Abstract
Background and Purpose
Differences in ischemic stroke (IS) between men and women have been mainly attributed to hormonal effects. However, sex differences in immune response to ischemia may exist. We hypothesized that differential expression of X-chromosome genes in blood immune cells contribute to differences between men and women with IS.
Methods
RNA levels of 683 X-chromosome genes were measured on Affymetrix U133 Plus2.0 microarrays. Blood samples from IS were obtained at ≤3h, 5h and 24h (n=61; 183 samples) following onset and compared to controls without symptomatic vascular diseases (n=109). Sex difference in X-chromosome gene expression was determined using ANCOVA (FDR≤0.05, |fold change|≥1.2).
Results
At ≤3, 5 and 24h after stroke there were 37, 140 and 61 X-chromosome genes, respectively, that changed in women; and 23, 18 and 31 X-chromosome genes that changed in men. Female-specific genes were associated with post-translational modification, small-molecule biochemistry and cell-cell signaling. Male-specific genes were associated with cellular movement, development, cell-trafficking and cell death.
Altered sex specific X-chromosome gene expression occurred in two genes known to be associated with human stroke including GLA and IDS, mutations of which result in Fabry's Disease and Hunter Syndrome, respectively.
Conclusions
There are differences in X-chromosome gene expression between men and women with ischemic stroke. Future studies are needed to decipher whether these differences are associated with sexually dimorphic immune response, repair or other mechanisms following stroke, or whether some of them represent risk determinants.
Keywords: X-chromosome, gene expression profiling, ischemic stroke, gender, sex
Introduction
Clinical and epidemiological evidence suggests that ischemic stroke (IS) risk, etiology, response to treatment and outcome are different between men and women. Females tend to have more cardioembolic stroke and men more arterial and lacunar stroke1, 2. Differences in response to thrombolysis and functional outcome may also exist between the sexes1–3.
The sex differences in IS have frequently been attributed to hormonal differences including estrogen, progesterone and testosterone4–6. However, some X-linked diseases can affect brain, clotting and the immune response independent of hormonal effects such as Fragile X, hemophilia and Fabry's disease. Thus, we sought to determine whether there is sex-specific expression of genes on the X-chromosome after IS.
The human X-chromosome has many features that are unique in the human genome7–9. Females inherit one X-chromosome from each parent, while males inherit a single, maternal X-chromosome. Gene expression on one of the female X-chromosomes is silenced during development by X-chromosome inactivation (XCI). However, for XCI “escapees” (15–25% of the X-chromosome genes), differential expression, as well as developmental re-activation of the inactive copy have been reported10. In males, the short tips of the X-chromosome can recombine with the equivalent segments on the Y-chromosome (pseudoautosomal regions, PARs). Genes outside these regions of the X-chromosome are strictly X-linked and most do not have homologues on the Y-chromosome. There are homologous genes on the X-chromosome, outside of the PARs, but their functional similarity to the Y-chromosome paralogues is unclear. X/Y paralogues have a sex-specific pattern of expression which suggests these paralogues may not have equivalent functions11.
Using RNA isolated from men and women with acute ischemic stroke, we determined the expression pattern of X-chromosome genes in each sex. The different patterns of X-chromosome expression in women compared to men provide insight into the sexually dimorphic immune responses to IS.
Materials and methods
Subjects
Subjects with acute IS (n=61; 183 samples) were recruited through the CLEAR trial - a multicenter, randomized, double-blind safety study of recombinant tissue-plasminogen activator (rt-PA) and eptifibatide12 (NCT00250991 at Clinical-Trials.gov). The IRB at each site approved the protocol and informed consent was obtained prior to study entry. Eligible patients had a diagnosis of acute IS, therapy (either standard-dose rt-PA alone or combined low-dose rt-PA plus eptifibatide) initiated within 3h of stroke onset, and a National Institutes of Health Stroke Scale >5. The first blood sample (at < 3 hours) was drawn prior to any treatment. After treatment, two blood samples were drawn at 5 hours and 24 hours after the onset of the stroke.
The control group (n=109) was composed of subjects with no history of symptomatic vascular disease. Subjects were recruited from Wake Forest University Baptist Medical Center (Dr. C. Bushnell), University of Cincinnati, UC Davis and UC San Francisco.
RNA and array processing
Whole venous blood was collected into PAXgene tubes (PreAnalytiX, Germany) and RNA processed as previously described13. Whole blood contains different cell types. Thus, RNA was derived primarily from leukocytes-polymorphonuclear leukocytes (neutrophils, basophils, and eosinophils) and agranulocytes (lymphocytes, monocytes, and macrophages), as well as from immature red blood cells and immature platelets. Each RNA sample was hybridized on Affymetrix Human U133 Plus 2.0 GeneChips (Affymetrix Santa Clara, CA).
Raw expression values (probe level data) were imported into Partek software (Partek Inc., St. Louis, MO). They were log-transformed and normalized using RMA (Robust Multichip Average) and our previously reported internal gene normalization method14. All statistical analyses were performed using Partek Genomics Suite 6.04.
X-chromosome probe sets
There are 1384 X-chromosome probe sets on the Affymetrix U133 Plus 2.0 expression array. We filtered out probe sets annotated as x_at and s_at because they can cross-hybridize and are less specific. This resulted in 888 probe sets corresponding to 638 X-chromosome genes
Statistical analysis
We adopted an analytical approach to decrease bias related to hormonal differences between the sexes. Females with stroke were compared to female controls without stroke; and males with stroke were compared to male controls without stroke. This approach was adopted because it is the only way to examine the expression of the X-chromosome genes in females without any contribution from males, and vice versa. In addition, a number of X-chromosome genes escape inactivation, thus the dosage in the two sexes may not be equivalent. In addition, expression of X/Y paralogues may differ since several studies suggest higher expression of X-chromosome genes of the X/Y paralogues10, 15. Once the X-chromosome genes regulated in females and males were identified, then these were compared to identify stroke-induced, sex-related differential expression of genes on the X-chromosome.
Using the above approach, the analyses addressed changes of gene expression at each time point (Objective 1) and across time points (Objective 2). For Objective 1 an Analysis of Covariance (ANCOVA) identified genes whose expression pattern indicates significant sex-by-diagnosis interaction in blood of IS patients at ≤ 3h (untreated), 5h (treated) and 24h (treated) following IS compared to non-stroke controls (separate analyses for females and males). Gene expression was analyzed as a function of diagnosis, age, sex, and batch, and included a sex-by-diagnosis interaction. Genes with Benjamini-Hochberg FDR corrected p≤0.05 (multiple comparison correction) and |Fold Change|≤1.2 were considered differentially expressed. A fold change cut-off was added (in addition to FDR corrected p-value) based on our power analysis, which showed we can detect an effect size (fold change) of 1.2 with significance (α) = 0.05 and power (β) = 0.8. Objective 1 is based on same-sex comparisons of IS patients versus controls at each time point.
For Objective 2, a mixed model ANOVA identified differences in the temporal X-chromosome gene expression following IS for females and males, separately. Gene expression was analyzed as a function of subject, sex, time, and batch, with sex-by-time interaction. The same significance criteria were used: Benjamini-Hochberg FDR-corrected p≤0.05 and |FC|≥1.2. Objective 2 was performed using the same-sex comparisons of IS patients between time points.
Regulated genes from the separate same-sex analyses of females and males from the above analyses were then compared. The male and female-specific stroke genes expressed on the X-chromosome derived from the non-overlapping gene lists for each objective are reported and discussed.
Gene ontology classification and cytoband over-representation
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases were used to classify the differentially expressed genes (http://david.abcc.ncifcrf.gov). An EASE score (modified Fisher's Exact maximum probability) ≤ 0.1 was used to identify statistically significant cytoband-enrichment.
Results
Subject demographics
There were no significant differences in the age between male and female IS subjects, as well as between male and female control subjects (p=0.91 and p=0.62, respectively). There was a significant difference in the age of IS and control male, as well as between IS and control female subjects (p<0.05) (Table 1). There were no significant differences in the race distribution between male control and male IS subjects, as well as female control and female IS subjects (p=0.49 and p=0.38, respectively). There was a significant difference in the race distribution of IS male and IS female subjects (p=0.01) (Table 1). There were no significant differences between the vascular risk factors in male and female IS subjects –type II diabetes mellitus (p=0.80), hypertension (p=0.13), atrial fibrillation (p=0.27) and hyperlipidemia (p=0.77). Similarly, there were no significant differences in stroke etiology (p=0.33), prior stroke (p=0.35) and myocardial infarction (p=0.32) (data not presented).
Table 1.
Demographics of ischemic stroke (IS) and control subjects.
| CONTROL | IS | |||
|---|---|---|---|---|
|
| ||||
| Male | Female | Male | Female | |
| Subjects, n | 41 | 68 | 35 | 26 |
| Age, years (mean ± SD) | 50.2±17.5* | 48.6±12.8** | 67.1±12.5* | 66.7±13.3** |
| Q1 | 34.0 | 40.3 | 59.7 | 63.4 |
| Median | 53.0 | 52.0 | 71.3 | 68.1 |
| Q3 | 62.0 | 57.5 | 78.3 | 77.3 |
| Race | ||||
| Caucasian, n | 35.0 | 50.0 | 32.0 | 16.0 |
| Non-Caucasian, n | 6.0 | 18.0 | 3.0 | 10.0 |
| Total, n | 41.0 | 68.0 | 35.0*** | 26.0*** |
| NIHSS, < 3h | ||||
| Q1 | N/A | N/A | 6.5 | 10.0 |
| Median | 12.0 | 13.0 | ||
| Q3 | 15.5 | 18.0 | ||
| NIHSS, 5h | ||||
| Q1 | N/A | N/A | 5.0 | 5.0 |
| Median | 8.0 | 9.0 | ||
| Q3 | 15.0 | 15.0 | ||
| NIHSS, 24h | ||||
| Q1 | N/A | N/A | 4.5 | 3.0 |
| Median | 10.0 | 7.0 | ||
| Q3 | 14.5 | 14.0 | ||
Significant difference between the ages of IS and control male subjects.
Significant difference between the ages of IS and control female subjects.
Significant difference in race distribution of IS female and male subjects.
Sex-specific gene expression differences at <3h in IS patients compared to controls
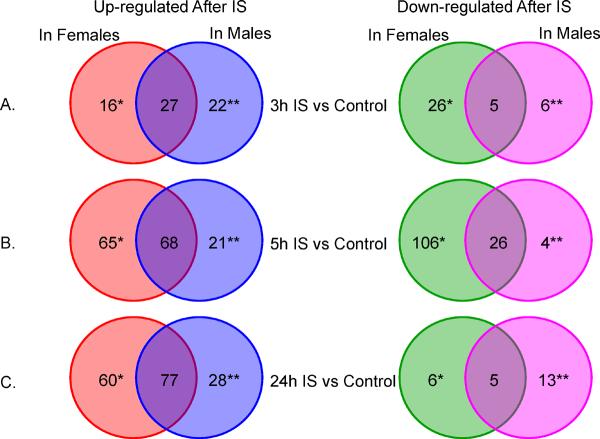
At <3h after IS there were 14 up-regulated and 23 down-regulated female-specific X-chromosome genes, represented by 16 and 26 probesets, respectively (Figure 1A, Supplementary Table 1). There were 18 up-regulated and 5 down-regulated male-specific X-chromosome genes (22 and 6 probesets, respectively) (Figure 1A, Supplementary Table 1).
Figure 1.
Numbers of probe sets (to specific genes) on the X-chromosome regulated at 3, 5 and 24 h after ischemic stroke (IS). * Female-specific stroke genes. ** Male-specific stroke genes. For these analyses IS females were compared to control females; and IS males were compared to control males. No gene was expressed in opposite directions in males and females. Red = up regulated in females. Blue = up regulated in males. Green = down regulated in females. Pink = down regulated in males.
To identify differentially expressed genes which cluster in specific defined chromosomal locations, we performed cytoband-enrichment analysis. This would reveal whether there is a clustering of differentially expressed genes for each of the gene lists. In addition, by identifying the cytobands it is possible to relate the findings of this study to previous and future linkage and GWAS studies using query databases such as the Cytoband Query System (CQS)16. Cytoband over-representation is shown in Table 2 and Supplementary Table 2. Notably, there are nearly as many up-regulated X-chromosome genes common for females and males as there are unique for each sex (Figure 1). The genes common to females and males in Figures 1A, B and C are provided in Supplementary Tables 1, 3, and 4 but are not discussed because they are not unique for either sex.
Table 2.
Cytoband over-representation on the X-chromosome in the differentially expressed gene lists for ischemic stroke (IS). See Figures 2 to 4 for graphical representation of the X-chromosome regions.
| Gene List | Cytoband | Count | List Total | % | P-value | Fold Enrichment |
|---|---|---|---|---|---|---|
| 3h IS vs Control Female Specific |
Xp11.3-p11.23 | 3 | 40 | 7.5 | 2.14E-02 | 11.6 |
| 5h IS vs Control Female Specific |
Xq22.1 | 8 | 147 | 5.4 | 7.91E-02 | 2.0 |
| 24h IS vs Control Female Specific |
Xq23 | 5 | 64 | 7.8 | 3.43E-02 | 3.7 |
| 24h IS vs Control Male Specific |
Xp22.3 | 4 | 39 | 10.3 | 2.49E-02 | 5.8 |
| 24h IS vs 5h IS Female Specific |
Xq22.2 | 6 | 112 | 5.4 | 6.47E-02 | 2.6 |
| 24h IS vs 3h IS Female Specific |
Xq22.2 | 4 | 30 | 13.3 | 1.92E-02 | 6.4 |
Since racial background could affect gene expression, we used an alternative model for both objectives in all of the analyses, where Race was included as an additional covariate in the ANCOVA models. Among the sex-specific genes reported here, the expression of seven X-chromosome, stroke-regulated genes was affected by race. However, they were also significant for the sex-stroke diagnosis interaction. No genes affected by age were amongst the reported sex-specific genes.
Hormone Replacement Therapy (HRT) or other hormone-containing compounds can affect gene expression. We had HRT status/hormone medication information recorded for all IS females (n=23 not on hormone medication, n=3 on hormone medication). However, it was recorded only for some of the female controls (n=29 not on hormone medications, n=5 on hormone medications; n=34 hormone medication status unknown). To address the question of a possible influence of hormone-containing compounds on gene expression in this study, we performed a sub-analysis on the females with known hormone medication status, including hormone medication as a dichotomous variable in the ANOVA model. The effect of hormone medication as well as the interaction between Hormone Medication and Group on the sources of variation in gene expression was very small, much smaller than the effect of Group (IS female vs. Control female) itself (Supplementary Figure 1). In addition, none of the differentially expressed genes between females on hormone medications and on no hormone medications are among the ones in our female-specific stroke gene lists.
The main analysis in our paper was performed by utilizing all of the available samples to maximize sample size, while accounting for confounders by including them in the ANCOVA model. This resulted in the ratio of Controls to IS being different in the female population (68 Controls/26 IS, a ratio of 2.62), compared to the male population (41 Controls/26 IS, a ratio of 1.17), which can bias the results. Thus we performed a sub-analysis, where we matched 26 IS males, 26 control males and 26 control females to the smallest sample size we had of 26 IS females. The matching was performed based on age, race and risk factors. The only significant difference was age (p=0.003 between IS and Control females) which was discussed in the main analysis approach and results. The results of this sub-analysis are presented in Supplementary Tables 9A (3hIS vs Control), 10A (5hIS vs Control) and 11A (24hIS vs Control). The overlapping genes of both analyses are presented in Supplementary Table 9B (for 3hIS vs Control), 10B (for 5hIS vs Control) and 11B (for 24hIS vs Control). The overlap of the genes between the two analyses was statistically significant (p<0.05; binomial probability test performed in Stata) for each comparison. The relative number of genes regulated in females compared to males at each time point in the sub-analysis (Supplementary Figure 2) was very similar to the all-samples analysis (Figure 1). The genes we focused on in the Discussion were regulated in both analysis.
Sex-specific gene expression differences in 5h and 24h IS patients compared to controls
At 5h following IS there were 55 up-regulated and 85 down-regulated female-specific X-chromosome genes (65 and 106 probe sets, respectively) (Figure 1B, Supplementary Table 3). There were 16 up-regulated and 3 down-regulated male-specific X-chromosome genes (21 and 4 probe sets, respectively) (Figure 1B, Supplementary Table 3). Cytoband over-representation is shown in Table 2 and Supplementary Table 2. At 24h following IS, there were 55 up-regulated and 6 down-regulated female-specific X-chromosome genes (60 and 6 probe sets, respectively) (Figure 1C, Supplementary Table 4). There were 19 up-regulated and 12 down-regulated male-specific X-chromosome genes (28 and 13 probe sets, respectively) (Figure 1C, Supplementary Table 4). Cytoband over-representation is presented in Table 2 and Supplementary Table 2. DHRSX and SPRY3 are located in the PARs and thus the contribution to the observed expression level of the X- and the Y- homologues in males cannot be distinguished for the 24h IS vs controls.
Several members of the MAGE (melanoma antigen family) gene family (MAGEA8, MAGEB18, MAGEB6, MAGEC1, MAGED1, MAGEE1, MAGEH1) had female-specific expression following IS.
Temporal sex-specific gene expression differences in IS patients
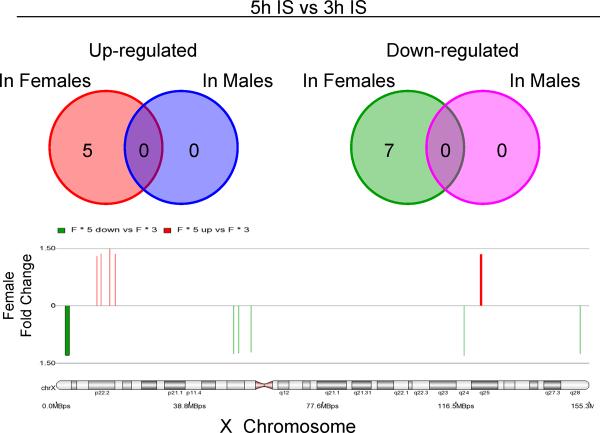
The second objective was to examine changes in gene expression over time in female and male patients with IS. No X-chromosome genes changed expression in males from 3h to 5h after the onset of the IS. In females, however, there were 5 up-regulated X-chromosome genes (REPS2, TLR8, BMX, ODZ1 and MSL3L1); and 5 down-regulated X-chromosome genes (7 probe sets) (SEPT6, TSPYL2, ZNF275, MAGED1 and LOC550643) (Figure 2, Supplementary Table 5).
Figure 2.
Temporal gene expression in females and males following ischemic stroke (IS). The numbers of probe sets on the X-chromosome are shown for females and males that were significantly regulated between 3h and 5h after IS. The widths of the probe sets shown on the X-chromosome are proportional to the Affymetrix target region. Colors as in Figure 1.
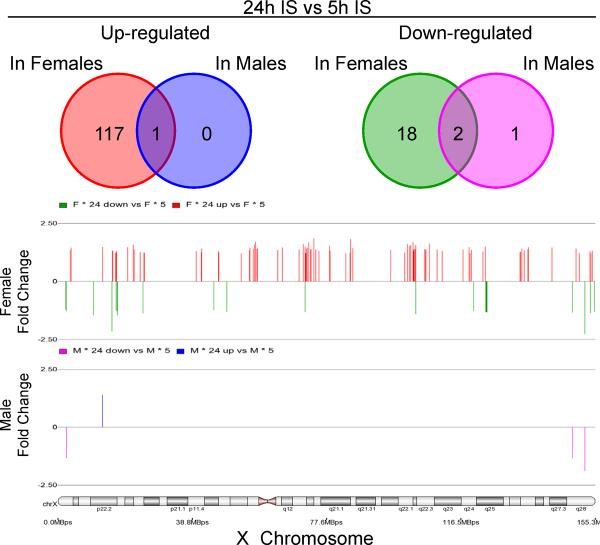
There were many more changes of gene expression from 5h to 24h in females compared to males (Figure 3, Supplementary Table 6). There was female-specific up-regulation of 98 X-chromosome genes (117 probe sets) and down-regulation of 16 genes (18 probe sets). In contrast, only the IDS gene showed male-specific change (decrease) of expression between 5h and 24h post stroke (Figure 3, Supplementary Table 6).
Figure 3.
Temporal gene expression in females and males following ischemic stroke (IS). The numbers of probe sets on the X-chromosome are shown for females and males that were significantly regulated between 5h and 24h after IS. Legend as in Figure 2.
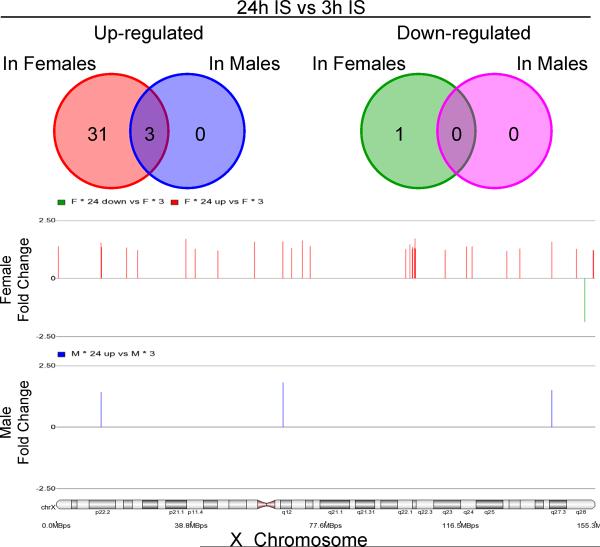
Between 3h and 24h post stroke, there were no X-chromosome genes that showed male-specific expression changes (Figure 4). There were 29 up-regulated X-chromosome genes (31 probe sets) and 1 down-regulated gene (LOC100287428) with a female-specific expression pattern (Figure 4, Supplementary Table 7). Cytoband over-representation is presented in Table 2 and Supplementary Table 2.
Figure 4.
Temporal gene expression in females and males following ischemic stroke (IS). The numbers of probe sets on the X-chromosome are shown for females and males that were significantly regulated between 3h and 24h after IS. No down-regulated genes passed significance with q≤0.05 and |FC|≥1.2 in males. Legend as in Figure 2.
Discussion
Biological sex affected X-chromosome gene expression in blood following IS. Though some of the X-chromosome gene expression changes were identical in both sexes, some were unique to males or females. These findings suggest that the X-chromosome contributes to differences that exist between men and women with ischemic stroke. Though a number of the biological processes presented below have been implicated in playing a key role in the response to stroke, most of the individual genes have not been associated with vascular risk factors or stroke, nor have their sexually dimorphic patterns of expression been suggested in human studies. Sexually dimorphic X-chromosome gene expression at <3 hours in IS subjects compared to controls
Some molecular mechanisms of neuronal cell death and survival following ischemia are different in males compared to females5. This may apply to the immune system and the results of this study as well. In addition, cross-talk between the brain and adaptive and innate immunity is highly relevant for tissue damage, systemic inflammation and regeneration after stroke 17. We observed female-specific expression of X-chromosome genes involved in Natural Killer cell signaling, TNFR1 signaling and axon guidance, TGF-beta signaling and IL17 signaling (TIMP1, TIMP metallopeptidase inhibitor 1). TIMP1, up-regulated in females at 3h and 5h following IS, is an inhibitor of the matrix metalloproteinases (MMPs). MMP9, which is expressed in neutrophils, degrades extracellular matrix and basal lamina which disrupts the blood brain barrier following stroke17. TIMPs also promote cell proliferation, can have anti-apoptotic functions and at least TIMP1 may be neuroprotective during cerebral ischemia17.
The expression of cytokines, chemotactic factors and adhesion molecules modulates leukocyte-endothelial interactions 17. Following stroke there was a male-specific down-regulation following stroke of EFNB1, which is a ligand of Eph-related receptor tyrosine kinases. It plays a role in cell adhesion, nervous system development, axon guidance and regulation of T cell proliferation. Male-specific up-regulation after stroke was noted for CYSLTR1 which is involved in cell-mediated immune response, chemotaxis and T cell migration; and for IGBP1 which is involved in proliferation, cell-cell interaction and differentiation of B cells.
Female-specific up-regulation occurred at 3h following IS for DDX3X (DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, X-linked). DDX3X is involved in translation initiation, nuclear and mitochondrial splicing, and ribosome and spliceosome assembly which are disrupted early after brain ischemia. Many of the above sex effects on expression of X-chromosome genes are probably related to differences associated with IS. These changes are occurring within the therapeutic window for treatment intervention, and as such might preferentially guide the search for future sex-based acute stroke treatment.
Sexually dimorphic X-chromosome gene expression at 5h and 24h in IS patients compared to controls
Recent studies demonstrate that some ischemic cell death pathways may differ in the male and female brain. Females are sensitive to caspase-mediated cell death, whereas males are more sensitive to other cell death pathways including those involved with apoptosis inducing factor (AIF) and Poly(ADP-ribose) polymerase (PARP) activation 18. Female-specific regulation at 5h following stroke was observed for genes that may be involved in caspase-related apoptosis: IKBKG, which encodes the regulatory subunit of the inhibitor of kappaB kinase (IKK) complex and PRKX, a protein kinase, X-linked.
Galactosidase A (GLA) showed female-specific up-regulation at 5h following stroke as compared to controls, while male-specific up-regulation occurred at 24h following stroke as compared to controls. GLA mutations cause Fabry disease, a rare lysosomal storage disorder. It leads to damaged vascular endothelium and is one of the rare single gene disorders of large and small vessel stroke. Another X-linked disorder associated with stroke involves mutations of the IDS gene which is required for lysosomal degradation of heparan sulfate and dermatan sulfate. Indeed, IDS exhibited male-specific expression from 5h to 24h (down-regulated) following stroke. Mutations of IDS cause recessive X-linked Mucopolysaccharidosis Type II (Hunter Syndrome) which is associated with ischemic stroke. Whether the sex differences of GALA and IDS expression in leukocytes relates to sex differences in response to stroke is unknown. However, genetic dysfunction of both genes is known to be associated with stroke in males.
Toll-like receptor signaling has been shown to play an important role in stroke and preconditioning, and serves as a link between the CNS and periphery following stroke 19. In this study male-specific up-regulation of TLR7 occurred at 24h following IS. TLR8 (toll-like receptor 8) was up-regulated in IS vs controls at all time points in both males and females. TLR8 was similarly expressed in males at all time points, whereas TLR8 increased expression from 3h to 5h, and then plateaued at 5h to 24h in females. TLR8, and related TLR7 and TLR9, recognize pathogen-derived nucleotides in intracellular compartments. TLR7 and TLR9 respond to host-derived nucleotides as well, and have been implicated in a variety of autoimmune diseases. In the context of stroke it is notable that TLR8 is expressed in males and females and TLR7 is male specific, possibly contributing to sex-specific immune and possibly autoimmune differences associated with IS.
MAOA (monoamine oxidase A) displayed female specific up-regulation following stroke at all time points when compared to controls. MAOB (monoamine oxidase B) showed female specific up-regulation only at 24h. MAOA is involved in dopamine, norepinephrine and serotonin metabolism. MAOB catalyzes the oxidative deamination of biogenic and xenobiotic amines and metabolizes neuroactive and vasoactive amines, particularly dopamine. These findings show differences of catecholamine genes induced in females compared to males following IS and might point in differences of the stress response in the two sexes following IS.
The MAGE family of genes was also specifically regulated in females following IS. These are members of the cancer/testes (CT) antigen group, which is disproportionately represented on the X chromosome. They are characterized by their expression in a number of cancer types, while their expression in normal tissue is mainly reported in testes. They have been proposed as targets for immunosuppressive therapy 20. Their female-specific differential expression may suggest their involvement in the female-specific stroke-related differences in the periphery to ischemic brain injury and/or interaction with the thrombolytic treatment.
There were several female-specific X-chromosome genes whose expression peaked at 5h and decreased at 24h including BMX, ODZ1 and REPS2. BMX is involved in the regulation of proliferation, differentiation, motility and apoptosis including the differentiation of endothelial cells and formation of the blood brain barrier (BBB). This could suggest that molecules expressed in inflammatory cells interact with endothelial cells to affect the BBB in a sex-specific manner.
The gene expression patterns over time are important for interpreting the results of this study. Genes expressed within the first 3 hour time window are among the most interesting since they are unaffected by treatment, and are being induced during the time when acute stroke therapy is likely to be beneficial. Genes that change expression in the 3 to 5 hour time period might be those most likely to be affected by treatment since treatment was initiated after the first blood sample but before 3h after stroke. Genes expressed at 24 hours likely represent the full complement of the immune cell changes associated with cell death, cellular phagocytosis as well as in the beginnings of repair. The effects of tPA can be short and long term 21. Our animal tPA studies show that some genes regulated following IS can be related to the tPA itself rather than to stroke 22. Thus, a number of genes regulated at 5 and 24 hours could represent changes produced by the IS, treatment or an interaction of sex, stroke and treatment.
Limitations and Conclusions
As patients at the 5h and 24h time-points were treated, the observed sex-specific differences in the expression of X-chromosome genes may be due, at least in part, to interaction with the treatment at these times. There were differences in the age and race for some of the comparisons. However, inclusion of these factors as co-variates in the ANCOVA models demonstrated that the identified sex-specific changes of gene expression on the X chromosome following stroke were independent of age and race. There was an unbalanced distribution of IS and Control samples between the male and female cohorts, which could bias the analysis. A sub-analysis of matched sample sets revealed a similar pattern in male vs female regulated genes to the pattern observed in the all-samples analysis. Given the demographic differences and the multiple comparisons made, however, a follow up study is needed to confirm the findings. Future studies investigating gene expression in specific blood cell types are needed to refine gene expression changes in individual cell types. Hormone medication status was not recorded on all of the female subjects in the study. Thus, some of the female specific genes may be due to hormone medication differences. However, our sub-analysis on the female subjects with known hormone medication status as well as the fact that there were a significant number of genes that changed expression over time following stroke argues in favor that most of the observed differences are sex-stroke related. Due to the case-control design of the study, causality cannot be determined. In addition, because demographic variables were not precisely matched in the IS and control groups, even with the analysis designed to minimize the effect of these differences, it is not possible to be absolutely sure that any given gene was not over-expressed prior to stroke in the ischemic stroke patients compared to the matched controls. However, similar to the argument above, the fact that the expression of many differentially expressed genes changed over time supports the hypothesis that many are sex-stroke genes. This study examines the transcript levels which may or may not relate to the rate of transcription as measured by promoter strength, or to changes in protein levels due to post-transcriptional regulation. A study with a larger population size is needed to decipher the relevance of the observed gene expression differences in terms of outcome.
Since a majority of the above genes have not been previously implicated in stroke, it is difficult to know whether the novel sexually dimorphic expression differences reported here affect outcomes differently in the two sexes. However, determining the mechanisms of sexually dimorphic pathophysiology, etiology and outcome in ischemic stroke is important, since the findings could eventually guide development of sex-specific treatments.
Supplementary Material
Acknowledgments
Funding Sources This study was supported by NIH grants (FRS, AP, ECJ, JPB) and the American Heart Association Bugher Foundation (FRS).
Disclosure/Conflict of Interest JPB received non-NIH support from Genentech, Schering Plough, PhotoThera and Oakstone Medical Publishing. AP received non-NIH support from Research Grant EKR Therapeutics, Genentech and Schering Plough.
Footnotes
Supplementary information is available at the Journal of Stroke website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forster A, Gass A, Kern R, Wolf ME, Ottomeyer C, Zohsel K, et al. Gender differences in acute ischemic stroke: Etiology, stroke patterns and response to thrombolysis. Stroke. 2009;40:2428–2432. doi: 10.1161/STROKEAHA.109.548750. [DOI] [PubMed] [Google Scholar]
- 2.Caso V, Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, et al. Gender differences in patients with acute ischemic stroke. Womens Health (Lond Engl) 2010;6:51–57. doi: 10.2217/whe.09.82. [DOI] [PubMed] [Google Scholar]
- 3.Kent DM, Buchan AM, Hill MD. The gender effect in stroke thrombolysis: Of cases, controls, and treatment-effect modification. Neurology. 2008;71:1080–1083. doi: 10.1212/01.wnl.0000316191.84334.bd. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: Are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009;27:163–179. doi: 10.3233/RNN-2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, Muzny D, et al. The DNA sequence of the human x chromosome. Nature. 2005;434:325–337. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Disteche CM. Sex differences in brain expression of x- and y-linked genes. Brain Res. 2006;1126:50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Vawter MP, Evans S, Choudary P, Tomita H, Meador-Woodruff J, Molnar M, et al. Gender-specific gene expression in post-mortem human brain: Localization to sex chromosomes. Neuropsychopharmacology. 2004;29:373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in x-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, et al. The combined approach to lysis utilizing eptifibatide and rt-pa in acute ischemic stroke: The clear stroke trial. Stroke. 2008;39:3268–3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamova B, Xu H, Jickling G, Bushnell C, Tian Y, Ander BP, et al. Gene expression profiling of blood for the prediction of ischemic stroke. Stroke. 2010;41:2171–2177. doi: 10.1161/STROKEAHA.110.588335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamova BS, Apperson M, Walker WL, Tian Y, Xu H, Adamczy P, et al. Identification and validation of suitable endogenous reference genes for gene expression studies in human peripheral blood. BMC Med Genomics. 2009;2:49. doi: 10.1186/1755-8794-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Deng X, Disteche CM. Sex-specific expression of the x-linked histone demethylase gene jarid1c in brain. PLoS ONE. 2008;3:e2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen KH, Lee C, Liu HS, Ho CL. A precise and scalable method for querying genes in chromosomal banding regions based on cytogenetic annotations. Bioinformatics. 2005;21:3469–3474. doi: 10.1093/bioinformatics/bti566. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 19.Leung PY, Packard AE, Stenzel-Poore MP. It's all in the family: Multiple toll-like receptors offer promise as novel therapeutic targets for stroke neuroprotection. Future Neurol. 2009;4:201–208. doi: 10.2217/14796708.4.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caballero OL, Chen YT. Cancer/testis (ct) antigens: Potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: More than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Jickling GC, Zhan X, Ander BP, Turner RJ, Stamova B, Xu H, et al. Genome response to tissue plasminogen activator in experimental ischemic stroke. BMC Genomics. 2010;11:254. doi: 10.1186/1471-2164-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.