Abstract
The development of treatment protocols that result in a complete response to chemotherapy has been hampered by free drug toxicity and the low bioavailability of nano-formulated drugs. Here, we explore the application of temperature-sensitive liposomes that have been formulated to enhance stability in circulation. We formed a pH-sensitive complex between doxorubicin (Dox) and copper (CuDox) in the core of lysolipid-containing temperature-sensitive liposomes (LTSLs). The complex remains associated at neutral pH but dissociates to free Dox in lower pH environments. The resulting CuDox-LTSLs were injected intravenously into a syngeneic murine breast cancer model (6 mg Dox/kg body weight) and intravascular release of the drug was triggered by ultrasound. The entire tumor was insonified for 5 min prior to drug administration and 20 min post drug injection. A single-dose administration of CuDox-LTSLs combined with insonation suppressed tumor growth. Moreover, after twice per week treatment over a period of 28 days, a complete response was achieved in which the NDL tumor cells and the tumor interstitium could no longer be detected. All mice treated with ultrasound combined with CuDox-LTSLs survived, and tumor was undetectable 8 months post treatment. Iron and copper-laden macrophages were observed at early time points following treatment with this temperature sensitive formulation. Systemic toxicity indicators, such as cardiac hypertrophy, leukopenia, and weight and hair loss were not detected with CuDox-LTSLs after the 28-day therapy.
Keywords: Extended survival, Doxorubicin, Temperature-sensitive liposomes, Toxicity, Hyperthermia
1. Introduction
Incorporation of lysolipid in thermally-sensitive liposomal formulations facilitates rapid release of the drug cargo under mild hyperthermia (40–42°C) [1–4]. The intravascular release of small drug molecules and their subsequent diffusion into the tumor can enhance delivery, as compared with strategies that involve the extravasation of large drug carriers such as liposomes through permeable tumor vasculature [5–10]. In addition, hyperthermia augments chemotherapeutic efficacy by enhancing perfusion, extravasation, drug penetration and increasing cell sensitization to the treatment [11–15]. However, the field of stabilized, yet releasable, therapeutics is still in its infancy and the efficacy of this strategy is not yet established [16, 17].
ThermoDox® (doxorubicin-loaded LTSLs) has reached phase III clinical trials for liver cancer and phase II trials in breast cancer recurrence at the chest wall [18, 19]. Through the incorporation of a lysolipid, ThermoDox® was designed to release doxorubicin within the blood pool in response to mild hyperthermia or during extended circulation. Approximately 50% of the drug is released at 60 min post intravenous administration in the absence of hyperthermia [17, 20, 21]. ThermoDox® has been prescribed with a single dose administration at the maximum tolerated dose (MTD) of 50 mg/m2 [22, 23].
Here, we used a different approach, enhancing the circulation stability of the nanoparticles to reduce systemic toxicity with the goal of achieving a nanoparticle that could be applied in multiple administrations over a period of weeks. We previously reported that the formation of a complex between doxorubicin and copper (II) in long-circulating liposomes reduces the toxicity of a long-circulating liposomal formulation similar to Doxil® without affecting the drug efficacy [24]. Small copper-doxorubicin (CuDox) crystals are formed at a neutral pH and liberate free drug in low pH environments [25], whereas fibrous-bundle precipitates of Dox, created by either pH or ammonium sulfate gradient loading methods, form in low pH and dissociate to Dox in neutral pH environments [25–32]. The pH gradient that has been the basis of loading in liposomes is subject to collapse in circulation and results in drug leakage [26].
We demonstrated earlier that CuDox within long circulating liposomes retained the drug longer in circulation and reduced toxicity [24]. As a mechanism for the reduced toxicity, we hypothesized that formation of the copper-doxorubicin complex reduces the reactivity of doxorubicin to available transition metals such as copper or iron, which otherwise would result in the generation of reactive and harmful oxygen radicals [33, 34].
In order to enhance efficacy, we apply the CuDox concept to stabilize doxorubicin in LTSLs. We loaded Dox in LTSLs containing 100 mM copper and an optimized concentration of triethanolamine (TEA) at neutral pH to create a stable CuDox complex. Ultrasound was employed to create real-time, controllable, focused hyperthermia [35, 36]. The resulting combination of CuDox-LTSLs and hyperthermia was tested with a high- and multiple-dosing schedule in order to achieve complete tumor remission in an aggressive mouse model of breast cancer. We demonstrate an enhanced drug pharmacokinetic profile and treatment effectiveness with reduced treatment toxicity.
2. Materials and Methods
A detailed description of the experimental procedures is found in the Supplementary Information.
2.1. Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (MPPC), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (MSPC), and 1,2 distearoyl-sn-glycero-3-phosphoethanolamine-N-Methoxy polyethyleneglycol-2000 (DSPE-PEG2k) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). Copper (II) gluconate, triethanolamine (TEA), ammonium sulfate, and doxorubicin hydrochloride were from Sigma (St. Louis, MO). The neu deletion (NDL) metastatic mammary carcinoma cell line was obtained from the Alexander Borowsky Laboratory (UC Davis) [37, 38].
2.2. Copper liposome preparation, drug loading and in vitro evaluation
Temperature-sensitive liposomes (TSLs) without a lysolipid were composed of DPPC:DSPC:DSPE-PEG2k:cholesterol (65:5:5:25, molar ratio). Lysolipid-containing temperature-sensitive liposomes (LTSLs) were composed of DPPC:DSPE-PEG2k:MPPC (86:4:10, molar ratio). Liposomes were prepared by the hydration method as described previously [24, 39]. Briefly, the dried lipid was hydrated in 0.3 mL of either 250 mM ammonium sulfate (AS) at pH 5.4 or 100 mM copper (II) gluconate including triethanolamine at 540 mM (pH 8.4). The multi-lamellar lipid solution at a final concentration of 50 mg/mL was extruded above the phase transition temperature of the lipid mixture through a polycarbonate membrane with a pore diameter of 100 nm. To induce a salt gradient across the liposomal membrane, ammonium sulfate-loaded or copper/TEA-loaded liposomes were separated from non-encapsulated ammonium sulfate or copper/TEA by passing the extruded liposomal suspension through a spin column of Sephadex G-75 (5 × 1 cm, GE Healthcare, Biosciences, Piscataway, NJ) equilibrated with 20 mM HEPES/150 mM sodium chloride, pH 7.4 (HES) and saline (0.9% sodium chloride), respectively. The liposomal diameters were ~100 nm (115 nm ± 18 nm) as measured using a NICOMP™ 380 ZLS submicron particle analyzer (Particle Sizing System Inc., Santa Barbara, CA). Lipid concentration was measured using the Phospholipids C assay kit (Wako Chemicals USA, Richmond, VA) according to manufacturer’s instructions.
Doxorubicin was added to ammonium sulfate-loaded (ASDox) or copper-loaded liposomes at a drug-to-lipid ratio of 0.2:1 (wt:wt) and incubated at 37°C for 1.5 h. Doxorubicin-loaded liposomes using the ammonium sulfate method were then separated from non-encapsulated Dox using Sephadex G-75 spin columns equilibrated with HES, whereas liposomal copper-doxorubicin with 100% loading efficiency was used without separation.
The efficacy of the liposomal formulations was first evaluated in vitro for either 50h of continuous incubation or a 30 min incubation with drug on ice followed by two rinses and a 24h incubation in media in the absence of the drug
2.3. Experimental protocol
All animal experiments were conducted under a protocol approved by the University of California, Davis, Animal Care and Use Committee (IACUC). In efficacy studies, a total of 46 NDL-tumor bearing mice were studied among which 23 mice were implanted with bilateral tumors and 23 mice with unilateral tumors for a survival study. Mice were randomized among several groups including drug treatment with ultrasound, ultrasound only, drug treatment only and no treatment. Mice bearing uni- or bilateral NDL tumors of ~4 mm (≥30 mm3) in longitudinal diameter were injected intravenously with liposomal doxorubicin (~6 mg Dox/kg body weight and ~30 mg lipid/kg body weight) twice a week with a total Dox injected dose of 267 mg/m2 over 4 weeks and compared to the control animals that received saline. For animals in the drug treatment with ultrasound and ultrasound-only groups, one tumor per animal was insonified for 5 min at 42°C prior to administration of drug and saline, respectively; the tumor insonation was continued for an additional 20 min at 42°C post injection. A custom dual-mode linear array transducer coupled with a conventional ultrasound scanner (Sonoline Antares, Siemens Medical Systems, Inc., Issaquah, WA) was used for generating mild hyperthermia in the tumor regions of interest. The ultrasound pulses consisted of 100-cycle bursts at 1.54 MHz center frequency and 1.1 MPa peak negative pressure, with a variable pulse-repetition frequency (PRF) ranging from 100 Hz up to 5 kHz.
2.4. In vivo multi-spectral fluorescence imaging
Mice (n=102) with and without NDL tumors were imaged in vivo and ex-vivo with hyperspectral optical techniques to assess the blood pharmacokinetics and to quantify drug release and accumulation of free and liposomal doxorubicin (each 6 mg/kg, including the liposomal formulations as above).
3. Results
3.1. In vitro protocol development
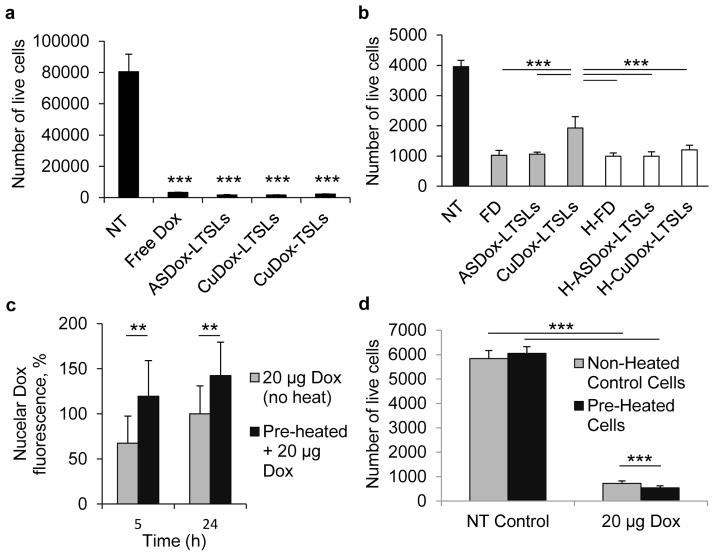
Cytotoxicity of the stabilized liposomal copper-doxorubicin in TSLs and LTSLs was tested via an MTT assay in vitro in NDL cells with and without heat-triggered release and compared with ASDox-LTSLs and free drug. CuDox-LTSLs and ASDox-LTSLs incubated with NDLs at a Dox concentration of 20 μg/mL media for 50 h at 37°C produced more than 95% cell death, similar to free drug (Figure 1a). Cell viability assays were also performed under conditions that reduced cellular particle uptake by incubating cells at 4°C for 30 min in the presence of drug followed by 24 h incubation at 37°C in the absence of drug. Under these conditions, liposomal copper-doxorubicin, CuDox-LTSLs, and ASDox-LTSLs, activated by heat (42°C for 15 min) prior to the addition of the drug to the cells, exerted similar levels of cytotoxicity compared to free drug (Figure 1b).
Figure 1. In vitro cytotoxicity of CuDox-LTSLs and the heat-drug sequencing.
a–b) Number of viable NDL cells treated with free (FD) or liposomal doxorubicin in TSLs (CuDox) and LTSLs (ASDox, CuDox) at 20 μg Dox/mL media for a) 50h under continuous incubation and b) a 30 min incubation with drug on ice followed by a rinse and a 24h incubation in media in the absence of the drug. H-FD, H-ASDox-LTSLs, and H-CuDox-LTSLs represent free (FD) and liposomal drug in LTSLs (ASDox, CuDox) heated prior to incubation with cells. c) Doxorubicin fluorescence in the nucleus at 5 h and 24 h. d) Number of live cells at 24 h after an initial incubation of preheated (5 min at 42°C) or non-heated NDL cells with and without the addition of 20 μg Dox/mL media for 30 min followed by rinsing in media and incubation in media in the absence of doxorubicin. Statistical analyses were performed using one-way ANOVA followed by the Tukey Post Hoc test (b) and Student’s t-test (a, c, d). **p < 0.01, ***p<0.001.
The timing of the application of heat relative to drug administration was then optimized in vitro using NDL cells. Pre-heating cells at 42°C for 5 min prior to addition of drug resulted in a 1.5- and 1.3-fold increase in nuclear accumulation of doxorubicin after 5 h and 24 h, respectively (Figure 1c, p<0.01), and consequently a 1.4-fold increase in drug cytotoxicity compared to non-heated control cells (Figure 1d, p<0.001). Pre-heating of NDL cells at 42°C for 5 min had no effect on cell viability in the absence of drug, but significantly sensitized cells to doxorubicin (Figure 1d, p<0.001). Based on this result, we applied 42°C hyperthermia to NDL tumors for 5 min prior to drug injection in subsequent studies.
3.2. Doxorubicin loading and stability
Previously we reported that doxorubicin loading into cholesterol-containing long circulating liposomes (LCLs) was dependent on both intraliposomal concentrations of copper (II) gluconate and triethanolamine. For long circulating liposomes, a copper gluconate concentration of 100 mM reached 100% loading at 270 mM TEA (pH 7.4) with a drug/lipid ratio of 0.2 mg/mg following overnight incubation at 37 °C. For LTSLs, the application of 100 mM copper gluconate and 270 mM TEA (pH 7.4) loaded 0.2 mg doxorubicin per mg lipid in 90 min at 37 °C (Figure 2a). However, we tested the resulting CuDox-LTSLs in vivo (data not shown) and found that higher concentrations of TEA (≥540 mM) and a higher pH during loading are required to enhance drug retention in circulation. Therefore, for MPPC-containing liposomes, intraliposomal concentrations of 100 mM copper gluconate and 540 mM TEA at pH 8.4 were used for all in vivo and in vitro studies. In contrast, drug loading in LTSLs using the ammonium sulfate gradient was limited to 50% when using HES (pH 7.4) and 30% in saline (Figure 2a).
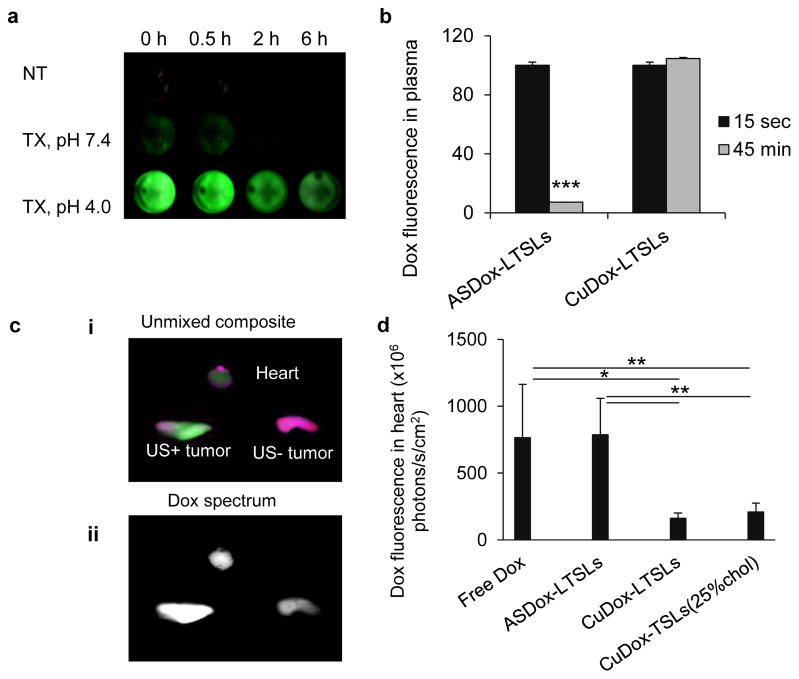
Figure 2. Doxorubicin loading and stability in LTSLs.
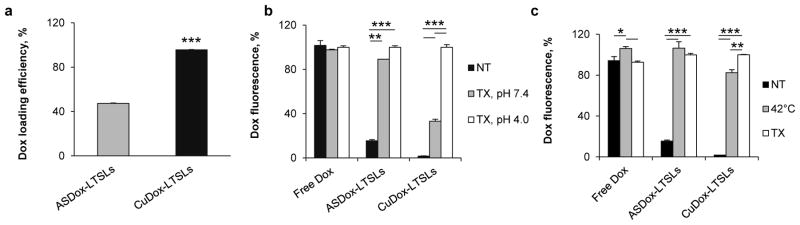
a) Loading efficiency of Dox in ASDox-LTSLs and CuDox-LTSLs. Doxorubicin was incubated with LTSLs at Dox:lipid, 0.2:1 wt/wt. ASDox-LTSLs were incubated in 20 mM HEPES/150 mM sodium chloride at pH 7.4. CuDox-LTSLs were incubated in 100 mM copper gluconate and 540 mM TEA at pH 8.4. b) Doxorubicin fluorescence after 30 min incubation of free Dox, ASDox-LTSLs, or CuDox-LTSLs with: 20 mM HEPES/150 mM sodium chloride at pH 7.4 and room temperature (NT), 0.25% Triton X-100 in 20 mM HEPES/150 mM sodium chloride at pH 7.4 and 42 °C (TX, pH 7.4) or 20 mM citrate buffer/150 mM sodium chloride at pH 4.0 and 42 °C (TX, pH 4.0). c) Doxorubicin fluorescence of free Dox, ASDox-LTSLs, and CuDox-LTSLs in three studies: 1) at pH 7.4 and room temperature (NT), 2) 10 min incubation at 42 °C at pH 4.0 in the absence of Triton X-100 (42 °C), or 3) 30 min incubation with 0.25% Triton X-100 at 42 °C and pH 4.0 (TX). Each is shown as a percentage of the fluorescence measured with 0.25% Triton X-100 at pH 4.0. Statistical analyses were performed using Student’s t-test (a) and one-way ANOVA followed by the Tukey Post Hoc test (b, c). *p < 0.05, **p < 0.01, ***p<0.001.
The release of CuDox from LTSLs was first evaluated in vitro in HES buffer (pH 7.4) and mouse serum. When diluted 100-fold, the fluorescence intensity of doxorubicin remained quenched in both ASDox-LTSLs and CuDox-LTSLs, as compared to the fluorescence of free drug. Disintegration of the liposomes using Triton X-100 in 30 mM EDTA resulted in nearly 100% release of doxorubicin from ASDox-LTSLs; however, the fluorescence of CuDox-LTSLs remained quenched (Figure 2b). The doxorubicin fluorescence was restored by lowering the pH which dissociated copper and doxorubicin, resulting in more than 90% release of the drug. The results demonstrate that the copper-doxorubicin complex remains associated post release at neutral pH in the presence of EDTA, but dissociates in low pH environments. Similar results were obtained for both formulations when incubated in mouse serum (data not shown). Activation of the liposomes by heat was then studied in vitro at 42°C in the absence of Triton X-100 in 20 mM citrate-buffered saline, pH 4.0. The release of doxorubicin was quantified as 100% and 83% from ASDox-LTSLs and CuDox-LTSLs after 10 min incubation at 42°C, respectively (Figure 2c), and 100% for both formulations when incubated under the same conditions in mouse serum (data not shown).
A preliminary in vitro study of intracellular trafficking was also conducted in NDL cells (data not shown). We observed that the fluorescence of the copper-doxorubicin complex was restored after the complex entered lysosomes during intracellular trafficking (data not shown).
3.3. CuDox-LTSLs are far more stable in circulation than ASDox-LTSLs
The fluorescence of doxorubicin in plasma isolated from mouse blood immediately after the administration of CuDox-LTSLs remained quenched (Figure 3a). In the isolated plasma, digestion of the circulating intact liposomes by Triton X-100 at 37 °C for 30 min did not change the fluorescence intensity. However, the fluorescence was fully restored by reducing the pH to 4, confirming our previous in vitro observation that drug is released as a stable CuDox complex in blood and liberation of free drug occurs in response to reduced pH values (Figure 3a). The fluorescence of CuDox in MPPC-containing liposomes was unchanged over the first 45 min (Figure 3b) and decreased to 40% and 20% of the initial value at 2 h and 6 h, respectively (Supplementary Figure 1). In contrast, the fluorescence decreased to ~10% of the initial value in blood isolated 45 min post administration of ASDox-LTSLs (Figure 3b).
Figure 3. Blood stability of CuDox-LTSLs.
a) Dox fluorescence in plasma isolated from mice at 0, 0.5, 2 and 6 h post injection of CuDox-LTSLs and incubated for 30 min with either 20 mM HEPES/150 mM sodium chloride at pH 7.4 and room temperature (NT), 0.25% Triton X-100 in 20 mM HEPES/150 mM sodium chloride, pH 7.4, at 37 °C (TX, pH 7.4), or 20 mM citrate buffer/150 mM sodium chloride, pH 4.0, at 42 °C (TX, pH 4.0). Green indicates Dox fluorescence. b) Dox fluorescence intensity in plasma isolated from mouse blood after 15 sec and 45 min post injection of either ASDox-LTSLs or CuDox-LTSLs at a Dox concentration of 6 mg/kg body weight. c) Ex-vivo images of the heart and tumors of a mouse treated with ASDox-LTSLs combined with insonation of the left tumor at 42 °C for 20 min post drug injection. Images were acquired immediately after ultrasound and ~30 min post drug administration and are presented as an unmixed composite (i) of doxorubicin fluorescence in green and background in purple and (ii) as the Dox spectrum alone showing Dox fluorescence intensity (white indicates higher fluorescence intensity). d) Dox accumulation in the mouse heart quantified 30 min post administration of free Dox, ASDox-LTSLs, CuDox-LTSLs, and CuDox-TSLs. Statistical analyses were performed using Student’s t-test (b) and one-way ANOVA followed by the Tukey Post Hoc test (d). *p < 0.05, **p < 0.01, ***p<0.001.
We assessed the systemic stability of drug in ASDox-LTSLs and CuDox-LTSLs post tail vein injection in mice bearing bilateral NDL tumors. One tumor was insonified bringing the local temperature to 42 °C for 20 min. Saline-perfused hearts were imaged immediately after tumor insonation (Figure 3c). The magnitude of the cardiac Dox fluorescence intensity for mice treated with ASDox-LTSLs was similar to those treated with free Dox and 5-fold higher than those of CuDox-LTSLs (Figure 3d).
3.4. Doxorubicin fluorescence validates delivery
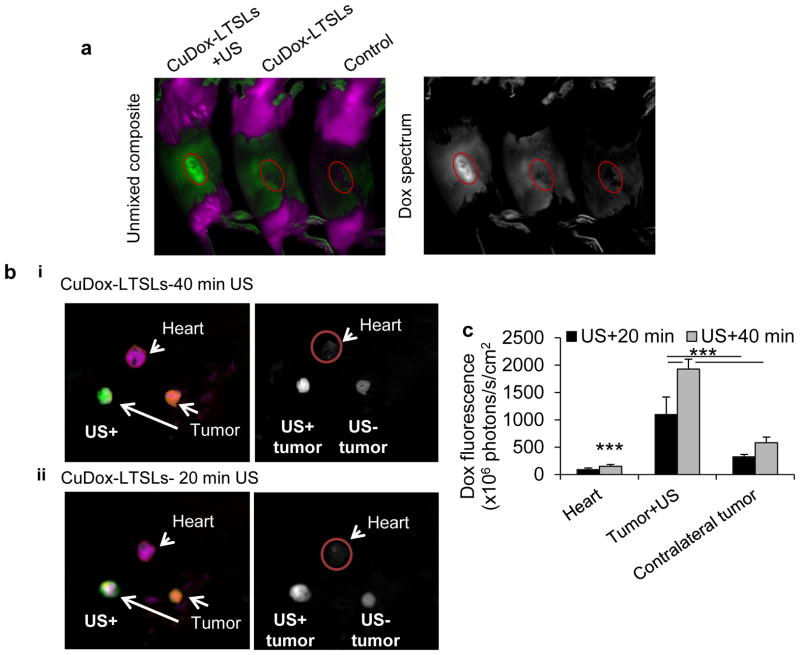
Hyperspectral optical imaging validated the increased delivery of doxorubicin to the CuDox-LTSL injected and insonified tumors (Figure 4). Doxorubicin fluorescence was greater in the tumors treated with CuDox-LTSL and 20 minutes of US, as compared with mice treated with CuDox-LTSL or saline without ultrasound (Figure 4a). For CuDox-LTSLs, doxorubicin fluorescence in the insonified tumors was 3-fold higher in the insonified tumors as compared with the contralateral, and the fluorescence in the heart was low (Figure 4b). a 30 min incubation with drug on ice followed by two rinses and a 24h after incubation in media in the absence of the drug
Figure 4. Dox fluorescence in tumors as a validation of triggered release of drug by ultrasound-mediated hyperthermia.
a–b) Hyperspectral optical imaging presented as both unmixed composite (left panels) of Dox fluorescence (green) and background (purple) and in Dox spectrum (right panels) with white indicating Dox fluorescence intensity (white indicates higher fluorescent intensity). a) In vivo images of NDL tumor-bearing mice injected with CuDox-LTSLs combined with insonation of right tumor at 42 °C for 5 min prior to and 20 min after injection (left) and without insonation (middle) and the control mouse injected with saline (right). Image was acquired ~30 min after injection, b) Ex-vivo images of the heart and tumors of a mouse treated with CuDox-LTSLs combined with insonation of one tumor (left) at 42 °C for 5 min prior to and either 40 min (i) or 20 min (ii) post injection. Images were acquired immediately after ultrasound and ~30 min post drug administration. c) Dox accumulation in mouse heart and tumors quantified after ~30 min post injection of CuDox-LTSLs. Dox was administrated at a concentration of 6 mg/kg body weight. Statistical analyses were performed using one-way ANOVA followed by the Tukey Post Hoc test. ***p<0.001.
We then studied the effect of 20 or 40 minutes of post-injection insonation on drug delivery to the insonified tumor and drug accumulation in the heart. Increasing the duration of ultrasound from 20 min to 40 min resulted in a 1.7-fold increase in the fluorescence signal in insonified tumors without a significant increase in contralateral tumors (Figure 4c). Dox fluorescence in the heart increased slightly with the increase in the time of insonation but remained lower than that observed in mice injected with ASDox-LTSLs (Figures 4b, 4c, 3c, 3d).
3.5. Systemic toxicity of CuDox-LTSLs
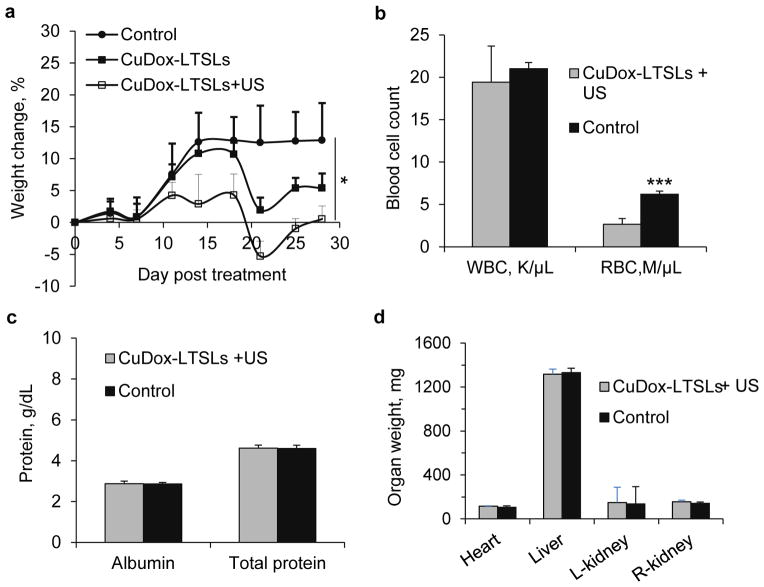
We then evaluated systemic toxicity resulting from multiple administrations of CuDox-LTSLs over a 28-day course of therapy. Thirty-four mice with unilateral tumors were randomly distributed among three treatment groups of “drug+US”, “drug only”, and “control” where treatment was repeated twice a week for 28 days. All animals showed a similar pattern of weight gain in the 1st week of treatment (Figure 5a); however, in the 2nd and later weeks of treatment the “drug +US” and “drug only” treated groups did not continue to gain weight. By the end of the 4-week therapy, the net weight gain was 0%, 5% and 15% for “drug+US” (p<0.05), “drug only”, and “control” mice, respectively. After the termination of therapy, mice in the “drug+US” and “drug only” groups gained weight and “drug+US” mice remain healthy 8 months following the conclusion of treatment.
Figure 5. Toxicity of CuDox-LTSLs assessed over 28-day administration of 6 mg/kg (33.4 mg/m2) twice per week (total of 266.7 mg/m2).
a) Weight change of NDL tumor bearing mice treated with CuDox-LTSLs with tumor insonation at 42 °C for 5 min prior to and 20 min post injection (CuDox-LTSLs+US), CuDox-LTSLs without ultrasound (CuDox-LTSLs) or saline injection (control) for 28 days. For b–d all tests involve CuDox-LTSLs+US or saline injection. b) White and red blood cell counts, c) protein (albumin and total protein) measurement, and d) organ weights. Statistical analyses were performed by Student’s t-test. *p<0.05, ***p<0.001.
After the 28-day therapy, the white blood cell counts for mice treated with CuDox-LTSLs and control mice were similar, but red blood cell counts for mice treated with CuDox-LTSLs (2.7±0.7 M/μL) were lower than for control mice (6.2±0.4 M/μL) (Figure 5b). Differential white blood cell counts were within the normal range for both CuDox-LTSLs-treated and control mice (data not shown). Circulating albumin and total protein were similar in CuDox-LTSLs-treated and control mice (Figure 5c) as were the levels of other types of blood proteins (data not shown).
Hypertrophy of heart, liver, and kidneys was not observed after 28 days of treatment with CuDox-LTSLs+US (Figure 5d). Dox fluorescence of organs and tissues dissected at the end of the 28-day therapy (four days after the last administration of CuDox-LTSLs and tumor insonation (25th day of treatment)) showed drug accumulation in tumors and organs associated with clearance, such as the spleen, liver and kidneys, but not in the heart and skin (Supplementary Figure 2).
3.6. In vivo efficacy of combination of CuDox-LTSLs and mild hyperthermia
We assessed the efficacy of CuDox-LTSLs combined with local hyperthermia to treat NDL tumors transplanted in mice uni- or bilaterally (n=40) with eight administrations of CuDox-LTSLs over 4 weeks. Control mice survived 16 days and tumor insonation in the absence of drug did not affect tumor growth rate (Figure 6a). Tumor growth was suppressed in mice treated with CuDox-LTSLs, extending survival to 28 days. However, the effect was further enhanced when the drug was combined with ultrasound (5 minutes before and 20 minutes after drug injection at 42 °C). In this group, at the 28 day time point, tumor volume had on average slightly increased with complete tumor regression in a subset of animals. The remaining tumors continued to regress and disappeared within a week after termination of the therapy, resulting in 100% complete regression (Figure 6a, Supplementary Figure 3). Over the period of 8 months since treatment, tumor regrowth and recurrence were not observed in mice that received a total of eight administrations of CuDox-LTSLs combined with ultrasound (Figure 6b).
Figure 6. In vivo treatment efficiency including ultrasound (US), and CuDox-LTSLs with and without US in NDL tumor mice.
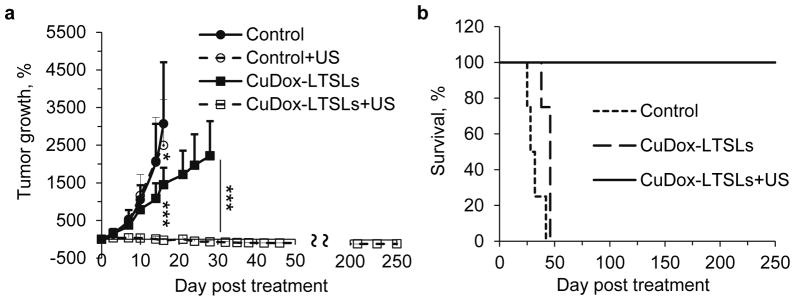
a) Tumor growth as a function of days post-treatment over 28-day treatment cycle presented as percent tumor growth. Initial tumor diameter was ~4 mm. Each mouse was injected intravenously with either saline or liposomal doxorubicin (~6 mg doxorubicin/kg body weight equivalent to ~33 mg/m2) and compared to control animals that received iv injection of saline. For treatment with ultrasound-mediated hyperthermia, one tumor per animal was insonified at 42°C for 5 min prior to and 20 min post-injection. b) Kaplan-Meier survival plot. Statistical analyses were performed using one-way ANOVA followed by the Tukey Post Hoc test. *p<0.05 and ***p<0.001.
3.7. Histology confirmed the treatment efficacy
Figure 7a compares the histological sections of tumors obtained from mice with bilateral NDL tumors and euthanized either after 16 days or at the end of the 28-day treatment. Tumors from the control mice that survived 16 days showed tightly packed and viable cancer cells similar to tumors treated with CuDox-LTSLs after 28 days of treatment.
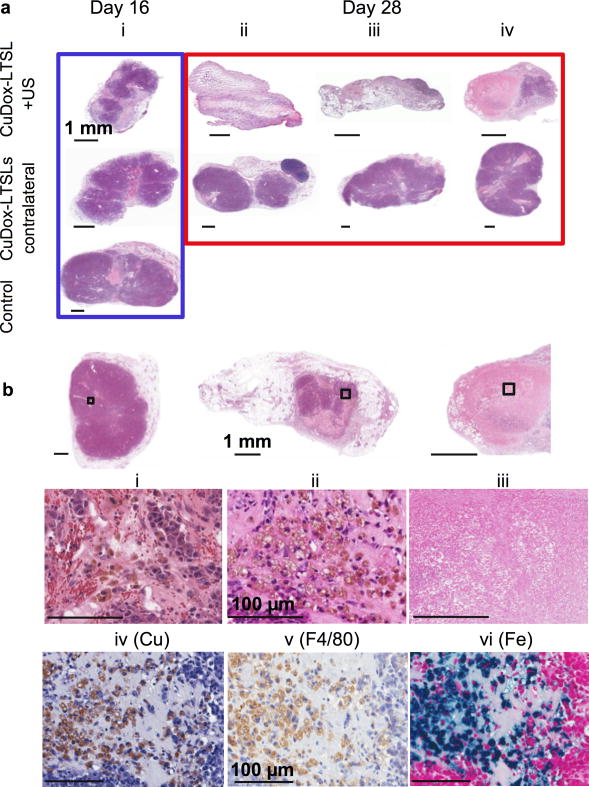
Figure 7. Histology of tumors treated with CuDox-LTSLs.
a) H&E of NDL tumors treated with CuDox-LTSLs with and without ultrasound post 16 (i, upper and middle images) and 28 days of treatment (ii-iv upper and middles images) compared to control at 16 days post intravenous injection of saline (i, lower image). For treatment CuDox-LTSLs combined with ultrasound, tumors were insonified at 42°C for 5 min prior to and 20 min post injection. b) H&E images of the whole tumor sections (upper panels) and the magnified views enclosed by black boxes (middle panels) post drug release by US at the early (i), middle (ii), and end of the 28-day therapy (iii). Additional sections of tumor (ii) were stained with rhodanine (iv), anti-mouse F4/80 (v), and Prussian blue (vi) to identify copper, macrophages, and iron, respectively. The scale bars correspond to 1 mm (a and upper panels of b) and 100 μm (middle and lower panels of b).
In contrast, tumors from the mice treated with CuDox-LTSLs combined with US-mediated hyperthermia were much smaller than measured with ultrasound and revealed distinct morphologies. CuDox-LTSL+US treatment for 16 days resulted in significant regression of the insonified tumor with a small volume of viable and apoptotic tumor cells remaining (Figure 7a-i). Continuation of treatment for the entire 28 days resulted in complete elimination of both the tumor and interstitium in some mice (Figure 7a-ii) or a small rim of seemingly viable tumor (Figures 7a-iii, 7a-iv).
Further, in the early stage of treatment, a magnified section of a closely packed tumor (6.897 mm in length) showed the presence of extravascular red blood cells (Figure 7b-i, red in color) along with released hemoglobin (pink), accompanied by macrophage-like cell populations containing brown pigment and seemingly associated with tumor cells (Figure 7b-ii). By repeating the treatment for 2 weeks, the outline of the tumor was reduced to 2.5 mm and the tumor contained only a few regions of viable tumor cells (Figure 7b-iii). By the end of the 4-week therapy, the tumor boundary had either disappeared or was filled with cell-lysate fluid and few or no viable tumor cells (Figure 7b-iii). Mouse macrophages were identified with anti-F4/80 (Figure 7b-v). Further staining of the histologic section with Prussian blue demonstrated abundant iron pigment in the macrophage cytoplasm, identifying them as hemosiderin-laden macrophages (Figure 7b-vi). These hemosiderin-laden macrophages stained with rhodanine also demonstrated a considerable deposition of copper (Figure 7b-iv). Hemosiderin-laden macrophages were not observed in our previous work with long circulating CuDox liposomes combined with ultrasound [24].
4. Discussion
To our knowledge, we show here for the first time the complete regression of a highly proliferative cancerous tumor via a uniquely-stabilized CuDox-LTSLs+US treatment strategy. A 4-week therapy was designed with twice per week intravenous administration of CuDox-LTSLs, combined with an ultrasound strategy that created a temperature of 42°C for 5 min prior to and 20 min post drug injection. Doxorubicin was encapsulated in lysolipid-containing liposomes with a lipid formulation developed by Mills and Needham [40], where the major difference in the liposomal particle applied here resulted from the copper-doxorubicin crystal with the enhanced drug stabilization. The LTSL formulations can release drug contents at high rates upon heating at ~40.5°C and ~41.3°C by incorporation of 10 mol% of MPPC or MSPC, respectively.
Comparing histological sections of tumors after two or four weeks of CuDox-LTSLs+US therapy confirms the efficacy and highlights the importance of multiple treatments to eliminate rapidly growing cancerous tumors. The first dose of CuDox-LTSLs, when combined with US, suppressed tumor growth significantly compared to those in the CuDox-LTSLs and the control groups, but did not eliminate tumors. Repeating the treatment twice a week effectively regressed tumors in the CuDox-LTSLs+US group to 30% of the initial average volume after 28 days. Upon termination of the treatment, the treated tumors in the CuDox-LTSLs+US group continued to regress without treatment and completely disappeared 53 days post treatment, without recurrence for 8 months post treatment. Neither hyperthermia alone nor CuDox-LTSLs alone produced a complete response.
The small numbers of tumor cells remaining at the tumor margin after the 4-week therapy could reflect the ultrasonic half-power beam profile which is slightly less than the tumor area in cross section; the beam profile will be increased in future work. Interestingly, the remaining tumor fractions resolved over time without further treatment.
Along with the finding that the repeated treatment eliminated all viable tumors, we were surprised to find that within 4 weeks after the conclusion of treatment (and 8 weeks from the start of treatment) the tumor interstitium also could no longer be detected. Earlier in treatment, we observed hemorrhage and the presence of hemosiderin-laden and copper-laden macrophages. In our previous studies of free doxorubicin and combinations of ultrasound and CuDox in long circulating liposomes, we have never observed the metal-laden macrophages within the tumor; however, this study differs in that the drug is released within the hyperthermic tumor vasculature. Based on these observations, we hypothesize that drug release from CuDox-LTSLs within the heated tumor vasculature triggers an endothelial cell injury, which in turn enhances hemorrhage. Persistent erythrocyte extravasation results in phagocytosis of debris by hemosiderin-laden macrophages. Sindrilaru et al. have recently shown that with iron-overloading due to continuing erythrocyte extravasation and leukocyte trapping in tissue, an unrestrained proinflammatory M1 macrophage population may occur [41]. The M1 macrophage population, particularly in the presence of copper [II], has the potential to release high concentrations of TNF-α and hydroxyl radicals, which lead to enhanced DNA damage and tissue breakdown. The role of this copper and iron-induced macrophage population in the mechanisms underlying the anti-tumor response merits further investigation.
With the incorporation of lysolipids, the onset of release of drug occurs more rapidly at ~38°C for MPPC and ~39°C for MSPC than for DPPC:DSPE-PEG2k liposomes, resulting in low drug retention and high systemic toxicity. Despite the lower stability of MPPC at body temperature in LTSLs compared to MSPC (the lysolipid currently used in ThermoDox®), we chose to use MPPC in our liposomal formulation. Our rationale was that the lower melting temperature of MPPC would allow the mouse body temperature to be more readily maintained at 37 °C during tumor heating with ultrasound, which is highly technically challenging in small animal models. Furthermore, in a clinical setting, low-temperature hyperthermia (40–42 °C) is preferred in order to reduce side effects and facilitate a longer treatment that could enhance delivery [42].
Drug loading was maximized with a combination of 0.2 mg Dox/mg lipid with 100 mM copper and 270 mM TEA. Loading was 2-fold higher using the copper doxorubicin formulation as compared with the ammonium sulfate loading method. The copper-doxorubicin complex was previously confirmed by electron microscopy to form diffuse dots uniformly distributed in the core of liposomes, as opposed to the needle-like crystals doxorubicin forms in the absence of copper [24]. The resulting CuDox-LTSLs were responsive to heat similar to ASDox-LTSLs, but released drug in the form of the copper-Dox complex instead of free Dox in a neutral pH buffer. The complex remained associated even in the presence of EDTA, a metal chelator. Doxorubicin was freed from copper in a low pH environment and thus, fluorescence was restored.
Multispectral in vivo imaging allowed us to track drug delivery in tumors and organ/tissue accumulation using doxorubicin fluorescence. Liposomal copper-doxorubicin retained drug longer in circulation compared to ASDox-liposomes: 100% Dox in circulation compared to less than 10% Dox in circulation after 45 min, respectively. The systemic release of doxorubicin is a factor in acute cardiotoxicity, the primary dose-limiting toxicity reported for this drug[43]. The in vivo and ex vivo images acquired post administration of free or liposomal doxorubicin followed by insonation of one tumor per animal verified efficient drug delivery in insonified tumors compared to control tumors for both ASDox and CuDox liposomes. Further, accumulation of doxorubicin in the heart was significantly higher for ASDox-liposomes (similar to free drug) as compared to that of CuDox-liposomes.
We measured blood transit time in NDL tumors using microbubble contrast agents and ultrasound contrast imaging and found that the average time interval for a circulating microbubble to travel through a 5 mm NDL tumor is 10 seconds (data not shown). From Positron Emission Tomography (PET) data, the vascular volume of NDL tumors is estimated to be ~ 0.05 mL/g-tumor [44]. Assuming a 1.5-mL blood volume for a 20-g mouse and ~50% drug release during each 10-second passage of blood in the insonified tumor, we estimate that the tumor blood volume is refreshed 120 times during the 20 minute treatment. Thus, a 0.75 mL blood volume was exposed to heat and a minimum of 30 μg of doxorubicin released into the tumor (25% injected dose).
We monitored toxicity of liposomal CuDox in mice over the course of a 28-day therapy with twice-a-week administration of liposomal CuDox at 6mg Dox/kg animal body combined with insonation of one tumor. Animal weight loss, fur loss, and skin rash were not observed for mice treated with CuDox-LTSLs+US after 28-days of treatment compared to control mice, whereas these symptoms were previously observed for long circulating liposomes using ammonium sulfate loading [24]. The complete blood cell count at the end of therapy did not show leukopenia, but did show anemia for CuDox-LTSLs+US compared to control mice. The circulating albumin and total proteins were not altered by CuDox-LTSLs+US treatment. Organ hypertrophy was not detected for the treated animals. and accumulation was not significantly increased in the heart and skin compared to control (saline-injected) mice.
5. Conclusions
We loaded a stable copper-doxorubicin complex within LTSLs, rather than free drug alone, and demonstrated improved pharmacokinetics and reduced systemic toxicity. With minimal toxicity, we created an efficacious therapy through repeated drug administration over a multi-week regimen, combined with whole-tumor ultrasound-mediated hyperthermia to trigger intravascular release of drug. A complete response to the treatment was obtained in aggressive murine breast tumors and all mice have remained tumor free 8 months post treatment.
Supplementary Material
Acknowledgments
Funding was provided by NIHR01CA134659 and NIHR01CA103828.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Azadeh Kheirolomoom, Email: akheirolomoom@ucdavis.edu.
Chun-Yen Lai, Email: cylai@ucdavis.edu.
Sarah M. Tam, Email: smjohns@ucdavis.edu.
Lisa M. Mahakian, Email: lmmahakian@ucdavis.edu.
Elizabeth S. Ingham, Email: esingham@ucdavis.edu.
Katherine D. Watson, Email: kdwatson@ucdavis.edu.
References
- 1.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- 2.Koning GA, Eggermont AMM, Lindner LH, ten Hagen TLM. Hyperthermia and Thermosensitive Liposomes for Improved Delivery of Chemotherapeutic Drugs to Solid Tumors. Pharmaceutical Research. 2010;27:1750–1754. doi: 10.1007/s11095-010-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- 4.Yarmolenko PS, Zhao YL, Landon C, Spasojevic I, Yuan F, Needham D, Viglianti BL, Dewhirst MW. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int J Hyperth. 2010;26:485–498. doi: 10.3109/02656731003789284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. In: Weber G, editor. Advances in Enzyme Regulation. Vol. 41. Pergamon-Elsevier Science Ltd; Oxford: 2001. pp. 189–207. [DOI] [PubMed] [Google Scholar]
- 7.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Ponce AM, Viglianti BL, Yu DH, Yarmolenko PS, Michelich CR, Woo J, Bally MB, Dewhirst MW. Magnetic resonance imaging of temperature-sensitive liposome release: Drug dose painting and antitumor effects. Journal of the National Cancer Institute. 2007;99:53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 9.Viglianti BL, Ponce AM, Michelich CR, Yu DH, Abraham SA, Sanders L, Yarmolenko PS, Schroeder T, MacFall JR, Barboriak DP, Colvin OM, Bally MB, Dewhirst MW. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn Reson Med. 2006;56:1011–1018. doi: 10.1002/mrm.21032. [DOI] [PubMed] [Google Scholar]
- 10.Tailor TD, Hanna G, Yarmolenko PS, Dreher MR, Betof AS, Nixon AB, Spasojevic I, Dewhirst MW. Effect of Pazopanib on Tumor Microenvironment and Liposome Delivery. Mol Cancer Ther. 2010;9:1798–1808. doi: 10.1158/1535-7163.MCT-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karino T, Koga S, Maeta M. Experimental studies of the effects of local hyperthermia on blood flow, oxygen pressure and pH in tumors. Japanese Journal of Surgery. 1988;18:276–283. doi: 10.1007/BF02471444. [DOI] [PubMed] [Google Scholar]
- 12.Kusumoto T, Maehara Y, Baba H, Takahashi I, Kusumoto H, Ohno S, Sugimachi K. Sequence dependence of the hyperthermic potential of carboplatin-induced cytotoxicity and intracellular platinum accumulation in Hela cells. British Journal of Cancer. 1993;68:259–263. doi: 10.1038/bjc.1993.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TLM, Needham D, Dewhirst MW. Overcoming Limitations in Nanoparticle Drug Delivery: Triggered, Intravascular Release to Improve Drug Penetration into Tumors. Cancer Res. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 15.Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperth. 1999;15:79–107. doi: 10.1080/026567399285765. [DOI] [PubMed] [Google Scholar]
- 16.Tagami T, Ernsting MJ, Li SD. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Release. 2011;152:303–309. doi: 10.1016/j.jconrel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Tagami T, May JP, Ernsting MJ, Li SD. A thermosensitive liposome prepared with a Cu2+ gradient demonstrates improved pharmacokinetics, drug delivery and antitumor efficacy. J Control Release. 2012;161:142–149. doi: 10.1016/j.jconrel.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Celsion. Phase 3 study of ThermoDox with radiofrequency ablation (RFA) in treatment of hepatocellular carcinoma (HCC) [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2012 June 15]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00617981 NLM Identifier: NCT00617981. [Google Scholar]
- 19.Celsion. Phase 1/2 study of ThermoDox with approved hyperthermia in treatment of breast cancer recurrence at the chest wall (DIGNITY) [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [cited 2012 June 15]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00826085 NLM Identifier: NCT0826085. [Google Scholar]
- 20.de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J Control Release. 2011;150:102–110. doi: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Al-Jamal WT, Al-Ahmady ZS, Kostarelos K. Pharmacokinetics & tissue distribution of temperature-sensitive liposomal doxorubicin in tumor-bearing mice triggered with mild hyperthermia. Biomaterials. 2012;33:4608–4617. doi: 10.1016/j.biomaterials.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Poon RTP, Borys N. Lyso-thermosensitive liposomal doxorubicin: a novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin Pharmacother. 2009;10:333–343. doi: 10.1517/14656560802677874. [DOI] [PubMed] [Google Scholar]
- 23.Landon C, Park JY, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. The open Nanomedicine Journal. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheirolomoom A, Mahakian LM, Lai CY, Lindfors HA, Seo JW, Paoli EE, Watson KD, Haynam EM, Ingham ES, Xing L, Cheng RH, Borowsky AD, Cardiff RD, Ferrara KW. Copper-Doxorubicin as a Nanoparticle Cargo Retains Efficacy with Minimal Toxicity. Mol Pharm. 2010;7:1948–1958. doi: 10.1021/mp100245u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng M, Yang YL, He PG, Fang YZ. Spectroscopic studies of copper(II) and iron(II) complexes of adriamycin. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy. 2000;56:581–587. doi: 10.1016/s1386-1425(99)00157-2. [DOI] [PubMed] [Google Scholar]
- 26.Clerc S, Barenholz Y. Loading of amphipathic weak acids into liposomes in response to transmembrane calcium acetate gradients. Biochim Biophys Acta-Biomembr. 1995;1240:257–265. doi: 10.1016/0005-2736(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 27.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane Ammonium-Sulfate Gradients in Liposomes Produce Efficient and Stable Entrapment of Amphipathic Weak Bases. Biochimica Et Biophysica Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 28.Madden TD, Harrigan PR, Tai LCL, Bally MB, Mayer LD, Redelmeier TE, Loughrey HC, Tilcock CPS, Reinish LW, Cullis PR. The Accumulation of Drugs within Large Unilamellar Vesicles Exhibiting a Proton Gradient - a Survey. Chemistry and Physics of Lipids. 1990;53:37–46. doi: 10.1016/0009-3084(90)90131-a. [DOI] [PubMed] [Google Scholar]
- 29.Mayer LD, Bally MB, Hope MJ, Cullis PR. Uptake of Antineoplastic Agents into Large Unilamellar Vesicles in Response to a Membrane-Potential. Biochimica Et Biophysica Acta. 1985;816:294–302. doi: 10.1016/0005-2736(85)90497-3. [DOI] [PubMed] [Google Scholar]
- 30.Lasic DD, Ceh B, Stuart MCA, Guo L, Frederik PM, Barenholz Y. Transmembrane Gradient Driven Phase-Transitions within Vesicles - Lessons for Drug-Delivery. Biochim Biophys Acta-Biomembr. 1995;1239:145–156. doi: 10.1016/0005-2736(95)00159-z. [DOI] [PubMed] [Google Scholar]
- 31.Li XG, Hirsh DJ, Cabral-Lilly D, Zirkel A, Gruner SM, Janoff AS, Perkins WR. Doxorubicin physical state in solution and inside liposomes loaded via a pH gradient. Biochim Biophys Acta-Biomembr. 1998;1415:23–40. doi: 10.1016/s0005-2736(98)00175-8. [DOI] [PubMed] [Google Scholar]
- 32.Bolotin EM, Cohen R, Bar LK, Emanuel N, Ninio S, Lasic DD, Barenholz Y. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. Journal of Liposome Research. 1994;4:455–479. [Google Scholar]
- 33.Wallace KB. Nonenzymatic Oxygen Activation and Stimulation of Lipid-Peroxidation by Doxorubicin Copper. Toxicology and Applied Pharmacology. 1986;86:69–79. doi: 10.1016/0041-008x(86)90400-x. [DOI] [PubMed] [Google Scholar]
- 34.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: From bench to bedside. J Clin Oncol. 2008;26:3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie JW, Libutti SK, Li KCP, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma XP, Pan HX, Yi JL. Combination sonodynamic therapy with immunoadjuvant may be a promising new modality for cancer treatment. Med Hypotheses. 2009;72:418–420. doi: 10.1016/j.mehy.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. Embo Journal. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JK, Shattuck DL, Ingalla EQ, Yen LL, Borowsky AD, Young LJT, Cardiff RD, Carraway KL, Sweeney C. Suppression of the Negative Regulator LRIG1 Contributes to ErbB2 Overexpression in Breast Cancer. Cancer Res. 2008;68:8286–8294. doi: 10.1158/0008-5472.CAN-07-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kheirolomoom A, Kruse DE, Qin S, Watson KE, Lai CY, Young LJT, Cardiff RD, Ferrara KW. Enhanced in vivo bioluminescence imaging using liposomal luciferin delivery system. J Control Release. 2010;141:128–136. doi: 10.1016/j.jconrel.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills JK, Needham D. The materials engineering of temperature-sensitive liposomes. Methods Enzymol. 2004;387:82–113. doi: 10.1016/S0076-6879(04)87006-X. [DOI] [PubMed] [Google Scholar]
- 41.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, Dewhirst MW. Thresholds for thermal damage to normal tissues: An update. Int J Hyperth. 2011;27:320–343. doi: 10.3109/02656736.2010.534527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: Changing toxicity profiles. Drug Safety. 2001;24:903–920. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 44.Qin SP, Seo JW, Zhang H, Qi J, Curry FRE, Ferrara KW. An Imaging-Driven Model for Liposomal Stability and Circulation. Mol Pharm. 2010;7:12–21. doi: 10.1021/mp900122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.