Abstract
Fibrinogen binding to receptors on stimulated platelets is a prerequisite for platelet aggregation. To gain further insight into the role of fibrinogen in platelet aggregation and to identify the platelet fibrinogen receptor, we developed a monoclonal anti-platelet antibody that inhibited platelet aggregation. The purified antibody, designated A2A9, inhibited platelet aggregation stimulated by 10 microM ADP, 10 microM epinephrine, and thrombin at 1 unit/ml without inhibiting platelet shape change or platelet secretion. A2A9 was also a competitive inhibitor of fibrinogen binding to ADP-stimulated platelets. Fifty percent inhibition of fibrinogen binding occurred at 65 nM A2A9. Direct binding studies using radiolabeled A2A9 demonstrated 47,000 A2A9 binding sites on unstimulated platelets, with a dissociation constant of 60 nM. Platelets from two individuals with Glanzmann thrombasthenia bound essentially no A2A9. Therefore, these data support the hypothesis that receptor-bound fibrinogen mediates platelet aggregation. In order to identify the platelet fibrinogen receptor, A2A9 immobilized on agarose was used for affinity chromatography. Two platelet polypeptides with Mr = 140,000 and 93,000 were recovered from the immobilized A2A9. After disulfide reduction, these Mr values were altered to 125,000 and 116,000. The smaller polypeptide was also found to contain the PlA1 antigen. These data localize the epitope recognized by A2A9 to the platelet membrane glycoprotein IIb-IIIa complex and suggest that this complex forms the physiologic platelet fibrinogen receptor.
Full text
PDF
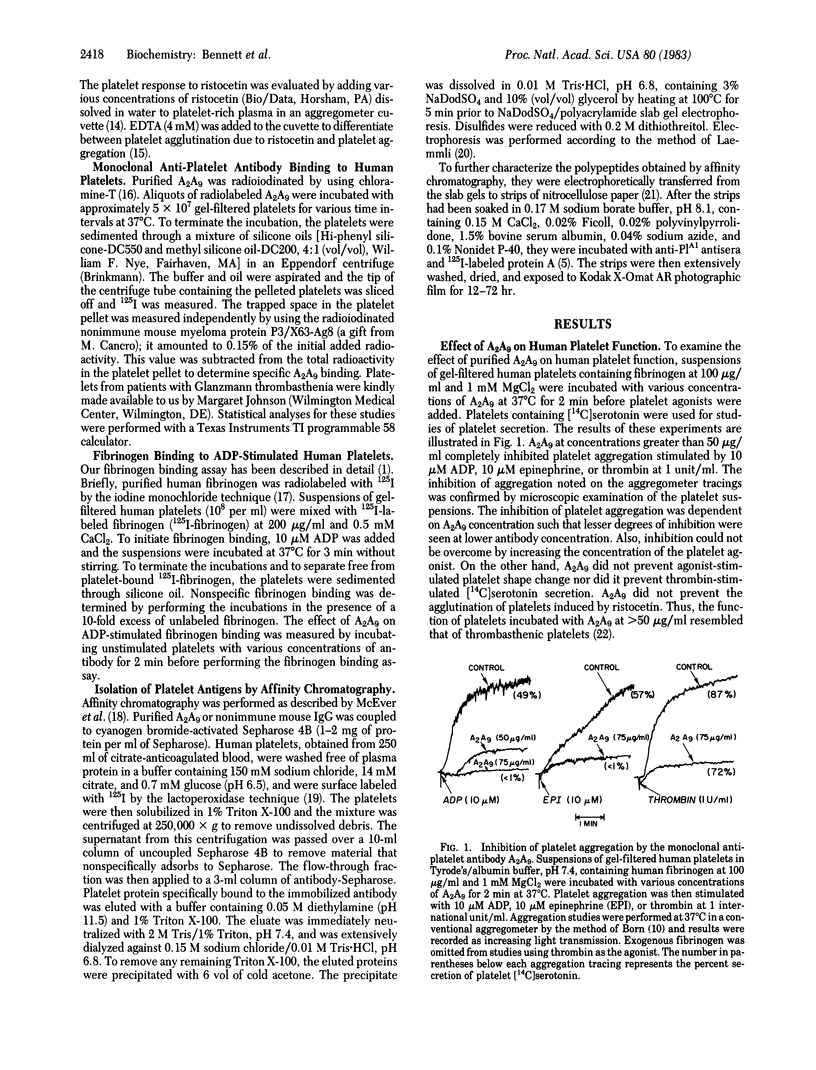
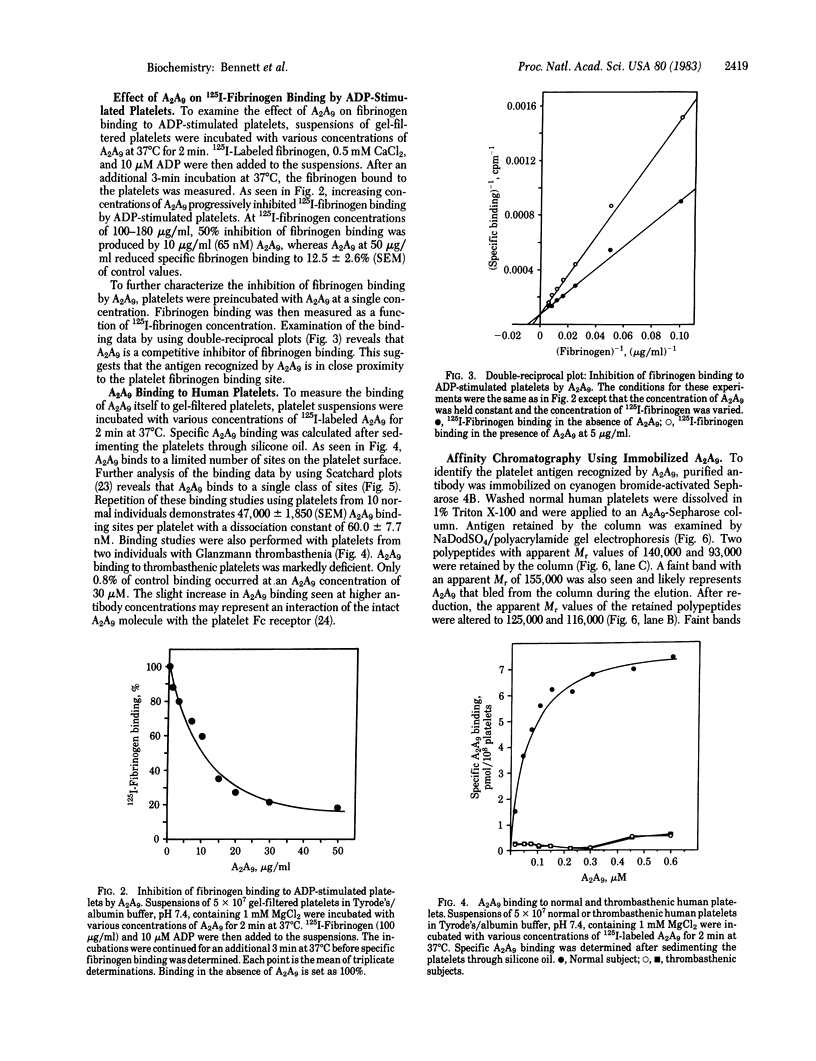
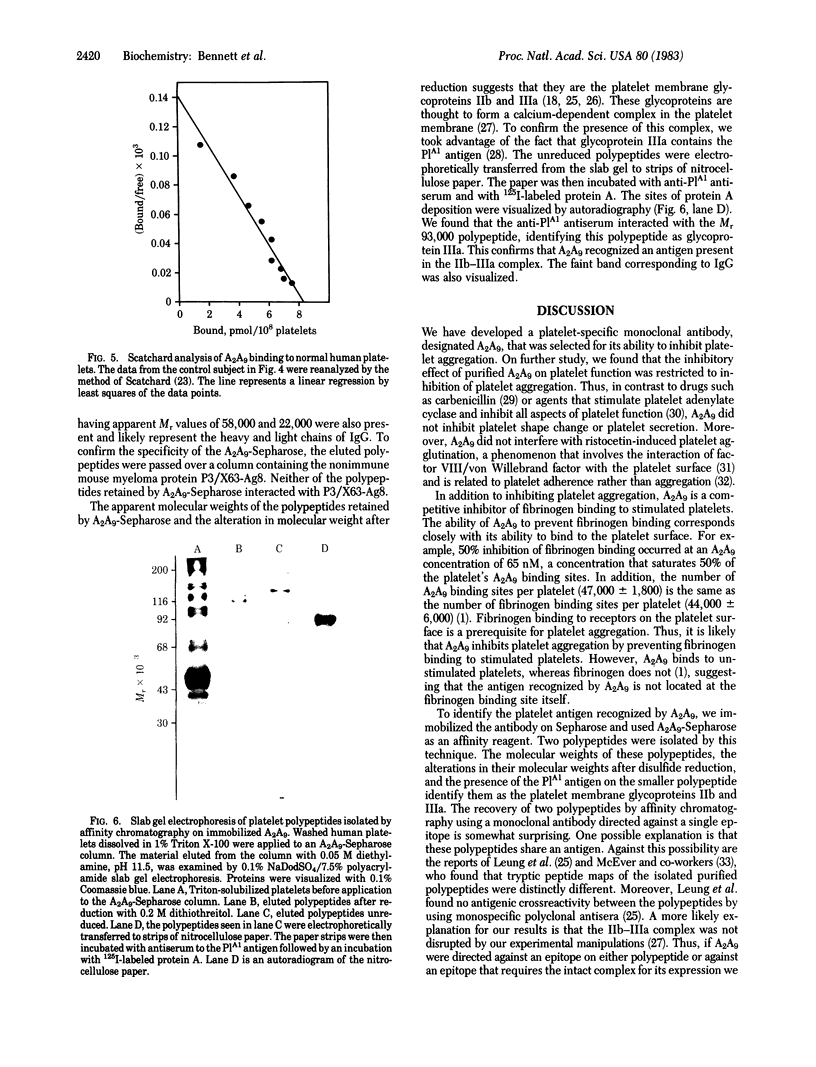

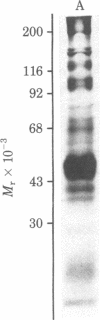
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Cines D. B. Identification of the fibrinogen receptor on human platelets by photoaffinity labeling. J Biol Chem. 1982 Jul 25;257(14):8049–8054. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Spiegelberg H. L. Release of serotonin from human platelets induced by aggregated immunoglobulins of different classes and subclasses. J Clin Invest. 1973 May;52(5):1282–1288. doi: 10.1172/JCI107296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M. A., Firkin B. G. Ristocetin--a new tool in the investigation of platelet aggregation. Thromb Diath Haemorrh. 1971 Oct 31;26(2):362–369. [PubMed] [Google Scholar]
- IZZO J. L., BALE W. F., IZZO M. J., RONCONE A. HIGH SPECIFIC ACTIVITY LABELING OF INSULIN WITH 131-I. J Biol Chem. 1964 Nov;239:3743–3748. [PubMed] [Google Scholar]
- Jerushalmy Z., Zucker M. B. Some effects of fibrinogen degradation products (FDP) on blood platelets. Thromb Diath Haemorrh. 1966 May 15;15(3):413–419. [PubMed] [Google Scholar]
- Kao K. J., Pizzo S. V., McKee P. A. Demonstration and characterization of specific binding sites for factor VIII/von Willebrand factor on human platelets. J Clin Invest. 1979 Apr;63(4):656–664. doi: 10.1172/JCI109348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Aster R. H. Isolation and immunologic characterization of the human platelet alloantigen, P1A1. Mol Immunol. 1979 Jun;16(6):353–360. doi: 10.1016/0161-5890(79)90100-7. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung L. L., Kinoshita T., Nachman R. L. Isolation, purification, and partial characterization of platelet membrane glycoproteins IIb and IIIa. J Biol Chem. 1981 Feb 25;256(4):1994–1997. [PubMed] [Google Scholar]
- Majerus P. W., Brodie G. N. The binding of phytohemagglutinins to human platelet plasma membranes. J Biol Chem. 1972 Jul 10;247(13):4253–4257. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- McEver R. P., Baenziger J. U., Majerus P. W. Isolation and structural characterization of the polypeptide subunits of membrane glycoprotein IIb-IIIa from human platelets. Blood. 1982 Jan;59(1):80–85. [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Phillips D. R. Effect of trypsin on the exposed polypeptides and glycoproteins in the human platelet membrane. Biochemistry. 1972 Nov 21;11(24):4582–4588. doi: 10.1021/bi00774a025. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Bennett J. S., McDonough M., Turnbull J. Carbenicillin and penicillin G inhibit platelet function in vitro by impairing the interaction of agonists with the platelet surface. J Clin Invest. 1980 Feb;65(2):329–337. doi: 10.1172/JCI109676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp T. B., Weiss H. J., Baumgartner H. R. Decreased adhesion of platelets to subendothelium in von Willebrand's disease. J Lab Clin Med. 1974 Feb;83(2):296–300. [PubMed] [Google Scholar]
- Walsh P. N., Gagnatelli G. Platelet antiheparin activity: storage site and release mechanism. Blood. 1974 Aug;44(2):157–168. [PubMed] [Google Scholar]
- Weiss H. J., Rogers J., Brand H. Defective ristocetin-induced platelet aggregation in von Willebrand's disease and its correction by factor VIII. J Clin Invest. 1973 Nov;52(11):2697–2707. doi: 10.1172/JCI107464. [DOI] [PMC free article] [PubMed] [Google Scholar]