Abstract
Cutaneous hyperpigmentations are frequent complaints, motivating around 8.5% of all dermatological consultations in our country. They can be congenital, with different patterns of inheritance, or acquired in consequence of skin problems, systemic diseases or secondary to environmental factors. The vast majority of them are linked to alterations on the pigment melanin, induced by different mechanisms. This review will focus on the major acquired hyperpigmentations associated with increased melanin, reviewing their mechanisms of action and possible preventive measures. Particularly prominent aspects of diagnosis and therapy will be emphasized, with focus on melasma, post-inflammatory hyperpigmentation, periorbital pigmentation, dermatosis papulosa nigra, phytophotodermatoses, flagellate dermatosis, erythema dyschromicum perstans, cervical poikiloderma (Poikiloderma of Civatte), acanthosis nigricans, cutaneous amyloidosis and reticulated confluent dermatitis
Keywords: Diagnosis, Hyperpigmentation, Melanosis, Pigmentation disorders, Therapeutics
ACQUIRED HYPERPIGMENTATIONS
Hyperpigmentations are a group of diseases that comprise both congenital forms, with different patterns of inheritance, and acquired forms secondary to cutaneous or systemic problems. The vast majority of them are linked to alterations in the melanin pigment and can be classified as epidermal, due to an increase in the number of melanocytes or the production of melanin or dermal, either melanocytic or not.
Pigmentary disorders are a frequent source of complaints, constituting the third most common reason for dermatological consultations, about 8.5% in our country. There is a different impact depending on the geographic region, being worse in places where the weather is always warm, and the skin becomes more exposed.1 Consultations due to hyperpigmentation vary with age group, being the second most common complaint between 15 and 30 years of age and the first in the range of 40 to 54 years, regardless of skin color and gender.1
This review will focus on the main acquired hyperpigmentation disorders associated with increased melanin, taking into account those most commonly found in clinical practice. It will also consider other pigmentation alterations which basic pathogenic mechanisms involve not only hyper-melanization but also associated factors such as hyperkeratosis with superficial oxidation of keratin and epithelial hyperproliferation. The main complaint in these dermatoses is the increase in color intensity, and they are relevant for their high frequency or for being markers of other diseases.
Melasma
The word melasma originates from the Greek, where melas means black. It appears as a symmetric acquired hypermelanosis, with stains in shades of brown to bluish gray, with irregular borders and located in more photo-exposed areas. It usually affects the face and neck, and less commonly, the arms and the sternal region, respecting the mucosal areas (Figures 1 and 2). Usually melasma has three clinical patterns: centrofacial with spots on the frontal region, nasal dorsum, cheekbones and chin areas, in 65% of the cases; malar in 20% of the cases, and mandibular, in about 15% of patients.2 Extra facial areas with the highest incidence of melasma are the extensor surface of the arms and forearms, the neckline, the upper third of the dorsal area of the trunk and the sides of the neck.3
FIGURE 1.
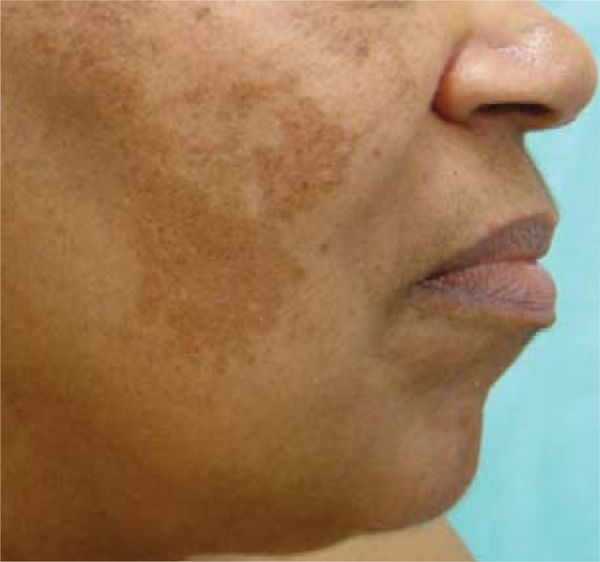
Acquired hyperpigmentations. Moderate melasma, brown spots with irregular edges affecting the right hemiface, following a mandibular pattern in a patient with phototype V
FIGURE 2.
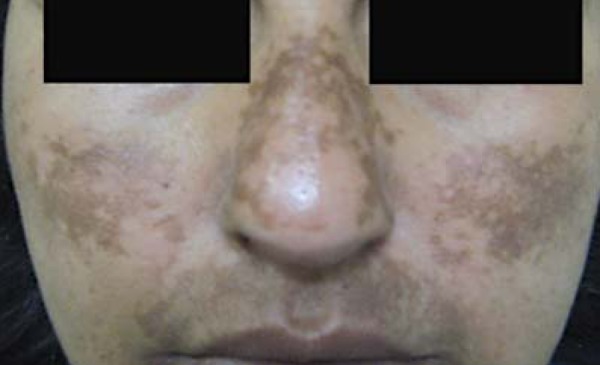
Acquired hyperpigmentations. Severe melasma. Patient with skin phototype IV, presenting a somewhat homogeneous lesions, but with intense pigmentation, predominantly in the middle facial region
Its occurrence is described within all racial and ethnic groups, but most commonly in individuals with higher skin phototypes living in areas of intense ultraviolet (UV) radiation, especially Latin American, Asians and Afrodescendants.4,5 Similarly, it is more common in young women, with men accounting for about 10% of all cases, with well-delimited geographical variations.6
Several factors have been linked to melasma. Among them, UV exposure and genetic predisposition appear to be the most significant ones.5,7-11 Ultraviolet radiation (UVR) induces melanocortin within melanocytes and keratinocytes, justifying the involvement of this hormone in the pathogenesis of melasma. More recently, it was suggested that high intensity visible light also contributes to the increased pigmentation in melasma, particularly in skin phototypes IV-VI, corroborating data derived from clinical observation.12,13
Recent histological and immunohistochemical studies demonstrated that melasma skin presents marked features of chronic sun damage. During sun exposure, physiological reactions occur, triggered by a network of cellular interactions between keratinocytes, mast cells, fibroblasts, the dermal vasculature over melanocytes and dermal inflammation, playing an important role in the hyperpigmentation and reactivation of melasma lesions.12-15
Other situations involved in the pathogenesis of melasma are: pregnancy, use of oral contraceptives (OC), endocrine disorders and hormonal treatments.16,17 The results of a large global study, that evaluated 324 women with melasma, suggested that a combination of known triggers, including pregnancy, hormonal birth control, age, family history and sun exposure affect the onset and recurrence of melasma lesions.11 Furthermore, the application of certain cosmetics and medications such as anticonvulsants and photosensitizing substances have also been described as possible causes or aggravating factors for hyperpigmentation.18,19
Melasma is usually classified into: epidermaltype (70% of patients), in which the pigmentation is enhanced during examination with ultraviolet A light (UVA), dermal type (10 to 15%) in which the pigmentation does not change during this same exam and mixed (20%).2 However, recent studies have questioned this diagnostic technique. Skin biopsies with melasma showed that the level of pigment deposit does not always correspond to the reading by UVA light, with most lesions having both dermal and epidermal components.20 Melasma lesions show increased density of dermal and epidermal melanin besides marked solar elastosis compared with the adjacent normal skin.9,20 It was verified, through immunohistochemistry, that the number of epidermal melanocytes may be either increased or equal to that of normal skin.10 However, these cells exhibit characteristics of hyperfunctioning cells with size enlargement and prominence of dendrites.
The goal of melasma treatment is to decrease the proliferation of melanocytes, inhibit the formation of melanosomes and promote their degradation.21 However, the chronic course of the disease and its relapses discourage the adherence to the proposed treatment, especially regarding the use of sunscreen.
Photoprotection is essential to the treatment and should be followed rigorously, since the lesions are aggravated by UVA, UVB and also by visible light. Sunscreens with a sun protection factor (SPF) greater than 30 and with physical photoprotective agents in their formulation are recommended. It is essential to apply them several times a day, and wear hats during outdoor activities, avoiding exposure during UV radiation peak hours. 21,22
The classical melasma treatment includes the use of topical hydroquinone (HQ), alone or combined with retinoic acid (RA) or glycolic acid (GA) (double combination) or RA and a topical corticosteroid (triple combination).23 Other agents that may be considered include GA and RA, isolated or associated with other agents; azelaic acid (AZ), arbutin, kojic acid and mequinol, among others (Table 1).23-26 The combined therapies have been more used as they present synergism in their actions, being therefore, more effective whilst with less adverse events. A recent study demonstrated that the triple combination is more costeffective than the double or single therapies and that the combination of 0.05% RA + 4% HQ plus 0.01% fluocinolone acetonide has the best grade and quality of evidence obtained through efficacy studies in melasma treatment.21-23,25,27 The Latin American Academy of Pigmentary Disorders (Pigmentary Disorders Academy-PDA) proposes an algorithm to better guide the treatment of melasma. According to it, patients with mild melasma can use HQ 4%, or triple combination therapy with 4% HQ + 0.05% RA + 0.01% fluocinolone acetonide, or dual therapy, or non-phenolic therapy if there is sensitivity to the cited agents.21 For cases of moderate to severe melasma, it is recommended to use triple therapy, when this is not available, dual therapy or 20% AZ may be used, achieving similar results to hydroquinone alone, but with fewer adverse events.28 In cases that are refractory to topical medications, the association with procedures such as peels and dermabrasion can be considered, with special care regarding the possibility of post inflammatory hyperpigmentation.29
TABLE 1.
Mechanism of action of the main depigmenting agents
| Tyrosinase inhibition | Stratum corneum exfoliation | Degradation / Decrease of melanosome transfer | Inhibition of DOPA and dopaquinone synthesis | Promotion of conversion of melanin to leukomelanin | Nonselective inhibition of melanogenesis |
|---|---|---|---|---|---|
| Hydroquinone | Alpha hydroxy acids | Hydroquinone | Kojic acid | Vitamins C and E | Corticosteroids (they should not be used in monotherapy) |
| Azelaic acid | Retinoids | Niacinamide | Glabridin | Glabridin | |
| Alpha arbutin | Soy extracts | ||||
| Gentisic acid | |||||
| Flavonoids | |||||
| Isoflavones | |||||
| Resveratrol |
Serial treatments with intense pulsed light may be indicated in selected cases, in patients with lighter skin types and no history of prior inflammatory hyperpigmentation and for those situations where pigment deposits are deeper. This recommendation is not fully accepted and represents relatively limited experience, however, some studies demonstrated excellent to good results in 60% of cases with a 6month follow-up.30,31 The use of Nd:YAG laser at low fluence showed encouraging results in case series, despite the possibility of occurring excessive depigmentation.32 Other authors have reported encouraging results with fractional thulium laser (1927nm) associated to microdermabrasion or peels.33-36 Some treatments suggested as adjuvants for depigmentation or to prevent the recurrence of melasma, both orally and topically, include substances such as tranexamic acid, antioxidant vitamins and botanical extracts.37-42
Post-inflammatory hyperpigmentation
Post-inflammatory hyperpigmentation (PIH) is characterized by increased pigmentation acquired after a cutaneous inflammatory process. People with higher skin phototypes, presumably, are more prone to this skin condition, because they already have a higher basal amount of epidermal melanin. Similarly, this hypermelanosis tends to be more intense and of longer duration in this group.3,43 The most common causes of PIH are: acne, atopic dermatitis, allergic contact dermatitis or secondary to irritants, trauma, psoriasis, lichen planus, drug eruptions, and nowadays, cosmetic procedures.44,45
The distribution of spots follows the location of the inflammatory processes, and retrieving this information from the patient is really important to establish the correct differential diagnoses (Figure 3). Histology shows melanin deposits both in free form and within the melanophages located in the upper dermis and around the blood vessels. The exact mechanism of PIH pathogenesis is still not well understood, but it is believed to be more related to the nature of the triggering inflammation, because the darkening is greater in chronic and recurrent inflammation processes and also in those that damage the basal layer.43,46 It is likely that the hyperpigmentation is caused by increased melanogenesis or abnormal distribution of the produced melanin, possibly resulting from the action of cytokines, inflammatory mediators and oxygen reactive species.45 These agents may act by stimulating the growth of melanocytes and dendritic proliferation, as well as increasing the activity of tyrosinase.47
FIGURE 3.
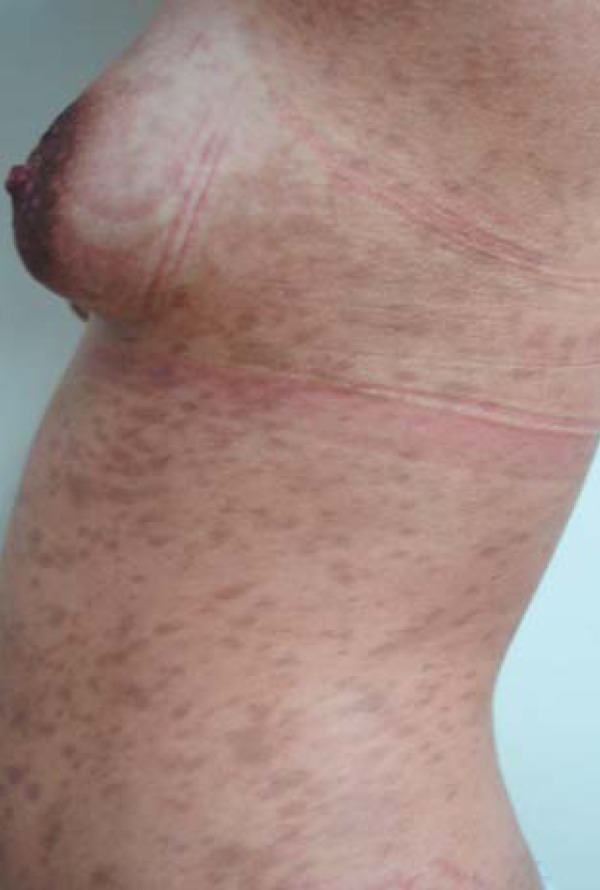
Acquired hyperpigmentations. Postinflammatory hyperpigmentation – bluish brown macules distributed around the trunk, secondary to extensive pityriasis rosea
The course and outcome of PIH treatment are unpredictable and relapses are frequent. Topical hydroquinone and tretinoin, alone or combined, are effective but require prolonged treatment.24,46 Hydroquinone has a limited effect when the pigment is primarily deposited in the dermis. Other bleaching agents such as kojic acid and azelaic acid have been used with varying degrees of success. Peels with glycolic acid or salicylic acid are particularly effective and well tolerated in patients with high phototype.48 The use of lasers is described in several reports, the most commonly used being Q-switched laser, but the results are variable, with risk of hyperpigmentation in darker skins.49,50 Recently, the application of fractional photothermolysis for PIH treatment showed promising results, but further studies are still needed.51
Periorbital Hyperpigmentation
Dark circles or periorbital hyperpigmentation (POH) is a common dermatological complaint. It affects individuals of both genders, of all ages and races. Despite being regarded as an anatomical feature or a physiological phenomenon, dark circles when very pronounced, interfere markedly with facial appearance.4,52
This condition is characterized by bilateral homogeneous hyperchromic areas in the infraorbital region (Figure 4). The lesions vary in intensity according to fatigue or lack of sleep and worsen with aging due to sagging, thinning of the skin, abnormal deposits of infraorbital fat and loss of subcutaneous tissue.4,53
FIGURE 4.

Acquired hyperpigmentations. Periorbital hyperpigmentations – Depression and shaded aspect, with visualization of the superficial vascular network in the infraorbital region of a 38 years-old woman
Histological features show that dark circles can be associated with multiple etiologic factors, such as dermal melanin deposition, post-inflammatory hyperpigmentation, superficial vasculature presentation and periorbital edema.4,52 Anatomical changes of the infraorbitary region also occur, especially malar region with a descending characteristic, prominent lacrimal sulcus, infraorbitary fat prolapse, sagging, edema, signs of chronic photodamage and hyperactivity of the periorbital musculature.4 POH is a frequent finding in individuals who are atopic or allergic to airborne substances, perhaps induced by the habit of rubbing the skin around the eyes, which triggers postinflammatory pigmentation.
Periorbital hyperpigmentation may also be a family feature or be part of other dermatoses, such as erythema dyschromicum perstans, and drug related eruptions.4
Dark circles treatment is unsatisfactory because of the necessity of dealing with multiple factors. Corrective makeup is a palliative solution, but with long-term average results. There are several therapeutic options, and it is often necessary to combine them.4,54-57
The drug of choice for the topical treatment of dark circles is hydroquinone, which inhibits the synthesis of DNA and RNA, induces the degradation of melanosomes and the destruction of melanocytes.58 The effects of depigmentation become apparent after 5-7 weeks, usually preceded by erythema and desquamation. Treatment should be continued for 3-12 months.
Hydroquinone can also be used in combination with other agents. The Kligman formulation (hydroquinone + tretinoin + dexamethasone) is the best known combination for this condition, however adverse reactions such as erythema, desquamation, colloid milium, irritant or allergic contact dermatitis and post-inflammatory paradoxical hypermelanosis, have been described.19,23,45
The use of 0.01% to 1% topical retinoic acid, reduce pigmentation by inhibiting tyrosinase transcription and significant thinning of the granular layer and the epidermis.24 The effects of RA become significantly evident after 24 weeks. Side effects commonly reported include erythema, peeling, burning and stinging. Other compounds used as depigmenting agents include azelaic acid and kojic acid, but different combined preparations have been used in order to increase effectiveness and reduce adverse events in the treatment of various hyperpigmentation disorders.12
Superficial peels with 15% to 25% or even higher concentrations of trichloroacetic acid (TCA) are widely used for the treatment of POH. TCA causes destruction of the epidermis and superficial dermis, inducing reepithelialization starting from the adnexae.53 Applications of 50% to 80% glycolic acid cause epidermolysis; the product must be applied for a few minutes, immediately followed by washing with water or sodium bicarbonate. The risk of adverse events increases with the depth of peels, skin type and sensitivity of individuals.53 Therefore, patients should be adequately screened before the medical procedure.
POH due to excessive pigmentation can also be treated with lasers such as Q-switched ruby laser (694 nm), Q-switched alexandrite laser (755 nm) and Nd:YAG (1064 nm).59,60 There are reports of higher than 50% improvements after the first session in 23,5% of patients and in 88,9% of them after the second session with Qswitched ruby laser.55 For POH secondary to sagging skin and age-related changes in the lacrimal groove, ablative and non-ablative lasers can provide satisfactory results, because they cause tissue contraction.59,60
Safety should be emphasized in the treatment of POH with lasers, because the eyes are particularly vulnerable to laser-induced injuries, so the use of glasses and / or ocular globe protectors is crucial.
When dark circles are caused predominantly by superficial presentation vasculature, thinning of the eyelid skin or the presence of marked lacrimal groove, autologous fat transplantation or the use of hyaluronic acid fillers are indicated.57 For cases with significant sagging, blepharoplasty is the method with the most satisfactory results in the majority of patients.53
Dermatosis papulosa nigra
Dermatosis papulosa nigra (DPN) consists of benign epithelial tumors characterized by multiple small papules and surface tubercles with 1 to 5mm in diameter, located over the malar, neck and chest regions.61 Biopsies of these lesions show the same histopathological findings of seborrheic keratosis.62 DPN carries a genetic predisposition and it is more frequent in women and African descendants, in whom the prevalence ranges from 10% to 35%, being even regarded as a racial variant of seborrheic keratosis.61-63 Typically, DPN lesions begin on the face and increase in size and number over the years, spreading to sun-exposed areas of the malar, neck and upper body regions without spontaneous regression.61
Although benign, DPN can be an aesthetically distressing condition, affecting interpersonal relationships. Its treatment includes various therapeutic modalities, such as curettage, cryotherapy, and superficial laser and electrofulguration.64,65 There is not a definite significant difference between these techniques, in general, the treatment is well tolerated without significant adverse events or recurrence.66 However, hypo or hyperpigmentation, scarring or keloid formation can sometimes occur, particularly if the procedures are performed in a rough manner or if patients are predisposed to these complications.62
Phytophotodermatoses
Phytophotodermatoses occur by contact with plants containing furocoumarins or psoralens that induce phototoxicity when activated by sunlight, particularly ultraviolet A radiation (UVAR) (320nm to 400nm).67
The lesions may be asymptomatic or present itching and burning, depending on the amount of contacting substance, the skin color and intensity of photo-exposure. The spots start with erythema and in a few days take on a brown coloration in those places where the contact happened (Figure 5).68,69 Vesicles or large blisters may be present and, unlike allergic photodermatitis, pruritus is not a common symptom. The evolution with hyperpigmented macules or patches is the main feature for the diagnosis and it may take weeks to months to regress.
FIGURE 5.
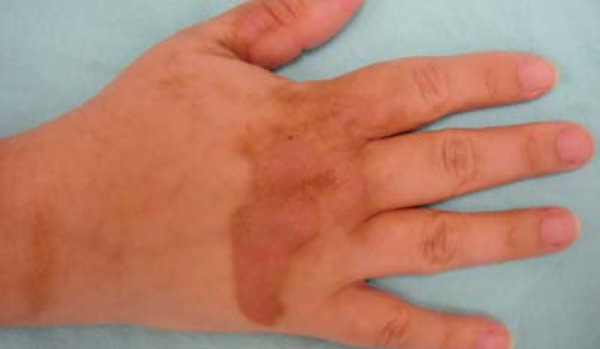
Acquired hyperpigmentations. Phytophotodermatoses – hyperchromic brown spots, with a leaked aspect, located on the dorsal area of the hand and fingers, secondary to accidental contact with lemon juice followed by sun exposure
Table 2 describes the plants most commonly linked to cases of phytophotodermatoses.
TABLE 2.
Plants that are the leading cause of phytophotodermatoses and their characteristics
| FAMILY | PLANTS | OBSERVATIONS |
|---|---|---|
| Rutaceae | Plants producing citrus fruits such as orange, rangpur, persian lime, tangerine or mandarin and rue. | Injuries are most common in the hands and contact surfaces. |
| Furocoumarins (methoxy-psoralen) are more concentrated in the fruit peel. | ||
| Apiacea (Umbelliferae) | Carrot, celery, angelica, fennel, parsley, dill, anise, coriander, fennel | Presence of psoralens at variable concentrations |
| Fabaceae (legumes) | imburana-de-cheiro (Amburana cearensis a tree found in northeastern Brazil whose major component is coumarin), vinhático (Plathymenia reticulata a tree used for woodworking, rich in psoralens) | Psoralea corylifolia is used in Chinese medicine to treat vitiligo and alopecia areata. The concentration of psoralen is highly variable, which may cause phytophotodermatites. |
| Its use is not recommended. | ||
| Moraceae | Fig Tree | It is a popular adjuvant agent for sun tanning, which even led to episodes of severe skin burns. |
Phototoxicity reactions may also be induced by other substances of vegetal origin, like the bergamot oil (bergapten) that contains 5-methoxy-psoralen in its composition. These plant extracts can be part of the composition of some perfumes and colognes, inducing the so called "Berloque dermatitis" or "au-de-cologne dermatitis" because the pigmentation stimulated by sunlight mimics embellishments, especially in retroauricular areas and on the sides of the neck where perfumes are usually applied.
Flagellate dermatosis
Flagellate dermatosis (FD) is characterized by the appearance of pruriginous, urticarial erythematous linear streaks, most commonly on the trunk (Figure 6). It was first described by Moulin et al. in 1971 and it is a specific reaction to bleomycin.70 DF occurs in about 8-20% of patients who use this medication for the treatment of lymphomas, germ cell tumors and squamous cell tumors with doses between 15mg and 285mg on reported cases.71,72
FIGURE 6.
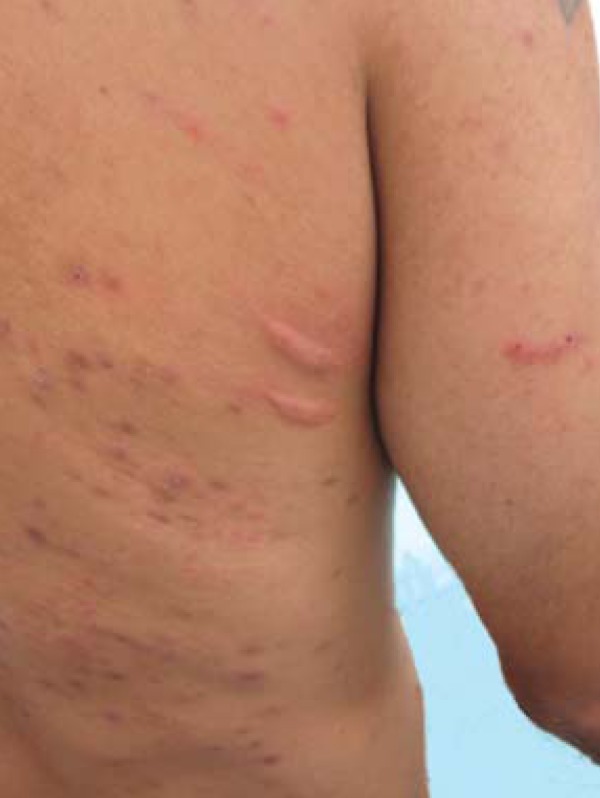
Acquired hyperpigmentations. Flagellate Dermatitis– Linear urticariform lesions, some with a slight superficial blistering, located on the lateral side of the chest, which appeared 15 days after the initiation of treatment with bleomycin
Bleomycin is a polypeptide derived from Streptomyces verticillus, discovered in Japan in 1965 by Umezawa. It has been used as an antineoplastic agent against different types of tumors because it inhibits the incorporation of thymidine to DNA, causing its fragmentation. The substance is distributed throughout the body and is inactivated by the hydrolase enzyme, capable of cleaving an ammonia group from their molecules. This enzyme does not exist in the lung or in the skin; therefore bleomycin is not inactivated in these organs. Thus, there is an increased concentration of the substance, explaining the higher skin and pulmonary toxicities observed.73 In most cases it is not necessary to institute any treatment for flagellate dermatosis. After stopping the medication the erythema gradually improves, but the residual characteristic hyperpigmentation may persist for several months.74
There are DF cases caused by shiitake mushroom (Lentinus edodes), the second most consumed mushroom in the world, which must be considered as differential diagnosis for bleomycin-induced flagellate dermatosis. After the mushroom ingestion, linear widespread erythematous pruritic lesions may appear in 24 to 48 hours.75 About two weeks are needed to achieve complete remission of the dermatosis induced by shiitake ingestion. The symptoms may be treated with antihistaminic drugs, topical corticosteroids and, in severe cases, oral corticosteroids for a short period of time. As the re-exposure could induce a new onset of symptoms, the ingestion of this mushroom should be avoided, especially when raw or undercooked.75
Erythema dyschromicum perstans
Erythema dyschromicum perstans (EDP) or gray dermatosis (dermatosis cinecienta) is a rare acquired and chronic dermatosis, characterized by asymptomatic progressive grayish macules. It was first described by Ramirez in 1957, in El Salvador. It is more common in the Latin American population, but it can affect all ethnic-racial groups, most frequently women and children on their first decade of life.76-78
EDP's etiology remains obscure. There are reports of associations with endocrinopathies, ingestion of ammonia nitrate and radiologic contrast, vitiligo, HIV and chronic hepatitis C infections, however most cases do not have an identified triggering factor.79-84 Due to the presence of interleukins and inflammatory mediators in the lesions, some studies point to an immune-mediated dermatosis, likely with a genetic predisposition to develop the disease.
EDP presents clinically as asymptomatic, grayish macules, with symmetric distribution, usually involving the trunk and proximal extremities. The lesions have variable sizes and frequently show erythematous, nondesquamative, palpable borders, suggesting the inflammatory process preceded the pigmented lesions (Figure 7).85 This dermatosis tends to affect mainly the extremities and trunk, sparing mucosae, hands, feet and scalp.
FIGURE 7.
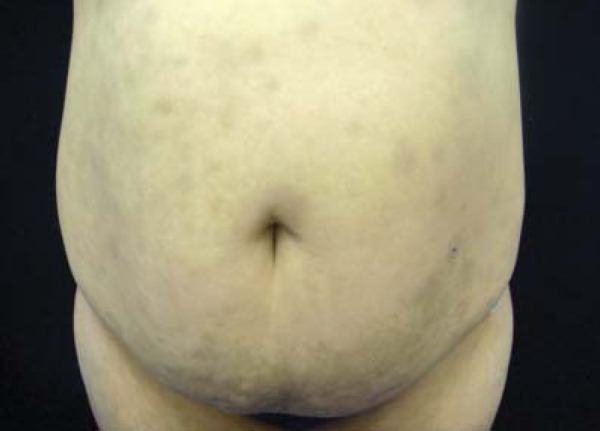
Acquired hyperpigmentations. Erythema Dyschromicum Perstans – Grayish macules of varying sizes, located on the anterior region of the thorax, abdomen and upper thighs of a 27 years-old woman
Diagnosis is based on clinical presentation and histopathological findings. On the active border of the lesions there is lichenoid dermatitis with vacuolization of basal layer cells and a strip of mononuclear infiltrate on the superficial dermis; at the center of the lesions the findings are compatible with post-inflammatory hyperpigmentation with melanophages in the dermis.86
Differential diagnosis for EDP include: lichen planus, idiopathic eruptive macular pigmentation, post-inflammatory hyperpigmentation, fixed pigmented erythema, Addison's disease and hemochromatosis.86 Treatment is based on the topical use of hydroquinone, topical corticosteroids and tretinoin, although the results may be unsatisfactory due to the dermic deposits of melanin. Clofazimine and dapsone were used with success in adult patients, possibly because of their anti-inflammatory and immunomodulatory activities. There are also reports of systemic use of corticosteroids, antibiotics, griseofulvin, isoniazid, antimalarials, phototherapy and psychotherapy.87,88
Cervical Poikiloderma or Poikiloderma of Civatte
Cervical idiopathic poikiloderma or poikiloderma of Civatte (PC) is a benign dermatosis first described in 1923 by the French dermatologist Achilles Civatte.89 It is characterized by irregular pigmentary alterations, with hypo and hyperpigmentation associated to superficial atrophies and telangiectasias, usually asymptomatic and rarely with discreet burning or pruritus. The pigmentation is reticulated, reddish to brown, with irregular and symmetrical distribution, affecting the hemifaces, neck and upper third of the chest, and sparing the shaded chin area (Figure 8). Its pathophysiology is not well understood, but it is probably related to cumulative sun exposure, aggravated by photoallergic or phototoxic reactions caused by fragrances and / or cosmetics in particularly susceptible individuals.90 The distribution by age and sex and familial occurrence suggest that hormonal and genetic factors may be involved in its onset.89,90
FIGURE 8.
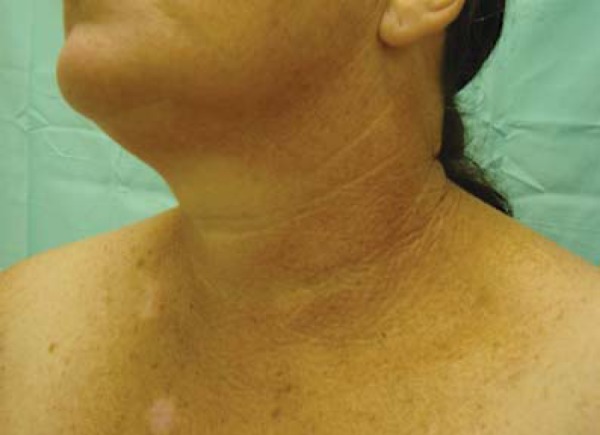
Acquired hyperpigmentations. Poikiloderma of Civatte. Note the grid-like hyperpigmentation, affecting the lateral areas and base of the neck in a 51 years-old phototype 3 woman. Observe photodamage lesions and a lighter area correspondings to the region shaded by the chin
PC is a chronic, progressive dermatosis, very common in some regions, affecting fair-skinned individuals, starting on the fourth decade of life, especially in postmenopausal women. Histopathological examination shows solar elastosis in the papillary dermis, associated with vasodilation, perivascular edema, hyperkeratosis and epidermal atrophy with effacement of the rete ridges, hydropic degeneration of basal cells, presence of dermal melanophages and sparse lymphocytic infiltrate.91
Cervical poikiloderma has a slow, progressive and irreversible course, if the aggravating factors persist. Differential diagnoses include: melasma, contact dermatitis caused by perfumes or cosmetics and follicular erythromelanosis.
The ideal treatment of PC includes the simultaneous elimination of pigmented and vascular components, with the combination of topical medications, especially depigmenting agents, procedures for the vascular component and mandatory broad-spectrum photoprotection.92 Chemical peels and topical retinoids may be adjuvants to oppose photoaging of the target area. Most recently argon lasers and flashlamppumped pulsed laser with yellow dye (FLPDL), have been recommended, with reasonable results, although requiring much caution as they can cause scarring, post-inflammatory hypo-and hyperpigmentation, erythema and post treatment purpura.92-95 Intense pulsed light (IPL), unlike laser systems, works on a wide range of wavelengths, providing a good reduction of pigment and telangiectasias as well as improving the skin texture, with less risk of complications.96
Acanthosis nigricans
Acanthosis nigricans (AN) appears as hyperchromic plaques, with vegetative, lichenified or papillomatous surfaces, dark brown to black coloration, located in the armpits, groin, neck and other intertriginous areas (Figure 9). More recently, it has been divided into 8 subtypes: benign, obesity-related, syndromic, malignant, acral, unilateral, secondary to medication and multifactorial. This dermatosis is often related to endocrine disorders, such as obesity, insulin resistance, type II diabetes mellitus and polycystic ovary syndrome (PCOS).97-99 Etiology involves a state of hyperinsulinemia caused by insulin resistance, with stimulation of insulin related growth factor (IGF1, insulin-like growth factor), and consequent proliferation of keratinocytes.100, 101 When associated with malignancy, AN may either precede or occur simultaneously with the neoplasm, with the most common tumor being gastric adenocarcinoma, although it has already been reported in uterine, liver, bowel, ovarian, renal, breast and lung cancers, most of them adenocarcinomas.101
FIGURE 9.
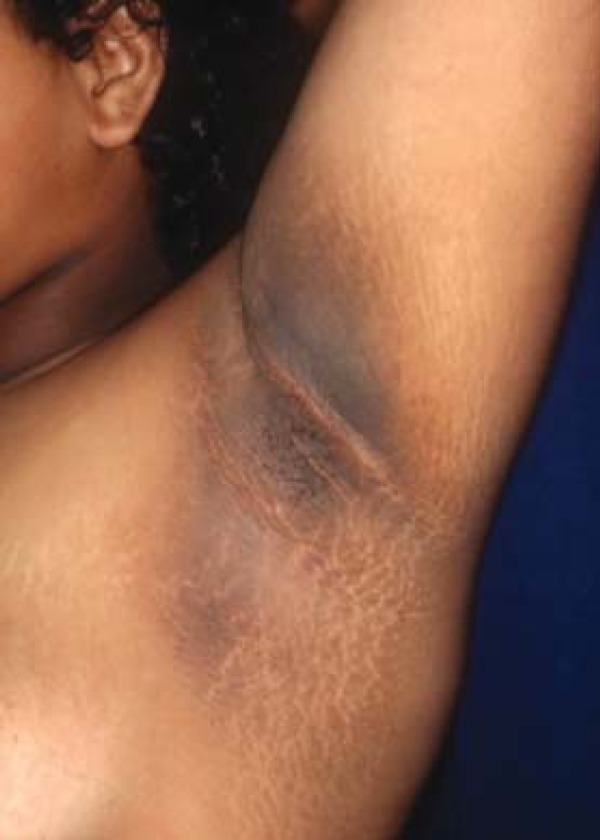
Acquired hyperpigmentations. Acanthosis nigricans – Linear and coalescent papular hyperpigmentation, with velvety aspect, located in the axillary region of an obese adolescent
Lesion improvement in AN is dependent on the resolution of the underlying disease. There are reports of the use of oral and topical retinoids with good results. In addition, other keratolytic agents such as 12% ammonium lactate or the combined triple therapy (4% HQ + 0.05% AR + 0.01% fluocinolone acetonide) can be used. Studies on the use of topical agents containing urea and salicylic acid reported variable results. Oral treatments include isotretinoin and metformin; the latter may be indicated in diseases that course with hyperandrogenism. Procedures include dermabrasion, Alexandrite laser and the application of trichloroacetic acid, described more recently.101,102
Primary localized cutaneous amyloidosis
Amyloidosis is a generic term applied to a group of diseases characterized by the deposit of a substance, mainly a fibrillar protein (amyloid) that can lead to compression and / or dysfunction in various organs, including the skin. The amyloidoses are divided into systemic or localized, and the latter can be of primary or secondary cause. Primary cutaneous forms include macular amyloidosis, amyloid lichen, and nodular or tumefactive amyloidosis.103,104 Systemic amyloidosis can be classified as primary, hereditary or not; and secondary to chronic or inflammatory diseases or cancer and also associated with hemodialysis. The distinction between primary localized cutaneous amyloidosis and systemic forms must be made through a careful physical examination and laboratory tests to exclude the presence of extracutaneous amyloid deposits and plasmocytic dyscrasias.105
Macular amyloidosis (AM) and amyloid lichen are more common variants of the primary localized form of amyloidosis, featuring amyloid deposits in the skin.106 Most occurrences are sporadic, but an autosomal dominant form of the disease may be present in up to 10% of cases, in places such as China, Central and South America.107 Sporadic forms occur more in women, with female to male rates of 4.5:1.103
The exact etiology and pathogenesis of cutaneous amyloidosis remains unknown, but it is believed that there are multiple factors involved: genetic, autoimmune, attrition and contact dermatitis.108-111 Recently, attention has been focused on the mechanisms associated with the so-called conformational diseases, in which a change in the spatial conformation of a protein occurs, resulting in an amyloidogenic structure. The exact mechanism responsible for this change has not yet been fully elucidated, however, systemic diseases such as Parkinson's and Alzheimer's disease appear to share the same etiopathogenic mechanism.112,113 Histology shows deposits of amyloid matter in the basal membrane or papillary dermis in both forms. In macular amyloidosis, the amyloid deposits can be quite subtle, and the skin does not show significant changes; in lichen amyloid the deposits are more pronounced, with the presence of hyperkeratosis, papillomatosis and moderate acanthosis. In some cases, there is vacuolization of basal cells, presence of intraepidermal cytoid bodies and signs of pigmentary incontinence.103,111
Macular amyloidosis presents clinically as macules, spots or pruritic hyperpigmented plaques with undulating surface (rippled patches), of a brownish or blackish color, with ill-defined borders, usually located in the interscapular region, which may be associated or coexist with notalgia paresthetica (Figure 10).114 In amyloid lichen, there are slightly pruritic coalescing papules either hyperpigmented or the same color of the skin, usually located in the legs or arms (Figure 11).106,114
FIGURE 10.

Acquired hyperpigmentations. Macular amyloidosis– a brownish itchy area, located in the dorsal region, corresponding to the range area of a dextral patient’s hand
FIGURE 11.
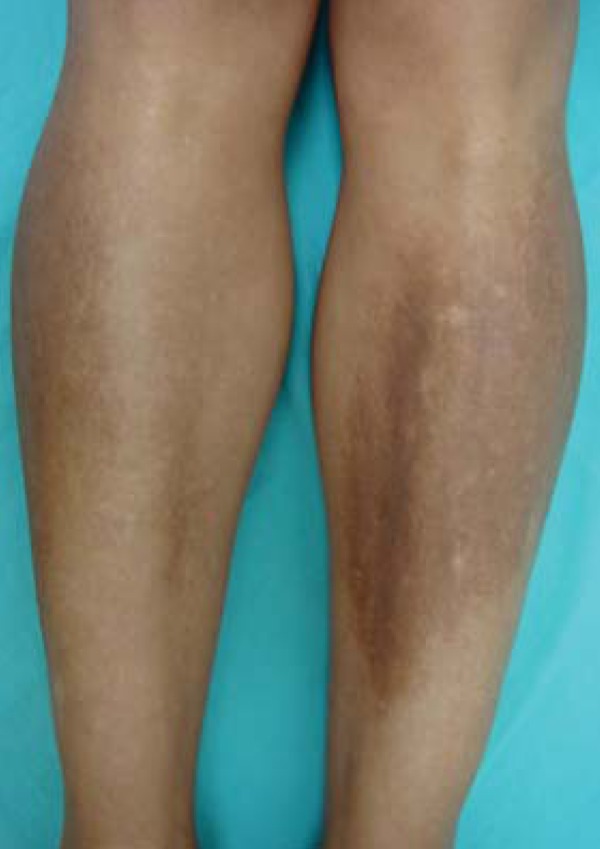
Acquired hyperpigmentations. Amyloid lichen – plaque formed by the confluence of small grayishbrown papules located in the pretibial region
Treatment options for cutaneous amyloidosis include intralesional and topical corticosteroids, calcineurin inhibitors, acitretin, UVB or UVA (PUVA) phototherapy, dermabrasion, laser and hydrocolloid dressings.114-116
Nodular or tumefactive amyloidosis is another type of localized cutaneous amyloidosis, although a less frequent one. It is characterized by diffuse amyloid deposits in the dermis, subcutaneous tissue and small dermal capillaries. Patients present with nodules or asymptomatic plaques of rosy to brownish color, either isolated or in multiples, and prone to affecting the face, especially the nose and periauricular regions, but also the genitals, trunk and limbs. These lesions are similar to those observed in primary systemic amyloidosis associated with plasma cell lymphoproliferative diseases. It occurs equally in both sexes, with a mean age at diagnosis of 60.8 years. The process can have a localized benign chronic course, but patients should be monitored for progression to systemic amyloidosis and plasmocytic dyscrasias, which occurs in 7-50% of patients.103,105,117
Dyschromic cutaneous amyloidosis is a rare subtype of primary cutaneous amyloidosis. This disease starts prior to puberty with small deposits of amyloid pigment beneath the epidermis.118 It is characterized by a reticulate hyperpigmentation interspersed with hypopigmented spots in almost all the skin and mild or absent pruritus. The treatment of this form of amyloidosis includes avoiding sunlight exposure, use of topical corticosteroids, capsaicin, dimethylsulfoxide, and CO2 laser with varying results. The use of systemic retinoids has been shown to be effective in a few reported cases.101
Confluent and reticulated papillomatosis of Gougerot and Carteaud
Confluent and reticulated papillomatosis (CRP) was described by French dermatologists Gougerot and Carteaud in 1927. It is a rare dermatosis of unknown etiology that occurs more frequently in women and melanodermic patients, aged 10 to 35 years.119
It is characterized by flat wrinkled papules, slightly salient, with variable coloration, hypochromic to pink or brownish that become confluent in the center and reticulated at the periphery. The lesions are mainly located in the interscapular, intermammary and epigastric regions. Its main differential diagnosis is pityriasis versicolor, but acanthosis nigricans, cutaneous amyloidosis, several keratinization disorders and some presentations of seborrheic dermatitis should also be considered.120 Criteria for its diagnosis have been recently proposed, including: 1) presence of macules and brownish desquamative spots, with reticulated and papillomatous aspect, 2) involvement of the upper chest and neck, 3) negative testing for fungus, 4) absence of response to antifungal drugs and 5) excellent response to minocycline.121
Many questions remain about this dermatosis, especially regarding its pathogenesis and treatment. Some hypotheses try to explain CRP genesis as vitamin A deficiency, genetic factors, photosensitivity, endocrine abnormalities, cutaneous amyloidosis, tissue reaction to skin colonization by lipophilic yeasts of the genus Malassezia, staphylococci or Propionibacterium acnes, and especially defects in keratinization.122,123
Minocycline (50-100 mg twice daily for 10 weeks) has been the treatment of choice. In addition, there are reports of improvements with azithromycin administered in a dose of 250-500mg three times per week. Other options are: clarithromycin 500 mg per day, erythromycin 1000 mg per day and tetracycline 500 mg, twice daily. Topical medications such as selenium sulfide, ketoconazole, tretinoin, tazarotene and calcipotriol have also been used, with varying results.122 Responses to retinoids support the theory that the disease may be caused by some keratinization disorder. The use of oral isotretinoin, 1 to 2mg/kg/day has been recommended, with favorable responses achieved in two months.120
QUESTIONS
1 - Regarding the triggering factors of melasma, it is CORRECT to state:
a) There is usually a conjunction of several factors for its initial onset, including genetic predisposition and exposure to ultraviolet radiation.
b) Treatment with ACE inhibitors can cause lesions in a large number of patients.
c) There is a clear relationship between pregnancy after the third decade of life and melasma, in most populations.
d) There is no evidence linking the predisposition to melasma with phototype and ancestry.
2 - Check the INCORRECT alternative:
a) Melasma lesions often show histological alterations consistent with chronic sun damage.
b) Cell interactions between keratinocytes, mast cells and fibroblasts contribute to increased activity of melanocytes in melasma.
c) The deposit of dermal pigment is most striking feature in melasma.
d) The number of melanocytes in melasma is similar to that of healthy skin, but the cells tend to be larger and with prominence of dendrites.
3 - Melasma treatment is not always easy and should be adapted to each patient. Check the MOST RECOMMENDED therapeutic orientation:
a) The association of azelaic and kojic acids and arbutin should be considered as first line treatment.
b) Classic depigmenting agents such as hydroquinone in progressive doses may be used. If no response is achieved in 46 weeks, medium peels or dermabrasion are the options with better response in most patients.
c) The combined therapies have synergistic action; the triple combination with hydroquinone, tretinoin, and mediumpower corticosteroids is the more cost-effective treatment.
d) No maintenance therapy is recommended due to the risk of tachyphylaxis. Re-treatment intervals should be of at least 2 months.
4 - Post-inflammatory hyperpigmentation may be secondary to various dermatoses. Select the CORRECT alternative:
a) There is no correlation between skin phototype and predisposition to post-inflammatory hyperpigmentation.
b) The histology of the lesions is characteristic and can give a good indication of the triggering cause of the dermatoses.
c) Acute conditions usually produce higher-intensity pigmentations that are more difficult to treat.
d) Hyperpigmentation is probably caused by increased melanogenesis and abnormal distribution of melanin, due to the action of inflammatory mediators.
5 - Regarding the post inflammatory hyperpigmentation check the TRUE option:
a) Response to topical treatment is excellent.
b) Recurrences are rare.
c) The distribution of spots is random and rarely accompanies the location of the inflammatory processes that preceded them.
d) Histology shows melanin deposits both in free form and within melanophages located in the upper dermis and around the vessels.
6 - The following histologic features are found on periorbital hyperpigmentation, EXCEPT:
a) Dermal melanin deposits
b) Superficial presentation of the vasculature
c) Tattoo secondary to chronic use of makeup
d) Hyperactivity of the periorbital muscles
7 - Regarding the periorbital hyperpigmentation, it is CORRECT to affirm that:
a) The treatment should be performed for a short period of time due to the high frequency of adverse events, such as erythema and desquamation, caused by depigmenting agents in this region.
b) As with melasma, combination therapy is the treatment of choice
c) The use of hydroquinone is justified in cases where there is a prevalence of hyperpigmentation, because it inhibits the synthesis of DNA and RNA, inducing melanosome degradation and melanocyte destruction.
d) Treatment options are similar, regardless of the predominant component.
8 - About dermatosis papulosa nigra, it is CORRECT to state that:
a) It is more frequent in white males and presents spontaneous regression.
b) It presents the same histopathological findings of seborrheic keratosis.
c) Typically, the lesions start on the face and show no changes in quantity or pattern over the years.
d) It usually regresses spontaneously in about 50% of patients.
9 - Different treatment modalities can be employed for the treatment of dermatosis papulosa nigra. THE LEAST indicated one is:
a) Curettage
b) Cryotherapy
c) Electrofulguration
d) Exeresis and suture
10 - The following statement is CORRECT about erythema dyschromicum perstans:
a) It is a common genodermatosis amongst the Latin American population.
b) In most cases the triggering factor is easily identified.
c) On the active border of the lesion there is lichenoid dermatitis with vacuolization of the basal cell layer and a strip of mononuclear infiltrate in the superficial dermis.
d) The response to treatment with hydroquinone is excellent.
11 - Acanthosis nigricans has several etiological and clinical characteristics. Mark the most CORRECT answer:
a) Depending on the clinical appearance of the lesions it is possible to determine the causative factor.
b) When associated with malignancy, AN appears simultaneously with cancer, never preceding that disease.
c) The most frequently associated tumor is the liver adenocarcinoma.
d) There are several therapeutic options for the treatment, and the appropriate management of the underlying disease is important.
12 - The following aspects are NOT associated with confluent papillomatosis of Gougerot Carteaud:
a) Rough, flat, slightly protruding papules with variable coloring, from hyperchromic to pink or brownish, that become confluent in the center and reticulated in the periphery.
b) Location mainly in the interscapular, intermammary and epigastric regions.
c) Good response to treatment with antifungals.
d) It is more frequent in women and melanodermic patients, aged 10 to 35 years.
13 - Amongst the differential diagnosis for confluent papillomatosis of Gougerot Carteaud the following may NOT be included:
a) Pityriasis versicolor
b) Seborrheic dermatitis
c) Lichenoid purpura of Gougerot Blum
d) Acanthosis nigricans
14 - Cervical poikiloderma presents peculiar clinical characteristics, EXCEPT:
a) Regular alterations in pigmentation, with hypo and hyper pigmentation
b) Superficial atrophy
c) Telangiectasias
d) It is never symptomatic
15 - Several factors seem to be linked to Poikiloderma of Civatte's pathogenesis or triggering. Indicate the INCORRECT alternative:
a) Cumulative sun exposure
b) Photoallergic or phototoxic reactions caused by fragrances and/or cosmetics in predisposed individuals
c) Hormonal factors
d) Trauma
16 - About the histological findings in primary localized cutaneous amyloidosis, it is possible to affirm that:
I. Both in macular amyloidosis and in amyloid lichen, there are deposits of amyloid material in the basal membrane or in the papillary dermis.
II. In macular amyloidosis, the amyloid deposits are always abundant and the skin may not show significant changes.
III.In amyloid lichen, the protein deposit is discreet and the epidermis often presents hyperkeratosis, papillomatosis and moderate acanthosis.
a) Only I is correct
b) Statements I and III are correct
c) All are correct
d) None is correct
17 - Complete the second column in accordance with the first one:
(1) Macular amyloidosis
(2) Amyloid lichen
( ) Macules, itchy spots or plaques, with undulating surface, hyperpigmented and with ill-defined borders
( ) Coalescing and slightly pruritic hyperpigmented or normochromic papules
( ) Most commonly located in the legs or arms
( ) Located usually in the interscapular area
( ) May be associated or coexist with notalgia paresthetica
a) 2 1 1 2 2
b) 1 2 2 1 1
c) 2 1 2 1 1
d) 1 2 1 2 2
18. The substances responsible for phytophotodermatoses caused by the Rutaceae (citrus-producing plants) are:
a) Furocoumarins
b) Bergapten
c) Apiacea
d) Fabaceae
19. The flagellate dermatitis is classically linked to bleomycin. More recently, however, there have been reports of its association with:
a) Bergamot oil (bergapten) which contains 5-methoxy-psoralen in its composition
b) Shiitake Mushroom (Lentinus edodes)
c) Metformin
d) ACE inhibitors
20. Acanthosis nigricans has a multifactorial etiology. On this point we can affirm that:
a) Its appearance cannot be assigned to the use of medications.
b) Its etiology may involve a state of hyperinsulinemia caused by insulin resistance, with stimulation of insulin-related growth factor (IGF1, insulin-like growth factor)
c) Its appearance during childhood has not been described.
d) Keratinocytes proliferation is reduced in this dermatosis
Answer key.
Giant congenital melanocytic nevus. An Bras Dermatol. 2013;88(6):863-78
| 1) C | 6) D | 11) D | 16) A |
| 2) C | 7) C | 12) B | 17) B |
| 3) D | 8) C | 13) C | 18) B |
| 4) B | 9) C | 14) C | 19) D |
| 5) C | 10) A | 15) B | 20) C |
Papers
Information for all members: The EMC-D questionnaire is now available at the homepage of the Brazilian Annals of Dermatology: www.anaisdedermatologia.org.br. The deadline for completing the questionnaire is 30 days from the date of online publication.
Footnotes
Work performed at the Dermatology Department at Porto Alegre Clinics Hospital - Rio Grande do Sul Federal University (HCPA-UFRGS) - Porto Alegre (RS), Brazil.
Financial Support: None.
Conflict of Interest: None.
REFERENCES
- 1.Sbd.org Censo Dermatologico da SBD. Brazilian Society of Dermatology Web page - Dermatological census. [Acesso 1 Dez. 2012]. [Internet] Disponível em: http://www.sbd.org.br/down/censo_dermatologico2006pdf2012.
- 2.Sanchez NP, Pathak MA, Sato S, Fitzpatrick TB, Sanchez JL, Mihm MC., Jr Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698–710. doi: 10.1016/s0190-9622(81)70071-9. [DOI] [PubMed] [Google Scholar]
- 3.Ritter CG, Fiss DV, Borges da Costa JA, de Carvalho RR, Bauermann G, Cestari TF. Extra-facial melasma: clinical, histopathological, and immunohistochemical case-control study. J Eur Acad Dermatol Venereol. 2013;27:1088–1094. doi: 10.1111/j.1468-3083.2012.04655.x. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SC. Epidemiology of skin diseases in ethnic populations. Dermatol Clin. 2003;21:601–607. doi: 10.1016/s0733-8635(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 5.Tamega Ade A, Miot LD, Bonfietti C, Gige TC, Marques ME, Miot HA. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27:151–156. doi: 10.1111/j.1468-3083.2011.04430.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar R, Puri P, Jain RK, Singh A, Desai A. Melasma in men: a clinical, aetiological and histological study. J Eur Acad Dermatol Venereol. 2010;24:768–772. doi: 10.1111/j.1468-3083.2009.03524.x. [DOI] [PubMed] [Google Scholar]
- 7.Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380–382. doi: 10.4103/0019-5154.84722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cestari T, Benvenuto-Andrade C. Hyperpigmentation and melasma: a physiopathologic review for the clinical dermatologist. Cosm Dermatol. 2005;18:703–706. [Google Scholar]
- 9.Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146:228–237. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- 10.Miot LD, Miot HA, Silva MG, Marques ME. Physiopathology of melasma. An Bras Dermatol. 2009;84:623–635. doi: 10.1590/s0365-05962009000600008. [DOI] [PubMed] [Google Scholar]
- 11.Ortonne JP, Arellano I, Berneburg M, Cestari T, Chan H, Grimes P, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009;23:1254–1262. doi: 10.1111/j.1468-3083.2009.03295.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang HY, Ortonne JP. What should be considered in the treatment of melasma? Ann Dermatol. 2010;22:373–378. doi: 10.5021/ad.2010.22.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092–2097. doi: 10.1038/jid.2010.95. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Barrera R, Torres-Alvarez B, Castanedo-Cazares JP, Oros-Ovalle C, Moncada B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin Exp Dermatol. 2008;33:305–308. doi: 10.1111/j.1365-2230.2008.02724.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin J, Kim JH, Kim EK. Repeated exposure of human fibroblasts to UVR induces secretion of stem cell factor and senescence. J Eur Acad Dermatol Venereol. 2012;26:1577–1580. doi: 10.1111/j.1468-3083.2011.04223.x. [DOI] [PubMed] [Google Scholar]
- 16.Jang YH, Lee JY, Kang HY, Lee ES, Kim YC. Oestrogen and progesterone receptor expression in melasma: an immunohistochemical analysis. J Eur Acad Dermatol Venereol. 2010;24:1312–1316. doi: 10.1111/j.1468-3083.2010.03638.x. [DOI] [PubMed] [Google Scholar]
- 17.Pérez M, Sánchez JL, Aguiló F. Endocrinologic profile of patients with idiopathic melasma. J Invest Dermatol. 1983;81:543–545. doi: 10.1111/1523-1747.ep12522896. [DOI] [PubMed] [Google Scholar]
- 18.Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195–202. doi: 10.1111/j.1473-2165.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 19.Sheth VM, Pandya AG. Melasma: a comprehensive update: part I. J Am Acad Dermatol. 2011;65:689–689. doi: 10.1016/j.jaad.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96–101. doi: 10.1097/01.dad.0000154419.18653.2e. [DOI] [PubMed] [Google Scholar]
- 21.Cestari T, Arellano I, Hexsel D, Ortonne JP, Latin American Pigmentary Disorders Academy Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760–772. doi: 10.1111/j.1468-3083.2009.03251.x. [DOI] [PubMed] [Google Scholar]
- 22.Arellano I, Cestari T, Ocampo-Candiani J, Azulay-Abulafia L, Bezerra Trindade P, Neto, Hexsel D, et al. Preventing melasma recurrence: prescribing a maintenance regimen with an effective triple combination cream based on long-standing clinical severity. J Eur Acad Dermatol Venereol. 2012;26:611–618. doi: 10.1111/j.1468-3083.2011.04135.x. [DOI] [PubMed] [Google Scholar]
- 23.Rajaratnam R, Halpern J, Salim A, Emmett C. Interventions for melasma. Cochrane Database Syst Rev. 2010: doi: 10.1002/14651858.CD003583.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Kang HY, Valerio L, Bahadoran P, Ortonne JP. The role of topical retinoids in the treatment of pigmentary disorders: an evidence-based review. Am J Clin Dermatol. 2009;10:251–260. doi: 10.2165/00128071-200910040-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kang HY, Valerio L, Bahadoran P, Ortonne JP. Treatment of melasma. Am J Clin Dermatol. 2009;10:251–260. doi: 10.2165/00128071-200910040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Sheth VM, Pandya AG. Melasma: a comprehensive update: part II. J Am Acad Dermatol. 2011;65:699–714. doi: 10.1016/j.jaad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Alikhan A, Daly M, Wu J, Balkrishnan R, Feldman SR. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276–281. doi: 10.3109/09546630903200612. [DOI] [PubMed] [Google Scholar]
- 28.Farshi S. Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J Cosmet Dermatol. 2011 Dec;10(4):282–287. doi: 10.1111/j.1473-2165.2011.00580.x. [DOI] [PubMed] [Google Scholar]
- 29.Magalhães GM, Borges MFB, Queiroz ARC, Capp AA, Pedrosa SV, Diniz MS. Estudo duplo-cego e randomizado do peeling de ácido retinoico a 5% e 10% no tratamento do melasma: avaliação clínica e impacto na qualidade de vida. Doubleblind randomized study of 5% and 10% retinoic acid peels in the treatment of melasma: clinical evaluation and impact on the quality of life. Surg Cosmet Dermatol. 2011;1:317–322. [Google Scholar]
- 30.Zaleski L, Fabi S, Goldman MP. Treatment of melasma and the use of intense pulsed light: a review. J Drugs Dermatol. 2012;11:1316–1320. [PubMed] [Google Scholar]
- 31.Zoccali G, Piccolo D, Allegra P, Giuliani M. Melasma treated with intense pulsed light. Aesthetic Plast Surg. 2010;34:486–493. doi: 10.1007/s00266-010-9485-y. [DOI] [PubMed] [Google Scholar]
- 32.Na SY, Cho S, Lee JH. Intense pulsed light and low-fluence Q-switched Nd:YAG laser treatment in melasma patients. Ann Dermatol. 2012;24:267–273. doi: 10.5021/ad.2012.24.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora P, Sarkar R, Garg VK, Arya L. Lasers for treatment of melasma and postinflammatory hyperpigmentation. J Cutan Aesthet Surg. 2012;5:93–103. doi: 10.4103/0974-2077.99436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan HH, Kono T. The use of lasers and intense pulsed light sources for the treatment of pigmentary lesions. Skin Therapy Lett. 2004;9:5–7. [PubMed] [Google Scholar]
- 35.Kauvar AN. Successful treatment of melasma using a combination of microdermabrasion and Q-switched Nd:YAG lasers. Lasers Surg Med. 2012;44:117–124. doi: 10.1002/lsm.21156. [DOI] [PubMed] [Google Scholar]
- 36.Niwa Massaki AB, Eimpunth S, Fabi SG, Guiha I, Groff W, Fitzpatrick R. Treatment of melasma with the 1,927-nm fractional thulium fiber laser: A retrospective analysis of 20 cases with long-term follow-up. Lasers Surg Med. 2013;45:95–101. doi: 10.1002/lsm.22100. [DOI] [PubMed] [Google Scholar]
- 37.Cho HH, Choi M, Cho S, Lee JH. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS Nd:YAG laser. J Dermatolog Treat. 2013;24:292–296. doi: 10.3109/09546634.2011.643220. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick RE, Lupton JR. Successful treatment of treatment-resistant laser-induced pigment darkening of a cosmetic tattoo. Lasers Surg Med. 2000;27:358–361. doi: 10.1002/1096-9101(2000)27:4<358::aid-lsm9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Handog EB, Galang DA, de Leon-Godinez MA, Chan GP. A randomized, doubleblind, placebo-controlled trial of oral procyanidin with vitamins A, C, E for melasma among Filipino women. Int J Dermatol. 2009;48:896–901. doi: 10.1111/j.1365-4632.2009.04130.x. [DOI] [PubMed] [Google Scholar]
- 40.Hwang SW, Oh DJ, Lee D, Kim JW, Park SW. Clinical efficacy of 25% L-ascorbic acid (C'ensil) in the treatment of melasma. J Cutan Med Surg. 2009;13:74–81. doi: 10.2310/7750.2008.07092. [DOI] [PubMed] [Google Scholar]
- 41.Sobhi RM, Sobhi AM. A single-blinded comparative study between the use of glycolic acid 70% peel and the use of topical nanosome vitamin C iontophoresis in the treatment of melasma. J Cosmet Dermatol. 2012;11:65–71. doi: 10.1111/j.1473-2165.2011.00599.x. [DOI] [PubMed] [Google Scholar]
- 42.Wanick FBF, Zink BS, Lopes RF. Avaliação da eficácia do licopeno, betacaroteno e Lactobacillus johnsonii no tratamento de manutenção do melasma durante o verão: um estudo comparativo. Efficacy evaluation of lycopene, beta-carotene and Lactobacillus johnsonii in the maintenance treatment of melasma during the summer: a comparative study. Surg Cosmet Dermatol. 2011;3:297–301. [Google Scholar]
- 43.Taylor S, Grimes P, Lim J, Im S, Lui H. Postinflammatory hyperpigmentation. J Cutan Med Surg. 2009;13:183–191. doi: 10.2310/7750.2009.08077. [DOI] [PubMed] [Google Scholar]
- 44.Chan HH, Manstein D, Yu CS, Shek S, Kono T, Wei WI. The prevalence and risk factors of post-inflammatory hyperpigmentation after fractional resurfacing in Asians. Lasers Surg Med. 2007;39:381–385. doi: 10.1002/lsm.20512. [DOI] [PubMed] [Google Scholar]
- 45.Grimes PE. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28:77–85. doi: 10.1016/j.sder.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Stratigos AJ, Katsambas AD. Optimal management of recalcitrant disorders of hyperpigmentation in dark-skinned patients. Am J Clin Dermatol. 2004;5:161–168. doi: 10.2165/00128071-200405030-00004. [DOI] [PubMed] [Google Scholar]
- 47.Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. J Investig Dermatol Symp Proc. 2008;13:10–14. doi: 10.1038/jidsymp.2008.7. [DOI] [PubMed] [Google Scholar]
- 48.Javaheri SM, Handa S, Kaur I, Kumar B. Safety and efficacy of glycolic acid facial peel in Indian women with melasma. Int J Dermatol. 2001;40:354–357. doi: 10.1046/j.1365-4362.2001.01149.x. [DOI] [PubMed] [Google Scholar]
- 49.Chan HH. Effective and safe use of lasers, light sources, and radiofrequency devices in the clinical management of Asian patients with selected dermatoses. Lasers Surg Med. 2005;37:179–185. doi: 10.1002/lsm.20244. [DOI] [PubMed] [Google Scholar]
- 50.Cho SB, Park SJ, Kim JS, Kim MJ, Bu TS. Treatment of post-inflammatory hyperpigmentation using 1064-nm Q-switched Nd:YAG laser with low fluence: report of three cases. J Eur Acad Dermatol Venereol. 2009;23:1206–1207. doi: 10.1111/j.1468-3083.2009.03123.x. [DOI] [PubMed] [Google Scholar]
- 51.Katz TM, Goldberg LH, Firoz BF, Friedman PM. Fractional photothermolysis for the treatment of post inflammatory hyperpigmentation. Dermatol Surg. 2009;35:1844–1848. doi: 10.1111/j.1524-4725.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 52.Freitag FM, Cestari TF. What causes dark circles under the eyes? J Cosmet Dermatol. 2007;6:211–215. doi: 10.1111/j.1473-2165.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 53.Roh MR, Chung KY. Infraorbital dark circles: definition, causes, and treatment options. Dermatol Surg. 2009;35:1163–1171. doi: 10.1111/j.1524-4725.2009.01213.x. [DOI] [PubMed] [Google Scholar]
- 54.Mitsuishi T, Shimoda T, Mitsui Y, Kuriyama Y, Kawana S. The effects of topical application of phytonadione, retinol and vitamins C and E on infraorbital dark circles and wrinkles of the lower eyelids. J Cosmet Dermatol. 2004;3:73–75. doi: 10.1111/j.1473-2130.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 55.Momosawa A, Kurita M, Ozaki M, Miyamoto S, Kobayashi Y, Ban I, et al. Combined therapy using Q-switched ruby laser and bleaching treatment with tretinoin and hydroquinone for periorbital skin hyperpigmentation in Asians. Plast Reconstr Surg. 2008;121:282–288. doi: 10.1097/01.prs.0000293869.00522.ec. [DOI] [PubMed] [Google Scholar]
- 56.Ohshima H, Mizukoshi K, Oyobikawa M, Matsumoto K, Takiwaki H, Kanto H, et al. Effects of vitamin C on dark circles of the lower eyelids: quantitative evaluation using image analysis and echogram. Skin Res Technol. 2009;15:214–217. doi: 10.1111/j.1600-0846.2009.00356.x. [DOI] [PubMed] [Google Scholar]
- 57.Roh MR, Kim TK, Chung KY. Treatment of infraorbital dark circles by autologous fat transplantation: a pilot study. Br J Dermatol. 2009;160:1022–1025. doi: 10.1111/j.1365-2133.2009.09066.x. [DOI] [PubMed] [Google Scholar]
- 58.Palumbo A, d'Ischia M, Misuraca G, Prota G. Mechanism of inhibition of melanogenesis by hydroquinone. Biochim Biophys Acta. 1991;1073:85–90. doi: 10.1016/0304-4165(91)90186-k. [DOI] [PubMed] [Google Scholar]
- 59.Ma G, Lin XX, Hu XJ, Jin YB, Chen H. Treatment of venous infraorbital dark circles using a long-pulsed 1,064-nm neodymium-doped yttrium aluminum garnet laser. Dermatol Surg. 2012;38:1277–1282. doi: 10.1111/j.1524-4725.2012.02457.x. [DOI] [PubMed] [Google Scholar]
- 60.Xu TH, Yang ZH, Li YH, Chen JZ, Guo S, Wu Y, et al. Treatment of infraorbital dark circles using a low-fluence Q-switched 1,064-nm laser. Dermatol Surg. 2011;37:797–803. doi: 10.1111/j.1524-4725.2011.01956..x. [DOI] [PubMed] [Google Scholar]
- 61.Niang SO, Kane A, Diallo M, Choutah F, Dieng MT, Ndiaye B. Dermatosis papulosa nigra in Dakar, Senegal. Int J Dermatol. 2007;46:45–47. doi: 10.1111/j.1365-4632.2007.03465.x. [DOI] [PubMed] [Google Scholar]
- 62.Katz TM, Goldberg LH, Friedman PM. Dermatosis papulosa nigra treatment with fractional photothermolysis. Dermatol Surg. 2009;35:1840–1843. doi: 10.1111/j.1524-4725.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 63.Rajesh G, Thappa DM, Jaisankar TJ, Chandrashekar L. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483–488. doi: 10.4103/0378-6323.82408. [DOI] [PubMed] [Google Scholar]
- 64.Garcia MS, Azari R, Eisen DB. Treatment of dermatosis papulosa nigra in 10 patients: a comparison trial of electrodesiccation, pulsed dye laser, and curettage. Dermatol Surg. 2010;36:1968–1972. doi: 10.1111/j.1524-4725.2010.01769.x. [DOI] [PubMed] [Google Scholar]
- 65.Polder KD, Landau JM, Vergilis-Kalner IJ, Goldberg LH, Friedman PM, Bruce S. Laser eradication of pigmented lesions: a review. Dermatol Surg. 2011;37:572–595. doi: 10.1111/j.1524-4725.2011.01971.x. [DOI] [PubMed] [Google Scholar]
- 66.Kundu RV, Joshi SS, Suh KY, Boone SL, Huggins RH, Alam M, et al. Comparison of electrodesiccation and potassium-titanyl-phosphate laser for treatment of dermatosis papulosa nigra. Dermatol Surg. 2009;35:1079–1083. doi: 10.1111/j.1524-4725.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 67.Reis VM. Dermatosis due to plants (phytodermatoses) An Bras Dermatol. 2010;85:479–489. doi: 10.1590/s0365-05962010000400009. [DOI] [PubMed] [Google Scholar]
- 68.Mill J, Wallis B, Cuttle L, Mott J, Oakley A, Kimble R. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731–733. doi: 10.1016/j.burns.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299–308. doi: 10.1016/j.det.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Dantzig PI. Immunosupressive and cytotoxic drugs in dermatology. Arch Dermatol. 1974;110:393–406. [PubMed] [Google Scholar]
- 71.Al-Khenaizan S, Al-Berouti B. Flagellate pigmentation: a unique adverse effect of bleomycin therapy. Eur J Dermatol. 2011;21:146. doi: 10.1684/ejd.2011.1213. [DOI] [PubMed] [Google Scholar]
- 72.Mowad CM, Nguyen TV, Elenitsas R, Leyden JJ. Bleomycin-induced flagellate dermatitis: a clinical and histopathological review. Br J Dermatol. 1994;131:700–702. doi: 10.1111/j.1365-2133.1994.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 73.Silveira JCB, Cunha BM, Estrella RR. Bleomycin- induced flagellate dermatitis. An Bras Dermatol. 2006;81:83–85. [Google Scholar]
- 74.Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337–339. doi: 10.1001/archderm.139.3.337. [DOI] [PubMed] [Google Scholar]
- 75.Poppe LM, Anders D, Kneitz H, Bröcker EB, Benoit S. Flagellate dermatitis caused by shiitake mushrooms. An Bras Dermatol. 2012;87:463–465. doi: 10.1590/s0365-05962012000300017. [DOI] [PubMed] [Google Scholar]
- 76.Chakrabarti N, Chattopadhyay C. Ashy dermatosis: a controversial entity. Indian J Dermatol. 2012;57:61–62. doi: 10.4103/0019-5154.92684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cherobin AC, Oliveira FO, Baeta IG, Vale EC. Case for diagnosis. Ashy dermatosis. An Bras Dermatol. 2012;87:151–152. doi: 10.1590/s0365-05962012000100025. [DOI] [PubMed] [Google Scholar]
- 78.Vega-Memije ME, Domínguez-Soto L. Ashy dermatosis. Int J Dermatol. 2010;49:228–229. doi: 10.1111/j.1365-4632.2008.04032.x. [DOI] [PubMed] [Google Scholar]
- 79.Novick NL, Phelps R. Erythema dyschromicum perstans. Int J Dermatol. 1985;24:630–633. doi: 10.1111/j.1365-4362.1985.tb05712.x. [DOI] [PubMed] [Google Scholar]
- 80.Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis) Dermatologica. 1975;150:287–291. doi: 10.1159/000251444. [DOI] [PubMed] [Google Scholar]
- 81.Lambert WC, Schwartz RA, Hamilton GB. Erythema dyschromicum perstans. Cutis. 1986;37(1):42–44. [PubMed] [Google Scholar]
- 82.Henderson CD, Tschen JA, Schaefer DG. Simultaneously active lesions of vitiligo and erythema dyschromicum perstans. Arch Dermatol. 1988;124:1258–1260. [PubMed] [Google Scholar]
- 83.Nelson MR, Lawrence AG, Staughton RC, Gazzard BG. Erythema dyschromicum perstans in an HIV antibody-positive man. Br J Dermatol. 1992;127:658–659. doi: 10.1111/j.1365-2133.1992.tb14886.x. [DOI] [PubMed] [Google Scholar]
- 84.Kontochristopoulos GJ, Aroni K, Anagnostopoulos G, Nakopoulou L, Tassopoulos NC. Erythema dyschromicum perstans and hepatitis C virus infection. Int J Dermatol. 2001;40:346–348. doi: 10.1046/j.1365-4362.2001.01196-2.x. [DOI] [PubMed] [Google Scholar]
- 85.Torrelo A, Zaballos P, Colmenero I, Mediero IG, de Prada I, Zambrano A. Erythema dyschromicum perstans in children: a report of 14 cases. J Eur Acad Dermatol Venereol. 2005;19:422–426. doi: 10.1111/j.1468-3083.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 86.Domínguez Soto L, Vega Memije ME, Arenas R, Waxtein Morgenstein L. Dermatosis cinecienta. A clinico-pathological study of 20 patients (1989-1990) Gac Med Mex. 1992;128:623–627. [PubMed] [Google Scholar]
- 87.Baranda L, Torres-Alvarez B, Cortes-Franco R, Moncada B, Portales-Perez DP, Gonzalez-Amaro R. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). The effect of clofazimine therapy. Arch Dermatol. 1997;133:325–329. [PubMed] [Google Scholar]
- 88.Combemale P, Faisant M, Guennoc B, Dupin M, Heyraud JD. Erythema dyschromicum perstans: report of a new case and critical review of the literature. J Dermatol. 1998;25:747–753. doi: 10.1111/j.1346-8138.1998.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 89.Katoulis AC, Stavrianeas NG, Georgala S, Katsarou-Katsari A, Koumantaki-Mathioudaki E, Antoniou C, et al. Familial cases of poikiloderma of Civatte: genetic implications in its pathogenesis? Clin Exp Dermatol. 1999;24:385–387. doi: 10.1046/j.1365-2230.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- 90.Katoulis AC, Stavrianeas NG, Katsarou A, Antoniou C, Georgala S, Rigopoulos D, et al. Evaluation of the role of contact sensitization and photosensitivity in the pathogenesis of poikiloderma of Civatte. Br J Dermatol. 2002;147:493–497. doi: 10.1046/j.1365-2133.2002.04993.x. [DOI] [PubMed] [Google Scholar]
- 91.Katoulis AC, Stavrianeas NG, Panayiotides JG, Bozi E, Vamvasakis E, Kalogeromitros D, et al. Poikiloderma of Civatte: a histopathological and ultrastructural study. Dermatology. 2007;214(2):177–182. doi: 10.1159/000098580. [DOI] [PubMed] [Google Scholar]
- 92.Katoulis AC, Stavrianeas NG, Georgala S, Bozi E, Kalogeromitros D, Koumantaki E, et al. Poikiloderma of Civatte: a clinical and epidemiological study. J Eur Acad Dermatol Venereol. 2005;19:444–448. doi: 10.1111/j.1468-3083.2005.01213.x. [DOI] [PubMed] [Google Scholar]
- 93.Behroozan DS, Goldberg LH, Glaich AS, Dai T, Friedman PM. Fractional photothermolysis for treatment of poikiloderma of Civatte. Dermatol Surg. 2006;32:298–301. doi: 10.1111/j.1524-4725.2006.32061.x. [DOI] [PubMed] [Google Scholar]
- 94.Meijs MM, Blok FA, de Rie MA. Treatment of poikiloderma of Civatte with the pulsed dye laser: a series of patients with severe depigmentation. J Eur Acad Dermatol Venereol. 2006;20:1248–1251. doi: 10.1111/j.1468-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 95.Tierney EP, Hanke CW. Treatment of Poikiloderma of Civatte with ablative fractional laser resurfacing: prospective study and review of the literature. J Drugs Dermatol. 2009;8:527–534. [PubMed] [Google Scholar]
- 96.Rusciani A, Motta A, Fino P, Menichini G. Treatment of poikiloderma of Civatte using intense pulsed light source: 7 years of experience. Dermatol Surg. 2008;34:314–319. doi: 10.1111/j.1524-4725.2007.34064.x. [DOI] [PubMed] [Google Scholar]
- 97.Barbato MT, Criado PR, Silva AK, Averbeck E, Guerine MB, Sá NB. Association of acanthosis nigricans and skin tags with insulin resistance. An Bras Dermatol. 2012;87:97–104. doi: 10.1590/s0365-05962012000100012. [DOI] [PubMed] [Google Scholar]
- 98.Boza JC, Trindade EN, Peruzzo J, Sachett L, Rech L, Cestari TF. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220–1223. doi: 10.1111/j.1468-3083.2011.04265.x. [DOI] [PubMed] [Google Scholar]
- 99.Puri N. A study of pathogenesis of acanthosis nigricans and its clinical implications. Indian J Dermatol. 2011;56:678–683. doi: 10.4103/0019-5154.91828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199–203. doi: 10.2165/00128071-200405030-00008. [DOI] [PubMed] [Google Scholar]
- 101.Lee SS, Jung NJ, Im M, Lee Y, Seo YJ, Lee JH. Acral-type malignant acanthosis nigricans associated with gastric adenocarcinoma. Ann Dermatol. 2011;23:S208–S210. doi: 10.5021/ad.2011.23.S2.S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moore RL, Devere TS. Epidermal manifestations of internal malignancy. Dermatol Clin. 2008;26:17-29, vii. doi: 10.1016/j.det.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 103.Fernandez-Flores A. Cutaneous amyloidosis: a concept review. Am J Dermatopathol. 2012;34:1–14. doi: 10.1097/DAD.0b013e31823465c7. [DOI] [PubMed] [Google Scholar]
- 104.Chuang YY, Lee DD, Lin CS, Chang YJ, Tanaka M, Chang YT, et al. Characteristic dermoscopic features of primary cutaneous amyloidosis: a study of 35 cases. Br J Dermatol. 2012;167:548–554. doi: 10.1111/j.1365-2133.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 105.Souza Jd, Júnior, Schettini RA, Tupinambá WL, Schettini AP, Chirano CA, Massone C. Localized primary cutaneous nodular amyloidosis: case report. An Bras Dermatol. 2011;86:987–990. doi: 10.1590/s0365-05962011000500018. [DOI] [PubMed] [Google Scholar]
- 106.Tanaka A, Arita K, Lai-Cheong JE, Palisson F, Hide M, McGrath JA. New insight into mechanisms of pruritus from molecular studies on familial primary localized cutaneous amyloidosis. Br J Dermatol. 2009;161:1217–1224. doi: 10.1111/j.1365-2133.2009.09311.x. [DOI] [PubMed] [Google Scholar]
- 107.Sakuma TH, Hans-Filho G, Arita K, Odashiro M, Odashiro DN, Hans NR, et al. Familial primary localized cutaneous amyloidosis in Brazil. Arch Dermatol. 2009;145:695–699. doi: 10.1001/archdermatol.2009.107. [DOI] [PubMed] [Google Scholar]
- 108.Lee DD, Lin MW, Chen IC, Huang CY, Liu MT, Wang CR, et al. Genome-wide scan identifies a susceptibility locus for familial primary cutaneous amyloidosis on chromosome 5p13.1-q11. Br J Dermatol. 2006;155:1201–1208. doi: 10.1111/j.1365-2133.2006.07524.x. [DOI] [PubMed] [Google Scholar]
- 109.Dahdah MJ, Kurban M, Kibbi AG, Ghosn S. Primary localized cutaneous amyloidosis: a sign of immune dysregulation? Int J Dermatol. 2009;48:419–421. doi: 10.1111/j.1365-4632.2009.03799.x. [DOI] [PubMed] [Google Scholar]
- 110.Venkataram MN, Bhushnurmath SR, Muirhead DE, Al-Suwaid AR. Frictional amyloidosis: a study of 10 cases. Australas J Dermatol. 2001;42:176–179. doi: 10.1046/j.1440-0960.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- 111.Bandhlish A, Aggarwal A, Koranne RV. A clinico-epidemiological study of macular amyloidosis from north India. Indian J Dermatol. 2012;57:269–274. doi: 10.4103/0019-5154.97662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baxa U. Structural basis of infectious and non-infectious amyloids. Curr Alzheimer Res. 2008;5:308–318. doi: 10.2174/156720508784533367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sugiura K, Muro Y, Futamura K, Matsumoto K, Hashimoto N, Nishizawa Y, et al. The unfolded protein response is activated in differentiating epidermal keratinocytes. J Invest Dermatol. 2009;129:2126–2135. doi: 10.1038/jid.2009.51. [DOI] [PubMed] [Google Scholar]
- 114.Vijaya B, Dalal BS, Sunila, Manjunath GV. Primary cutaneous amyloidosis: a clinico-pathological study with emphasis on polarized microscopy. Indian J Pathol Microbiol. 2012;55:170–174. doi: 10.4103/0377-4929.97853. [DOI] [PubMed] [Google Scholar]
- 115.Garg A, Mahalingam M, Alavian C. Pruritic patches on the back and papules on the legs. Primary localized cutaneous amyloidosis (PLCA) (cutaneous lichen amyloidosis and macular amyloidosis) Arch Dermatol. 2007;143:255–260. doi: 10.1001/archderm.143.2.255-c. [DOI] [PubMed] [Google Scholar]
- 116.Melo BL, Costa IS, Goes Cde A, Tigre CA, André NF. An unusual presentation of macular amyloidosis. An Bras Dermatol. 2011;86:S24–S27. doi: 10.1590/s0365-05962011000700005. [DOI] [PubMed] [Google Scholar]
- 117.Oiso N, Yudate T, Kawara S, Kawada A. Successful treatment of lichen amyloidosus associated with atopic dermatitis using a combination of narrowband ultraviolet B phototherapy, topical corticosteroids and an antihistamine. Clin Exp Dermatol. 2009;34:e833–e836. doi: 10.1111/j.1365-2230.2009.03574.x. [DOI] [PubMed] [Google Scholar]
- 118.Fujisawa T, Shu E, Ikeda T, Seishima M. Primary localized cutaneous nodular amyloidosis that appeared in a patient with severe atopic dermatitis. J Dermatol. 2012;39:312–313. doi: 10.1111/j.1346-8138.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 119.Hudacek KD, Haque MS, Hochberg AL, Cusack CA, Chung CL. An unusual variant of confluent and reticulated papillomatosis masquerading as tinea versicolor. Arch Dermatol. 2012;148:505–508. doi: 10.1001/archdermatol.2011.2812. [DOI] [PubMed] [Google Scholar]
- 120.Ferreira LM, Diniz LM, Ferreira CJM. Confluent and reticulated papillomatosis of Gougerot and Carteaud: report of three cases. An Bras Dermatol. 2008;84:78–81. doi: 10.1590/s0365-05962009000100012. [DOI] [PubMed] [Google Scholar]
- 121.Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): a minocycline-responsive dermatosis without evidence for yeast in pathogenesis. A study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol. 2006;154:287–293. doi: 10.1111/j.1365-2133.2005.06955.x. [DOI] [PubMed] [Google Scholar]
- 122.Scheinfeld N. Confluent and reticulated papillomatosis: a review of the literature. Am J Clin Dermatol. 2006;7:305–313. doi: 10.2165/00128071-200607050-00004. [DOI] [PubMed] [Google Scholar]
- 123.Xia Y, Marquart LN, Gunning ST. What is your diagnosis? Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome) Cutis. 2007;80:184,201-2. [PubMed] [Google Scholar]


