitis (n=1), listeria meningitis (n=1), appendicitis (n=2) and clinical reactivation of Epstein Barr Virus (n=3).14, 18–20, 48, 51, 56, 65, 68 Of the 429 patients reviewed on ADA treatment, reported infections included: abscesses (n=8), staff folliculitis (n=1), C. Diff (n=1), scarlet fever (n=1), disseminated histoplasmosis (n=1), H1N1 (n=1), unspecified viral illnesses (n=1), Yesinia enterocolitica (n=1), device related sepsis (n=1), Aeromonas spp. (n=1) and sinusitis (n=1).24–29 Only one potentially treatment related infectious death occurred with IFX in an 11 year old boy who became septic from an abscess located near a colonic stenosis in the setting of malnutrition and leucopenia associated with azathioprine.70 Two potentially treatment related infectious deaths occurred with ADA, both with sepsis in the setting of central line placement.25
Overall, biologic therapy in pediatric patients appears to be safe and well tolerated. Attempts at reducing the incidence of potentially severe complications should be focused on exposure prevention, screening and vaccination where appropriate. Treatment should be avoided in those with acute infections and patients with abscesses should have surgical drainage before treatment. Those undergoing concomitant treatment with immunosuppressive therapy should be carefully monitored for neutropenia and leucopenia to avoid life threatening opportunistic illnesses.
Malignancy
Concern arises for the occurrence of malignancy given adult studies demonstrating an increased incidence of non-Hodgkins lymphoma with the use of anti-TNF agents and immunomodulators.81 In our review only one patient developed Hodgkin’s lymphoma resulting in an incidence rate of 3.8/10,000 PYF.80 This is numerically, but not statistically, different than the rates seen with thiopurine monotherapy (4.4/10,000 PYF; SIR=0.84, 95% CI 0.016–16.1) or the expected baseline rate in the general pediatric population for all lymphoid neoplasias (0.58/10,000 PYF; SIR=6.3, 95% CI 0.14–51.9) and Hodgkin’s lymphoma specifically (0.12/10,000 PYF; SIR=37.8, 95% CI 0.48–2693).80,82 With 73% of patients being treated concomitantly with thiopurines, including the one patient who developed Hodgkin’s lymphoma, it is unclear if this potential risk still exists with anti-TNF monotherapy in the pediatric population. Adult pooled data have suggested that IFX monotherapy carries no additional risk of lymphoma beyond that seen with placebo but further studies are needed in adult and pediatric patients.83
Another particularly serious type of lymphoma, hepatosplenic T-cell lymphoma (HSTCL), has been reported in inflammatory bowel disease patients. At the time of Kotylar’s 2011 publication on this topic, 36 cases had been reported between the ages of 12–58 years. Twenty of which had received IFX and thiopurine combination therapy with the remaining 16 receiving thiopurine monotherapy. Four patients received adalimumab after infliximab therapy. All except 2 patients were male.84 Since there is no accurate method to determine the number of male patients within this age range who have been exposed to anti-TNF agents or thiopurines, it is not possible to confidently determine a denominator of “at risk” patients. Therefore, the incidence of HSTCL cannot be accurately calculated. This risk with thiopurines appears to be duration- and gender-dependent, with male patients exposed for >2 years being at highest risk.84 Postulated mechanisms for this risk include dose and duration-dependent rates of thiopurine induced DNA damage resulting in abnormalities of chromosome 7, 8, 13 and Y.85
Based on our review, and adult literature, IFX therapy does not appear to significantly impact the rate of lymphoma in pediatric patients. To date, there have been no reported cases of Hodgkin’s lymphoma or HSTCL occurring with anti-TNF monotherapy and therefore attempts at reducing the risk for lymphoma may be accomplished through discontinuation of thiopurines after an initial response is seen or transitioning to alternative immunnosuppressants such as methotrexate. Large population-based studies however are required to more accurately understand the relationship of lymphoma development with biologics in pediatric IBD patients.
Other Reactions and Adverse Events
In our review, other reported adverse events attributable to IFX included: Psoriasis (n=1) or psoriaform lesions (n=12), anemia (n=11), neutropenia (n=3), transaminitis (n=6), artharlgias/joint pain (n=5), suicide attempt (n=2), pancreatitis (n=1), basal cell carcinoma (n=1), bradycardia (n=1), cardiac insufficiency (n=1), cardiomyopathy (n=2) and prolonged QTc resulting in death (n=1).14, 18–20, 30, 48, 51, 56, 61, 65, 69 The psoriaform lesions were easily treated with topical steroids and transaminitis typically resolved despite continued therapy. Of the 3 patients with neutropenia, 2 of these patients were on concomitant immunosuppressive therapy and 1 resolved after discontinuation of azathioprine.
Of the two cardiomyopathies noted in our review, one was found to have a familial cardiomyopathy after IFX was initiated and the other was found to have a cardiomyopathy with pericardial effusion which resolved after discontinuation of therapy.30, 56 The patient with prolonged QTc who unfortunately died had suffered a near cardiac death from an arrhythmia prior to IFX therapy, which questions the association with IFX at all.69 The risk of cardio toxicity with anti-TNF agents has been well-described. The ATTACH trial evaluated the use of biologics in adult patients with NYHA Class III–VI Heart Failure and demonstrated that when compared to placebo, IFX resulted in a 3 fold increased risk for the combined outcomes of death from any cause and hospitalization for heart failure.86 A pediatric pilot study investigated the role of electrocardiographic (ECG) and echocardiographic (ECHO) monitoring of heart function during IFX therapy in IBD patients. Twenty-six patients were enrolled, 12 IBD patients undergoing IFX therapy and 14 age- and sex-matched controls. Asymptomatic ultrasound evidence of cardiac involvement was present in 7 of the 12 IBD patients after initiation of IFX therapy. Although no differences were found in heart rate variability indexes when compared to controls, the investigators commented on the presence of a positive correlation between QT dispersion, left ventricular diastolic dimension (LVDD), and left ventricular systolic dimension (LVSD) which they felt suggested an increased risk for development of cardiac dysrrhythmias.87 Taken together, patients should be screened clinically for features of heart failure or familial syndromes that may predispose them to adverse events. At the current time, there are no recommendations for screening or monitoring average risk adult or pediatric patients with an ECG or ECHO.
Balancing risk and reward – Step-up, Top-Down, Mono- or Combination-Therapy?
Traditionally, pediatric IBD patients undergo a step-up strategy for treatment. It has recently been demonstrated in adult patients with Crohn’s disease that this pyramidal approach may be reversed to allow for earlier initiation of biologics with improved efficacy and long-term outcomes.88 In adult patients with Crohn’s disease, the SONIC trial demonstrated that combined therapy was most beneficial in immunosuppressive naïve patients.89 Despite various studies demonstrating that pediatric CD and UC patients treated earlier in their disease course have improved outcomes, data comparing a step-up with a top-down treatment strategy in pediatric patients are limited.13, 30, 52, 58, 60
A retrospective study compared three treatment strategies in 36 newly diagnosed pediatric CD patients. At 1 year follow-up, patients treated initially with IFX and azathioprine had lower rates of relapse (as defined by a PCADI >10) compared to those initially treated with steroids + azathioprine (23% vs. 62%, p=0.047) or steroids + mesalamine (23% vs. 80%, p=0.012).64 At 2 years, relapse rates were 39%, 77% and 90% respectively. Similarly in pediatric UC, patients with new onset disease and those with a shorter duration of disease activity were found to be more likely to respond to IFX suggesting that earlier initiation of biologics would result in improved outcomes.30
In summary, the adult literature supports the concept of the early use of combination therapy, and although this may also apply to pediatric patients, data are lacking to currently endorse this approach. However, as described above, the risks associated with anti-TNF therapy are really not significantly different as compared to thiopurine therapy, and perhaps in some cases safer. Therefore, we should be moving closer to the idea of using anti-TNF therapy early, with or without an immunomodulator. In the sickest patients, combination therapy probably adds benefit, and then once in remission, consideration can be given for stopping one of the medications, more likely the thiopurine.
Beyond the data: Who’s making treatment decisions and who’s qualified to do so?
Although the treatment benefit and safety profile of anti-TNF treatment is becoming clearer in pediatric IBD patients, there is still a strong emotional and communication barrier for patients and parents ultimately choosing to use these medications. The decision for treatment is significantly influenced by the data we present to patients and parents and the manner in which we present it. It has been demonstrated that parents of children with IBD are accepting of risks related to therapies, but require significant benefit to make this tradeoff.90, 91 As patients and parents of patients perceive risks and benefits differently,10 it is vital to understand the barriers to communication and to develop informative yet simple communication tools to overcome these misperceptions.
Health Literacy and Health care decisions – Barriers to success
Various factors influence the perception of risk and benefit, but none more so than health literacy. Health literacy, defined as a set of skills that are required to function well in the health care or public health setting, is a well recognized critical component of a high-quality health care system.92 A large proportion of parents in the United States however, nearly 1 in 4, have limited health literacy skills.93 This low parental literacy has been shown to correlate with worse health outcomes in pediatric patients.92, 93 Likely through a combination of perceived barriers to communication or health care access, difficulty understanding written material, particularly medication labels, and feelings of exclusion from the decision making process. 92–94 The ultimate result of this is a decreased participation in shared decision making by parents, greater perceived burden from the child’s illness and increased reliance of physicians to dictate therapy.94, 95
Adolescent health literacy appears to significantly impact health outcomes as well. Those with lower-than-average literacy rates appeared to have increased risk-taking or violent behaviors,92 and were less likely to participate in their own medical care and therefore more likely to be non-adherent with treatment.96, 97 This non-involvement results in adolescents not implementing the necessary level of self-care activities, particularly when transfer of care occurs to adult specialists. This further worsens health care outcomes and creates barriers to communication between adult physicians and now adult patients.92, 93
Traditionally we have been very poor at assessing health literacy and readiness for transition of care from parent to child.98 It is thus paramount to assess both parents and children for deficiencies in health literacy and identify high risk patients, particularly during the transition phase from adolescence to adult medicine. These deficits can then be overcome through a combination of written material and counseling with a variety of tools such as the My Health Passport for IBD, activity books, option grids (described below) or simply asking children about involvement.92, 97, 99
Children and medical decisions – what do they want and when should they get it?
The effort to make adolescent health care more patient-centered must also include a clear understanding of patient preferences and priorities. Only through this can we effectively communicate the benefits of therapy and improve patient satisfaction and compliance. Furthermore, involvement of the child may promote a sense of control, which in turn, may correlate to positive adjustment, increased adherence and ultimately improved outcomes. Much like adults, children prefer a high degree of involvement in the medial decision making process. A recent survey demonstrated that the factors most important to adolescents with chronic illnesses were: sense of respect and trust, assessment of pain and direct communication with the physician as opposed to through their parents.100, 101 The technical aspects of care, such as experience of the physician, also ranked highly but having a sense control over health care decisions appeared to be the most important feature of a good relationship. It also important to note that not all patients want to participate in shared decision making and this should be assessed on an individual basis.
Concerns arise for when to initiate these discussions and how to assess for readiness of involvement. Earlier literature has shown us that, children as young as 9 years of age appear to have the capacity to express their preferences regarding treatment. Those aged 14 years and older do not differ significantly from adults in regards to reasoning and understanding treatment options.102 Therefore, although this will be variable, early involvement of children in the shared decision making process is not only feasible but creates a sense of responsibility and improved communication resulting in improved outcomes and quality of life.
Shared decision making in pediatric IBD – a new tool in our armamentarium
Shared decision making is the process through which physicians inform and recommend treatment options to patients with the overall goal of enhancing patient involvement and facilitating ‘evidence-based patient choice’. In order to effectively communicate these data, physicians rely upon ‘decision aids’ – balanced presentations of particular treatment options for a decision that needs to be made by the patients with their provider.11 These can be as simple as paper handouts to as complex as web-based videos which can be updated regularly and allow for a broader distribution. A 2011 Cochrane analysis of over 80 patients’ decision aids showed that these tools can have a positive effect on patient-provider communication, and increase people’s involvement, and improve knowledge and realistic perception of outcomes.103
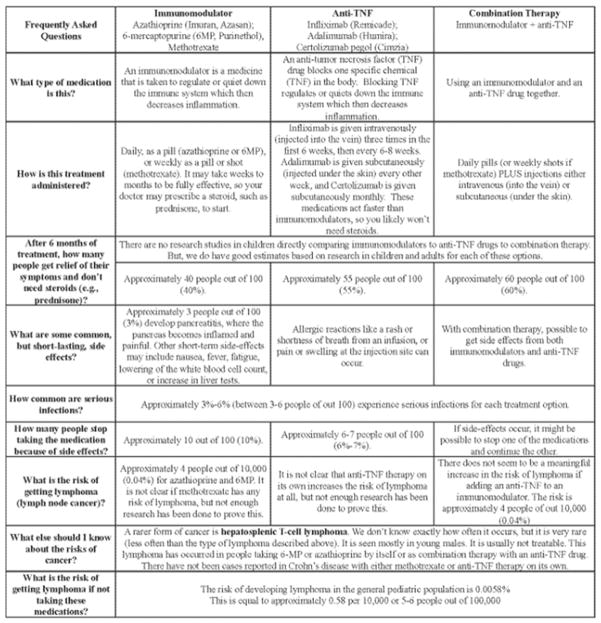
One type of decision aid that is recently gaining popularity is an “Option Grid.” An Option Grid is a one-page summary table answering frequently asked questions comparing head-to-head treatment options. It is designed to be used quickly and efficiently in a face-to-face clinical encounter to help a patient through a specific treatment decision. When studied, clinicians believed that Option Grids made it easier to explain treatment options to patients, and found that it enhanced patient involvement in collaborative decision making.104 An option grid has previously been created using adult data for patients with Crohn’s disease comparing thiopurine monotherapy to anti-TNF monotherapy to combination therapy (http://www.optiongrid.co.uk/resources/Crohns_disease_treatment-Option_Grid.pdf).
To help summarize the vast amount of pediatric data presented above, we have adapted the adult Crohn’s disease Option Grid for pediatric patients. It is specifically focused on the question of using thiopurine monotherapy versus anti-TNF monotherapy versus combination therapy (Figure 1). Data presented in the Option Grid include REACH,18, 21 IMAgINE 129, pooled data from our analysis regarding the risk of side effects, and expert opinion of the authors and two external reviewers. Due to a paucity of biologic and comparative benefit and risk data for pediatric ulcerative colitis, we did not create an Option Grid for ulcerative colitis. Option Grids should not be used in isolation instead of careful conversation with patients, but as a tool to facilitate a discussion over benefits and risks and to promote an informed shared medical decision. We hope that this Option Grid will help providers better communicate with patients and their parents, and will motivate others to develop and use such tools.
Figure 1.
Conclusion
The use of anti-TNF agents has transformed the way in which we care for pediatric IBD patients. It has resulted in not only improved disease control, but also patient growth and quality of life. Although these drugs carry the potential for serious adverse events such as infections and malignancy, overall biologic therapy in pediatric patients appears to be safe and well tolerated. Through careful patient selection and screening, the use of appropriate scheduled treatment protocols and avoidance of prolonged immunomodulator use we can keep the balance in favor of reward over risk. Irrespective of these presented data, the greatest barrier to success with biologics still lies in our ability to effectively communicate these outcomes to parents and patients in a simple yet informative manner. We hope that through the creation of our Option Grid providers will be able to not only address parent and patient concerns and misperceptions, but also enhance the shared decision making process allowing for improved parent and patient satisfaction, adherence and ultimately long-term outcomes.
Acknowledgments
Disclosures and acknowledgements: Dr. Siegel serves on the advisory board, as a consultant and received grant support from Abbott Labs, Janssen and UCB. Dr. Siegel is supported by Grant number K23DK078678 from the National Institute of Diabetes and Digestive and Kidney Diseases and Grant number 1R01HS021747-01from the Agency for Healthcare Research and Quality. Dr. Dubinsky serves as a consultant to Abbott, Janssen, UCB and Takeda. Dr. Dulai has nothing to disclose. The authors thank Dr. Jim Markowitz and Dr. Joel Rosh for their thoughtful review of the Option Grid.
References
- 1.Schildkraut V, Alex G, Cameron DJ, et al. Sixty-year study of incidence of childhood ulcerative colitis finds eleven-fold increase beginning in 1990s. Inflamm Bowel Dis. 2012 Apr 24; doi: 10.1002/ibd.22997. [DOI] [PubMed] [Google Scholar]
- 2.Martin-de-Carpi J, Rodriguez A, Ramos E, et al. on behalf of the SPIRIT-IBD Working Group of SEGHNP (Sociedad Espanola de Gastroenterologia, Hepatologia y Nutricion Pediatrica) Increasing incidence of pediatric inflammatory bowel disease in spain (1996–2009): The SPIRIT registry. Inflamm Bowel Dis. 2012 Apr 25; doi: 10.1002/ibd.22980. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm Bowel Dis. 2011 Jan;17(1):423–39. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 4.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2009 Nov;15(11):1755–64. doi: 10.1002/ibd.20919. [DOI] [PubMed] [Google Scholar]
- 5.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the montreal classification for inflammatory bowel disease: The paris classification. Inflamm Bowel Dis. 2011 Jun;17(6):1314–21. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 6.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009 Nov;361(21):2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009 Sep 18;31(3):401–11. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009 Nov;361(21):2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut. 2012 Nov;61(11):1619–35. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 10.Siegel CA, Levy LC, Mackenzie TA, et al. Patient perceptions of the risks and benefits of infliximab for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2008 Jan;14(1):1–6. doi: 10.1002/ibd.20283. [DOI] [PubMed] [Google Scholar]
- 11.Siegel CA. Shared decision making in inflammatory bowel disease: Helping patients understand the tradeoffs between treatment options. Gut. 2012 Mar;61(3):459–65. doi: 10.1136/gutjnl-2011-300988. [DOI] [PubMed] [Google Scholar]
- 12.Hyams JS, Markowitz J, Wyllie R. Use of infliximab in the treatment of crohn’s disease in children and adolescents. J Pediatr. 2000 Aug;137(2):192–6. doi: 10.1067/mpd.2000.107161. [DOI] [PubMed] [Google Scholar]
- 13.Kugathasan S, Werlin SL, Martinez A, et al. Prolonged duration of response to infliximab in early but not late pediatric crohn’s disease. Am J Gastroenterol. 2000 Nov;95(11):3189–94. doi: 10.1111/j.1572-0241.2000.03263.x. [DOI] [PubMed] [Google Scholar]
- 14.Baldassano R, Braegger CP, Escher JC, et al. Infliximab (REMICADE) therapy in the treatment of pediatric crohn’s disease. Am J Gastroenterol. 2003 Apr;98(4):833–8. doi: 10.1111/j.1572-0241.2003.07343.x. [DOI] [PubMed] [Google Scholar]
- 15.Cezard JP, Nouaili N, Talbotec C, et al. A prospective study of the efficacy and tolerance of a chimeric antibody to tumor necrosis factors (remicade) in severe pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2003 May;36(5):632–6. doi: 10.1097/00005176-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Borrelli O, Bascietto C, Viola F, et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with crohn’s disease. Dig Liver Dis. 2004 May;36(5):342–7. doi: 10.1016/j.dld.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Crandall W, Hyams J, Kugathasan S, et al. Infliximab therapy in children with concurrent perianal crohn disease: Observations from REACH. J Pediatr Gastroenterol Nutr. 2009 Aug;49(2):183–90. doi: 10.1097/MPG.0b013e3181a70f21. [DOI] [PubMed] [Google Scholar]
- 18.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe crohn’s disease in children. Gastroenterology. 2007 Mar;132(3):863, 73. doi: 10.1053/j.gastro.2006.12.003. quiz 1165–6. [DOI] [PubMed] [Google Scholar]
- 19.Ruemmele FM, Lachaux A, Cezard JP, et al. Efficacy of infliximab in pediatric crohn’s disease: A randomized multicenter open-label trial comparing scheduled to on demand maintenance therapy. Inflamm Bowel Dis. 2009 Mar;15(3):388–94. doi: 10.1002/ibd.20788. [DOI] [PubMed] [Google Scholar]
- 20.Crombe V, Salleron J, Savoye G, et al. Long-term outcome of treatment with infliximab in pediatric-onset crohn’s disease: A population-based study. Inflamm Bowel Dis. 2011 Oct;17(10):2144–52. doi: 10.1002/ibd.21615. [DOI] [PubMed] [Google Scholar]
- 21.Hyams J, Walters TD, Crandall W, et al. Safety and efficacy of maintenance infliximab therapy for moderate-to-severe crohn’s disease in children: REACH open-label extension. Curr Med Res Opin. 2011 Mar;27(3):651–62. doi: 10.1185/03007995.2010.547575. [DOI] [PubMed] [Google Scholar]
- 22.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with crohn’s disease: The CHARM trial. Gastroenterology. 2007 Jan;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for crohn disease previously treated with infliximab: A randomized trial. Ann Intern Med. 2007 Jun 19;146(12):829–38. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 24.Rosh JR, Lerer T, Markowitz J, et al. Retrospective evaluation of the safety and effect of adalimumab therapy (RESEAT) in pediatric crohn’s disease. Am J Gastroenterol. 2009 Dec;104(12):3042–9. doi: 10.1038/ajg.2009.493. [DOI] [PubMed] [Google Scholar]
- 25.Russell RK, Wilson ML, Loganathan S, et al. A british society of paediatric gastroenterology, hepatology and nutrition survey of the effectiveness and safety of adalimumab in children with inflammatory bowel disease. Aliment Pharmacol Ther. 2011 Apr;33(8):946–53. doi: 10.1111/j.1365-2036.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- 26.Viola F, Civitelli F, Di Nardo G, et al. Efficacy of adalimumab in moderate-to-severe pediatric crohn’s disease. Am J Gastroenterol. 2009 Oct;104(10):2566–71. doi: 10.1038/ajg.2009.372. [DOI] [PubMed] [Google Scholar]
- 27.Wyneski MJ, Green A, Kay M, et al. Safety and efficacy of adalimumab in pediatric patients with crohn disease. J Pediatr Gastroenterol Nutr. 2008 Jul;47(1):19–25. doi: 10.1097/MPG.0b013e318174e886. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbach Y, Hartman C, Shapiro R, et al. Adalimumab treatment in children with refractory crohn’s disease. Dig Dis Sci. 2010 Mar;55(3):747–53. doi: 10.1007/s10620-009-0791-7. [DOI] [PubMed] [Google Scholar]
- 29.Hyams JS, Griffiths A, Markowitz J, et al. Safety and efficacy of adalimumab for moderate to severe crohn’s disease in children. Gastroenterology. 2012 Aug;143(2):365, 74.e2. doi: 10.1053/j.gastro.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: A prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010 Jun;138(7):2282–91. doi: 10.1053/j.gastro.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 31.Hyams JS, Lerer T, Griffiths A, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010 Jun;105(6):1430–6. doi: 10.1038/ajg.2009.759. [DOI] [PubMed] [Google Scholar]
- 32.Heuschkel R, Salvestrini C, Beattie RM, et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008 Jun;14(6):839–49. doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 33.Kanof ME, Lake AM, Bayless TM. Decreased height velocity in children and adolescents before the diagnosis of crohn’s disease. Gastroenterology. 1988 Dec;95(6):1523–7. doi: 10.1016/s0016-5085(88)80072-6. [DOI] [PubMed] [Google Scholar]
- 34.Hildebrand H, Karlberg J, Kristiansson B. Longitudinal growth in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994 Feb;18(2):165–73. doi: 10.1097/00005176-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe crohn’s disease. Inflamm Bowel Dis. 2007 Apr;13(4):424–30. doi: 10.1002/ibd.20069. [DOI] [PubMed] [Google Scholar]
- 36.Malik S, Wong SC, Bishop J, et al. Improvement in growth of children with crohn disease following anti-TNF-alpha therapy can be independent of pubertal progress and glucocorticoid reduction. J Pediatr Gastroenterol Nutr. 2011 Jan;52(1):31–7. doi: 10.1097/MPG.0b013e3181edd797. [DOI] [PubMed] [Google Scholar]
- 37.Thayu M, Leonard MB, Hyams JS, et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric crohn’s disease: Results of the REACH study. Clin Gastroenterol Hepatol. 2008 Dec;6(12):1378–84. doi: 10.1016/j.cgh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Turner D, Travis SP, Griffiths AM, et al. Consensus for managing acute severe ulcerative colitis in children: A systematic review and joint statement from ECCO, ESPGHAN, and the porto IBD working group of ESPGHAN. Am J Gastroenterol. 2011 Apr;106(4):574–88. doi: 10.1038/ajg.2010.481. [DOI] [PubMed] [Google Scholar]
- 39.Turner D, Levine A, Escher JC, et al. Management of pediatric ulcerative colitis: Joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012 Sep;55(3):340–61. doi: 10.1097/MPG.0b013e3182662233. [DOI] [PubMed] [Google Scholar]
- 40.de Bie CI, Escher JC, de Ridder L. Antitumor necrosis factor treatment for pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2012 May;18(5):985–1002. doi: 10.1002/ibd.21871. [DOI] [PubMed] [Google Scholar]
- 41.Veres G, Baldassano RN, Mamula P. Infliximab therapy in children and adolescents with inflammatory bowel disease. Drugs. 2007;67(12):1703–23. doi: 10.2165/00003495-200767120-00005. [DOI] [PubMed] [Google Scholar]
- 42.Candon S, Mosca A, Ruemmele F, et al. Clinical and biological consequences of immunization to infliximab in pediatric crohn’s disease. Clin Immunol. 2006 Jan;118(1):11–9. doi: 10.1016/j.clim.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Miele E, Markowitz JE, Mamula P, et al. Human antichimeric antibody in children and young adults with inflammatory bowel disease receiving infliximab. J Pediatr Gastroenterol Nutr. 2004 May;38(5):502–8. doi: 10.1097/00005176-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in crohn’s disease. N Engl J Med. 2003 Feb 13;348(7):601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 45.Kugathasan S, Levy MB, Saeian K, et al. Infliximab retreatment in adults and children with crohn’s disease: Risk factors for the development of delayed severe systemic reaction. Am J Gastroenterol. 2002 Jun;97(6):1408–14. doi: 10.1111/j.1572-0241.2002.05784.x. [DOI] [PubMed] [Google Scholar]
- 46.Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: A large center experience. Am J Gastroenterol. 2003 Jun;98(6):1315–24. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 47.Wanty C, Stephenne X, Sokal E, et al. Long-term outcome of infliximab therapy in pediatric crohn disease. Arch Pediatr. 2011 Aug;18(8):863–9. doi: 10.1016/j.arcped.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 48.de Ridder L, Rings EH, Damen GM, et al. Infliximab dependency in pediatric crohn’s disease: Long-term follow-up of an unselected cohort. Inflamm Bowel Dis. 2008 Mar;14(3):353–8. doi: 10.1002/ibd.20329. [DOI] [PubMed] [Google Scholar]
- 49.Duricova D, Pedersen N, Lenicek M, et al. Infliximab dependency in children with crohn’s disease. Aliment Pharmacol Ther. 2009 Apr 1;29(7):792–9. doi: 10.1111/j.1365-2036.2009.03926.x. [DOI] [PubMed] [Google Scholar]
- 50.Wewer V, Riis L, Vind I, et al. Infliximab dependency in a national cohort of children with crohn’s disease. J Pediatr Gastroenterol Nutr. 2006 Jan;42(1):40–5. doi: 10.1097/01.mpg.0000189137.06151.33. [DOI] [PubMed] [Google Scholar]
- 51.De Bie CI, Hummel TZ, Kindermann A, et al. The duration of effect of infliximab maintenance treatment in paediatric crohn’s disease is limited. Aliment Pharmacol Ther. 2011 Jan;33(2):243–50. doi: 10.1111/j.1365-2036.2010.04507.x. [DOI] [PubMed] [Google Scholar]
- 52.Mamula P, Markowitz JE, Brown KA, et al. Infliximab as a novel therapy for pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2002 Mar;34(3):307–11. doi: 10.1097/00005176-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Mamula P, Markowitz JE, Cohen LJ, et al. Infliximab in pediatric ulcerative colitis: Two-year follow-up. J Pediatr Gastroenterol Nutr. 2004 Mar;38(3):298–301. doi: 10.1097/00005176-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Russell GH, Katz AJ. Infliximab is effective in acute but not chronic childhood ulcerative colitis. J Pediatr Gastroenterol Nutr. 2004 Aug;39(2):166–70. doi: 10.1097/00005176-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Eidelwein AP, Cuffari C, Abadom V, et al. Infliximab efficacy in pediatric ulcerative colitis. Inflamm Bowel Dis. 2005 Mar;11(3):213–8. doi: 10.1097/01.mib.0000160803.44449.a5. [DOI] [PubMed] [Google Scholar]
- 56.Fanjiang G, Russell GH, Katz AJ. Short- and long-term response to and weaning from infliximab therapy in pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2007 Mar;44(3):312–7. doi: 10.1097/MPG.0b013e31802e98d4. [DOI] [PubMed] [Google Scholar]
- 57.Cucchiara S, Romeo E, Viola F, et al. Infliximab for pediatric ulcerative colitis: A retrospective italian multicenter study. Dig Liver Dis. 2008 Jul;40( Suppl 2):S260–4. doi: 10.1016/S1590-8658(08)60535-6. [DOI] [PubMed] [Google Scholar]
- 58.McGinnis JK, Murray KF. Infliximab for ulcerative colitis in children and adolescents. J Clin Gastroenterol. 2008 Sep;42(8):875–9. doi: 10.1097/MCG.0b013e3181354417. [DOI] [PubMed] [Google Scholar]
- 59.Turner D, Griffiths AM. Acute severe ulcerative colitis in children: A systematic review. Inflamm Bowel Dis. 2011 Jan;17(1):440–9. doi: 10.1002/ibd.21383. [DOI] [PubMed] [Google Scholar]
- 60.Lionetti P, Bronzini F, Salvestrini C, et al. Response to infliximab is related to disease duration in paediatric crohn’s disease. Aliment Pharmacol Ther. 2003 Aug 15;18(4):425–31. doi: 10.1046/j.1365-2036.2003.01672.x. [DOI] [PubMed] [Google Scholar]
- 61.Lamireau T, Cezard JP, Dabadie A, et al. Efficacy and tolerance of infliximab in children and adolescents with crohn’s disease. Inflamm Bowel Dis. 2004 Nov;10(6):745–50. doi: 10.1097/00054725-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Afzal NA, Ozzard A, Keady S, et al. Infliximab delays but does not avoid the need for surgery in treatment-resistant pediatric crohn’ disease. Dig Dis Sci. 2007 Dec;52(12):3329–33. doi: 10.1007/s10620-007-8102-1. [DOI] [PubMed] [Google Scholar]
- 63.Wynands J, Belbouab R, Candon S, et al. 12-month follow-up after successful infliximab therapy in pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2008 Mar;46(3):293–8. doi: 10.1097/MPG.0b013e31815604cd. [DOI] [PubMed] [Google Scholar]
- 64.Lee JS, Lee JH, Lee JH, et al. Efficacy of early treatment with infliximab in pediatric crohn’s disease. World J Gastroenterol. 2010 Apr 14;16(14):1776–81. doi: 10.3748/wjg.v16.i14.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinitsky DM, Lemberg DA, Leach ST, et al. Infliximab improves inflammation and anthropometric measures in pediatric crohn’s disease. J Gastroenterol Hepatol. 2010 Apr;25(4):810–6. doi: 10.1111/j.1440-1746.2009.06195.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim MJ, Lee JS, Lee JH, et al. Infliximab therapy in children with crohn’s disease: A one-year evaluation of efficacy comparing ‘top-down’ and ‘step-up’ strategies. Acta Paediatr. 2011 Mar;100(3):451–5. doi: 10.1111/j.1651-2227.2010.01938.x. [DOI] [PubMed] [Google Scholar]
- 67.Kierkus J, Dadalski M, Szymanska E, et al. The impact of infliximab induction therapy on mucosal healing and clinical remission in polish pediatric patients with moderate-to-severe crohn’s disease. Eur J Gastroenterol Hepatol. 2012 May;24(5):495–500. doi: 10.1097/MEG.0b013e32835159f2. [DOI] [PubMed] [Google Scholar]
- 68.Stephens MC, Shepanski MA, Mamula P, et al. Safety and steroid-sparing experience using infliximab for crohn’s disease at a pediatric inflammatory bowel disease center. Am J Gastroenterol. 2003 Jan;98(1):104–11. doi: 10.1111/j.1572-0241.2003.07161.x. [DOI] [PubMed] [Google Scholar]
- 69.Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with crohn’s disease. Inflamm Bowel Dis. 2009 Jun;15(6):816–22. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- 70.de Ridder L, Escher JC, Bouquet J, et al. Infliximab therapy in 30 patients with refractory pediatric crohn disease with and without fistulas in the netherlands. J Pediatr Gastroenterol Nutr. 2004 Jul;39(1):46–52. doi: 10.1097/00005176-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Teitelbaum JE, Saeed S, Triantafyllopoulou M, et al. Infliximab in pediatric crohn disease patients with enterovesicular fistulas. J Pediatr Gastroenterol Nutr. 2007 Feb;44(2):279–82. doi: 10.1097/01.mpg.0000237933.38223.da. [DOI] [PubMed] [Google Scholar]
- 72.Afzal NA, Shenoy MU, Haque S, et al. Recognition and treatment of genitourinary complications in paediatric crohn’s disease using infliximab. Acta Paediatr. 2010 Jul;99(7):1042–6. doi: 10.1111/j.1651-2227.2010.01731.x. [DOI] [PubMed] [Google Scholar]
- 73.Keljo DJ, Markowitz J, Langton C, et al. Course and treatment of perianal disease in children newly diagnosed with crohn’s disease. Inflamm Bowel Dis. 2009 Mar;15(3):383–7. doi: 10.1002/ibd.20767. [DOI] [PubMed] [Google Scholar]
- 74.Crandall WV, Mackner LM. Infusion reactions to infliximab in children and adolescents: Frequency, outcome and a predictive model. Aliment Pharmacol Ther. 2003 Jan;17(1):75–84. doi: 10.1046/j.1365-2036.2003.01411.x. [DOI] [PubMed] [Google Scholar]
- 75.Friesen CA, Calabro C, Christenson K, et al. Safety of infliximab treatment in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2004 Sep;39(3):265–9. doi: 10.1097/00005176-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Jacobstein DA, Markowitz JE, Kirschner BS, et al. Premedication and infusion reactions with infliximab: Results from a pediatric inflammatory bowel disease consortium. Inflamm Bowel Dis. 2005 May;11(5):442–6. doi: 10.1097/01.mib.0000158166.88238.ea. [DOI] [PubMed] [Google Scholar]
- 77.Lahdenne P, Wikstrom AM, Aalto K, et al. Prevention of acute adverse events related to infliximab infusions in pediatric patients. Arthritis Care Res. 2010 Jun;62(6):785–90. doi: 10.1002/acr.20246. [DOI] [PubMed] [Google Scholar]
- 78.West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in crohn’s disease. Aliment Pharmacol Ther. 2008 Nov 1;28(9):1122–6. doi: 10.1111/j.1365-2036.2008.03828.x. [DOI] [PubMed] [Google Scholar]
- 79.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in crohn’s disease. Gastroenterology. 2009 Nov;137(5):1628–40. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 80.Dulai PS, Dubinsky MC, Siegel CA. Systematic review of serious infection and lymphoma risk with anti-TNF therapy for pediatric IBD. Submitted to DDW 2013. [Google Scholar]
- 81.Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of crohn’s disease: A meta-analysis. Clin Gastroenterol Hepatol. 2009 Aug;7(8):874–81. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashworth LA, Billett A, Mitchell P, et al. Lymphoma risk in children and young adults with inflammatory bowel disease: Analysis of a large single-center cohort. Inflamm Bowel Dis. 2012 May;18(5):838–43. doi: 10.1002/ibd.21844. [DOI] [PubMed] [Google Scholar]
- 83.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012 Jul;107(7):1051–63. doi: 10.1038/ajg.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011 Jan;9(1):36, 41.e1. doi: 10.1016/j.cgh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 85.Kotlyar DS, Blonski W, Diamond RH, et al. Hepatosplenic T-cell lymphoma in inflammatory bowel disease: A possible thiopurine-induced chromosomal abnormality. Am J Gastroenterol. 2010 Oct;105(10):2299–301. doi: 10.1038/ajg.2010.213. [DOI] [PubMed] [Google Scholar]
- 86.Chung ES, Packer M, Lo KH, et al. Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003 Jul 1;107(25):3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 87.Barbato M, Curione M, Viola F, et al. Cardiac involvement in children with IBD during infliximab therapy. Inflamm Bowel Dis. 2006 Aug;12(8):828–9. doi: 10.1097/00054725-200608000-00021. [DOI] [PubMed] [Google Scholar]
- 88.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed crohn’s disease: An open randomised trial. Lancet. 2008 Feb 23;371(9613):660–7. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 89.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for crohn’s disease. N Engl J Med. 2010 Apr 15;362(15):1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 90.Johnson FR, Ozdemir S, Mansfield C, et al. Are adult patients more tolerant of treatment risks than parents of juvenile patients? Risk Anal. 2009 Jan;29(1):121–36. doi: 10.1111/j.1539-6924.2008.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siegel CA. Making therapeutic decisions in inflammatory bowel disease: The role of patients. Curr Opin Gastroenterol. 2009 Jul;25(4):334–8. doi: 10.1097/MOG.0b013e32832b764b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeWalt DA, Hink A. Health literacy and child health outcomes: A systematic review of the literature. Pediatrics. 2009 Nov;124( Suppl 3):S265–74. doi: 10.1542/peds.2009-1162B. [DOI] [PubMed] [Google Scholar]
- 93.Yin HS, Johnson M, Mendelsohn AL, et al. The health literacy of parents in the united states: A nationally representative study. Pediatrics. 2009 Nov;124( Suppl 3):S289–98. doi: 10.1542/peds.2009-1162E. [DOI] [PubMed] [Google Scholar]
- 94.Yin HS, Dreyer BP, Vivar KL, et al. Perceived barriers to care and attitudes towards shared decision-making among low socioeconomic status parents: Role of health literacy. Acad Pediatr. 2012 Mar;12(2):117–24. doi: 10.1016/j.acap.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shone LP, Conn KM, Sanders L, et al. The role of parent health literacy among urban children with persistent asthma. Patient Educ Couns. 2009 Jun;75(3):368–75. doi: 10.1016/j.pec.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Runeson I, Martenson E, Enskar K. Children’s knowledge and degree of participation in decision making when undergoing a clinical diagnostic procedure. Pediatr Nurs. 2007 Nov-Dec;33(6):505–11. [PubMed] [Google Scholar]
- 97.Greenley RN, Kunz JH, Biank V, et al. Identifying youth nonadherence in clinical settings: Data-based recommendations for children and adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2012 Jul;18(7):1254–9. doi: 10.1002/ibd.21859. [DOI] [PubMed] [Google Scholar]
- 98.Huang JS, Tobin A, Tompane T. Clinicians poorly assess health literacy-related readiness for transition to adult care in adolescents with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012 Jun;10(6):626–32. doi: 10.1016/j.cgh.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 99.Benchimol EI, Walters TD, Kaufman M, et al. Assessment of knowledge in adolescents with inflammatory bowel disease using a novel transition tool. Inflamm Bowel Dis. 2011 May;17(5):1131–7. doi: 10.1002/ibd.21464. [DOI] [PubMed] [Google Scholar]
- 100.Knopf JM, Hornung RW, Slap GB, et al. Views of treatment decision making from adolescents with chronic illnesses and their parents: A pilot study. Health Expect. 2008 Dec;11(4):343–54. doi: 10.1111/j.1369-7625.2008.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Britto MT, DeVellis RF, Hornung RW, et al. Health care preferences and priorities of adolescents with chronic illnesses. Pediatrics. 2004 Nov;114(5):1272–80. doi: 10.1542/peds.2003-1134-L. [DOI] [PubMed] [Google Scholar]
- 102.Weithorn LA, Campbell SB. The competency of children and adolescents to make informed treatment decisions. Child Dev. 1982 Dec;53(6):1589–98. [PubMed] [Google Scholar]
- 103.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 104.Elwyn G, Lloyd A, Joseph-Williams N, et al. Option grids: Shared decision making made easier. Patient Educ Couns. 2012 Jul 31; doi: 10.1016/j.pec.2012.06.036. [DOI] [PubMed] [Google Scholar]