Abstract
The interaction of plasminogen with cell surfaces results in promotion of plasmin formation and retention on the cell surface. This results in arming cell surfaces with the broad spectrum proteolytic activity of plasmin. Over the past quarter century, key functional consequences of the association of plasmin with the cell surface have been elucidated. Physiologic and pathophysiologic processes with plasmin-dependent cell migration as a central feature include inflammation, wound healing, oncogenesis, metastasis, myogenesis, and muscle regeneration. Cell surface plasmin also participates in neurite outgrowth, and prohormone processing. Furthermore, plasmin-induced cell signaling also affects the functions of inflammatory cells, via production of cytokines, reactive oxygen species, and other mediators. Finally, plasminogen receptors regulate fibrinolysis. In this review we highlight emerging data that shed light on long-standing controversies and raise new issues in the field. We focus on 1) the impact of the recent X-ray crystal structures of plasminogen and the development of antibodies that recognize cell-induced conformational changes in plasminogen, on our understanding of the interaction of plasminogen with cells; 2) the relationship between apoptosis and plasminogen binding to cells; 3) the current status of our understanding of the molecular identitity of plasminogen receptors and the discovery of a structurally unique novel plasminogen receptor, Plg-RKT; 4) the determinants of the interplay between distinct plasminogen receptors and cellular functions; and 5) new insights into the role of co-localization of plasminogen and plasminogen activator receptors on the cell surface.
Keywords: α-enolase, actin, annexin A2, plasminogen receptors, Plg-RKT, S100A10
Introduction
Over twenty-five years ago it was first recognized that plasminogen specifically interacts with the surfaces of cells. This interaction is analogous to the interaction of plasminogen with fibrin in that plasminogen bound to cells is more efficiently activated by plasminogen activators compared with plasminogen in solution (1–10). Furthermore, plasmin remains associated with the cell surface where it is relatively protected from inactivation by α2-antiplasmin (11;12). This results in association of the broad spectrum proteolytic activity of plasmin with the cell surface leading to a wide array of physiological and pathological sequelae. In this review we will highlight emerging data that shed light on long-standing controversies and that raise new questions in the field. Plasminogen receptors are very broadly distributed on both prokaryotic and eukaryotic cells, including monocytes (13), monocytoid cells (11), macrophages (14), endothelial cells (3;15), fibroblasts (16), platelets (1), adrenal medullary cells (17;18) and carcinoma cells (19;20) as well as on cell-derived microvesicles (21;22). The structure and function of plasminogen receptors on prokaryotic cells have been comprehensively reviewed recently (23;24). Here, we focus on the structure and function of eukaryotic plasminogen receptors.
Physiological and Pathological Consequences of Localization of Plasminogen on Cell Surfaces
When cells become armed with the broad-spectrum activity of plasmin, they acquire the ability to degrade extracellular matrices and activate other matrix-associated growth factors and proteolytic enzymes that facilitate cell migration. Physiologic and pathophysiologic processes with plasmin-dependent cell migration as a central feature include inflammation (25–27), wound healing (28;29), oncogenesis (30;31), metastasis (32;33), myogenesis, and muscle regeneration (34–36). Cell surface plasmin also participates in neurite outgrowth (37;38), and prohormone processing (17;18;39;40). Furthermore, plasmin-induced cell signaling also affects the functions of inflammatory cells, via production of cytokines, reactive oxygen species, and other mediators (41;42). Finally, plasminogen receptors regulate fibrinolysis (43–47).
Structural Determinants within Plasminogen that Mediate its Interaction with Cells
The recent X-ray crystal structures of full-length human Glu-plasminogen (the native circulating form of plasminogen with N-terminal Glutamic acid) (48;49) have provided new insights into the structural determinants in the plasminogen molecule that mediate its interaction with cells. The interactions of plasminogen with all cell types evaluated are blocked by lysine and lysine analogs, e.g. ε-aminocaproic acid (EACA), as well as peptides with carboxyl terminal lysines. This implies that the lysine binding sites within the disulfide-bonded kringle domains of plasminogen are required for the interaction with cells. Indeed, isolated kringle-containing plasminogen domains can specifically bind to cells with the following order of potency: The kringle 1–3 domain > the kringle 5-protease domain > kringle 2 (50). Nonetheless, the crystal structures of plasminogen reveal that only the lysine binding site of kringle 1 is available in the closed form of Glu-plasminogen, suggesting that Kringle 1 mediates the initial recruitment of plasminogen to the cell surface (48).
Conformational Changes Induced in Plasminogen upon Binding to Cells and Their Relationship to Plasminogen Activation
Glu-plasminogen exists in a closed tight (T) conformation in the presence of Cl− ion (51). In the presence of lysine and lysine analogs, Glu-plasminogen adopts a more open relaxed (R) conformation that is much more readily activated by plasminogen activators (52). In addition, plasmin catalyzes hydrolysis of the N-terminal 77 amino acids of Glu-plasminogen, resulting in formation of a truncated form with an N-terminal lysine, Lys-plasminogen. Lys-plasminogen exists in an open conformation [relaxed (R) form] and is, consequently, more readily activated by plasminogen activators than Glu-plasminogen in the closed conformation (53–55).
When Glu-plasminogen binds to cells, its activation is markedly enhanced, compared with the reaction in solution due to a reduction in the Km (by 11 to 60-fold) for the plasminogen activation reaction in solution (1–10). This suggests that Glu-plasminogen on the cell surface adopts a conformation distinct from its conformation in solution. Direct evidence for such a conformational change was obtained recently using monoclonal anti-plasminogen antibodies that recognize receptor-induced binding sites (RIBS) in Glu-plasminogen upon its interaction with cells, but react poorly with soluble Glu-plasminogen (56–58). Previously, it has generally been accepted that Glu-plasminogen adopts a Lys-plasminogen-like open conformation when bound to the cell surface, to account for enhancement of activation of cell-associated Glu-plasminogen (59). However, soluble Lys-plasminogen did not compete for the interaction of anti-plasminogen RIBS mAbs with surface-associated Glu-plasminogen, suggesting that the conformation induced when Glu-plasminogen binds to cells is distinct from the conformation of Lys-plasminogen (56). Furthermore, conformational changes in Glu-plasminogen induced by lysine analogs were detected by an anti-plasminogen RIBS mAb, suggesting that the R form of Glu-plasminogen induced by its interaction with lysine analogs, is distinct from the conformation of Lys-plasminogen (56).
The crystal structure of full-length plasminogen has revealed that the plasmin cleavage site in the N-terminus (that produces Lys-plasminogen) is buried in the closed conformation of Glu-plasminogen, suggesting that a conformational rearrangement precedes the production of Lys-plasminogen (48). Plasmin proteolysis of Glu-plasminogen to Lys-plasminogen is promoted when Glu-plasminogen is bound to the cell surface (60–62). Taken together, these studies suggest that the conformation adopted by Glu-plasminogen when bound to cells is one that exposes the cleavage site for plasmin-mediated removal of the N-terminal 77 amino acids, as a mechanism for promoting the conversion of Glu-plasminogen to Lys-plasminogen on the cell surface. Analysis of the crystal structure of plasminogen (48) as well as earlier studies (38;63–65) suggest that this conformational rearrangement may be due to an interaction of the lysine binding site within plasminogen kringle 5 with plasminogen receptors on the cell surface.
Relationship Between Plasminogen Binding to Cells and Apoptosis
An evolving area of research strongly supports a role for plasminogen receptors and the plasminogen activation system in regulation of apoptosis. With adherent cells, plasmin promotes anoikis, depriving cells of a necessary survival signal due to the loss of cell/matrix interactions, leading to programmed cell death. For example, plasmin degrades the hippocampal extracellular matrix protein, laminin, in response to excitotoxin treatment, leading to neuronal cell detachment and cell death (66;67). Similarly, plasmin-dependent anoikis is observed with adherent smooth muscle cells (68;69), retinal cells (70) and fibroblast cell lines (71).
Plasminogen binding capacity is markedly enhanced on both early apoptotic and late apoptotic/necrotic monocytoid cells following treatment with either cycloheximide (72–74) or TNFα (74), consistent with a role for cell-associated plasminogen in regulation of apoptosis. In contrast to adherent cells, monocytoid cells do not depend on adherence for a survival signal. Interestingly, plasminogen has a cytoprotective effect on TNFα-induced apoptosis in monocytoid cells that is independent of anoikis and requires both plasmin activity and PAR1 (74). In addition, plasminogen has prosurvival effects on both spontaneous and Fas-induced neutrophil apoptosis and triggers Akt phosphorylation and ERK1/2 activation via an interaction of plasminogen with the integrin, αMβ2 (75). Thus, plasminogen receptors play a key role in the inflammatory responses of both monocytoid cells and neutrophils by regulating cellular apoptosis.
Plasminogen binding is also markedly higher on non-viable breast cancer cell lines compared with viable cancer cells (32). Cell-associated plasmin has recently been demonstrated to cleave the CUB domain containing protein 1 (CDCP1), resulting in outside-in signaling involving activation of Akt-related cell survival and suppression of PARP1-induced apoptosis in cancer cells (76;77). This signaling cascade ultimately regulates the survival potential of tumor cells in the late stages of the metastatic cascade, namely during extravasation and early tissue colonization. The results also suggest that the original link of the uPA-plasmin system with cancer may not all be via protease-mediated invasive migration, but also via plasmin cleavage and activation of cell survival signaling molecules.
The observation that plasminogen binding capacity is markedly enhanced on both early apoptotic and late apoptotic/necrotic cells (32;72–74) was made possible by the use of fluorescence activated cell sorting (FACS) analysis in which plasminogen binding, separately, to live, apoptotic and necrotic cell populations could be evaluated. Thus, it became incumbent upon investigators studying plasminogen receptors to use this method and it has since replaced earlier methods in which tagged plasminogen binding to whole cell populations was quantified. In addition, kinetic constants can be derived using quantitative FACS analysis (78;79). The relationship between plasminogen binding and apoptosis also serves as an impetus for re-examination of the broad distribution as well as the number of plasminogen binding sites on numerous viable cell types, since these former analyses may have included apoptotic/necrotic cell types. For example, previously, the only cell type examined (in analysis of more than twenty cell types that were not separated into viable/apoptotic/necrotic populations) that did not appreciably bind plasminogen was the erythrocyte (13). However, using FACS analysis, we were able to identify the first viable nucleated cell type, the murine macrophage progenitor line, Hoxa9ER4 (80), which did not detectably bind plasminogen unless differentiated to macrophages (79).
Molecular Identity of Plasminogen Receptors
A key concept regarding the mechanism by which an interaction with the eukaryotic cell surface promotes plasminogen activation is that a subset of carboxypeptidase B (CpB)-sensitive plasminogen binding proteins is responsible for enhancing plasminogen activation. When cells are treated with CpB, the ability to stimulate plasminogen activation is lost (81). Furthermore, plasminogen-dependent macrophage recruitment in the inflammatory response in vivo is mediated by CpB-sensitive plasminogen binding sites (82). Since CpB removes C-terminal basic residues, these results imply that plasminogen binding proteins exposing C-terminal basic residues on cell surfaces are responsible for stimulation of plasminogen activation.
Several distinct plasminogen receptors have been identified over the past decades, consistent with the high number of receptors determined/cell [ranging from 37,000/platelet (1) to > 107 sites/endothelial cell (15)] and also consistent with the diversity of cell types that bind plasminogen.
Until recently, known CpB-sensitive cellular plasminogen receptors could be divided into two classes: 1) proteins synthesized with C-terminal basic residues and having well established intracellular functions, including α-enolase (83;84), cytokeratin 8 (20;85), S100A10 (in complex with annexin A2 within the annexin A2 heterotetramer) (46;86;87), TIP49a (88) and histone H2B (89) and; 2) proteins requiring proteolytic processing in order to reveal a C-terminal basic residue (lysine), including actin (90;91). It was initially proposed that the annexin A2 monomer functioned directly as a plasminogen receptor after a proteolytic cleavage event to liberate a new C-terminal lysine (92). However, recent data suggest that the profibrinolytic role of annexin A2 is to transport and localize the plasminogen regulatory protein, S100A10, to the cell surface within the annexin A2 heterotetramer [reviewed in (31;46)]. It should be noted that there is a CpB-insensitive component of plasminogen binding to eukaryotic cells, as exemplified by tissue factor (93) and the non-proteinaceous gangliosides (94). However, this CpB-insensitive class of plasminogen receptors does not appreciably promote activation of cell-bound plasminogen (81). Integrins, including αIIbβ3 (95;96), αMβ2 (47;97) and α5β1 (97), as well as amphoterin (98) and GP330 (99;100) are plasminogen binding proteins not synthesized with C-terminal basic residues. Whether this group of proteins undergoes proteolysis to reveal C-terminal basic residues and/or are susceptible to CpB proteolysis has not been investigated.
Recently, we used a proteomics approach involving multidimensional protein identification technology (MudPIT) [reviewed in (101)] to probe the membrane proteome of differentiated, macrophage colony stimulating factor (M-CSF)-treated murine monocyte progenitor cells for the presence of integral membrane plasminogen receptor(s) exposing a C-terminal basic residue on the cell surface (79). Intact cells were biotinylated using a biotinylation reagent that reacts with carboxyl groups, rather than basic groups (thus, avoiding potential interference with the plasminogen-binding function of C-terminal basic residues). Because early apoptotic and non-viable/necrotic cells exhibit markedly enhanced plasminogen binding ability (72–74) we wished to focus on plasminogen receptors on viable cells and, therefore, passed the biotinylated cells over a dead cell removal column to enrich for live cells. The cells were then lysed and membrane fractions prepared and passed over a plasminogen-Sepharose affinity column and specifically eluted with EACA. Biotinylated cell surface proteins bound to the avidin column and were digested with trypsin while still on the column. The peptide digest was then subjected to MudPIT. In MudPIT, the peptide mixtures were first resolved by strong cation exchange liquid chromatography upstream of reversed phase liquid chromatography. The eluting peptides were electrosprayed onto an LTQ ion trap mass spectrometer and full MS spectra were recorded over a 400–1600 m/z range, followed by three tandem mass events. The resulting spectra were searched against a mouse protein database. Only one protein with a predicted transmembrane sequence and a C-terminal basic residue was identified: the hypothetical protein, C9orf46 homolog (IPI00136293), homologous to the protein predicted to be encoded by human chromosome 9, open reading frame 46. We have designated the protein, Plg-RKT, to indicate a plasminogen receptor with a C-terminal lysine and having a transmembrane domain (see below).
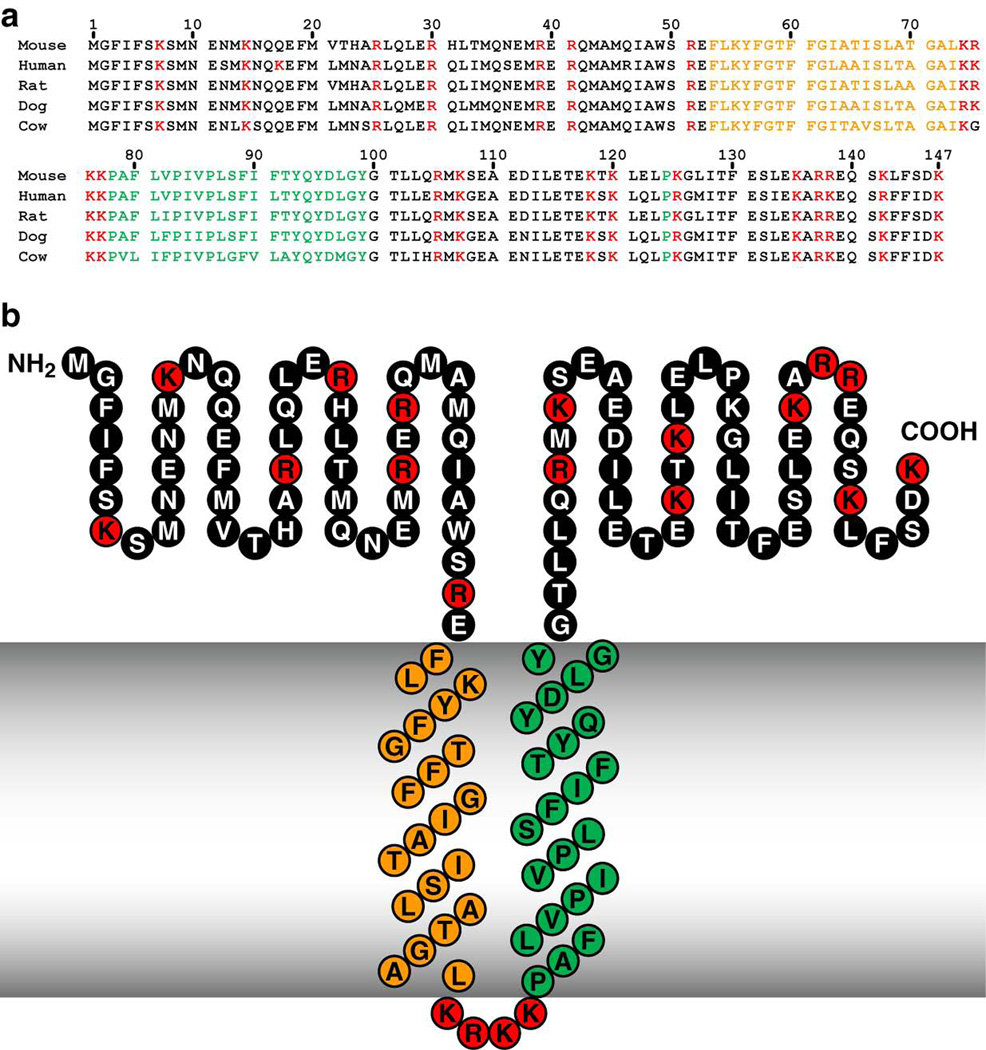
The C9orf46 homolog/Plg-RKT murine DNA sequence encodes a protein of 147 amino acids with a molecular mass of 17,261 Da and a C-terminal lysine (Figure 1, top line). We blasted the C9orf46 homolog/Plg-RKT sequence against all species using NCBI Blast and obtained unique human, rat, dog, cow, dog, giant panda, gibbon, horse, pig, rabbit, and rhesus monkey predicted orthologs, which exhibited high identity (e.g. human vs. chimpanzee = 99% identity) and no gaps in the sequence. Of key importance, a C-terminal lysine was predicted for all of the mammalian orthologs obtained in the blast search. In a query of the Ensembl Gene Report, DNA sequences of all 10 other sequenced mammalian orthologs encoded a C-terminal lysine (102).
Figure 1. Alignment of predicted amino acid sequences of mouse, human, rat, dog and cow orthologs of Plg-RKT (a) and the structural model of Plg-RKT (b) show high interspecies sequence homology.
Green indicates amino acids within the predicted primary transmembrane helix. Orange indicates amino acids within the predicted secondary transmembrane helix. Red indicates basic amino acids. This research was originally published in Blood, Andronicos, N.M., Chen, E.I., Baik, N., Bai, H., Parmer, C.M., Kiosses, W.B., Kamps, M.P., Yates, J.R., III, Parmer, R.J., Miles, L.A., Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation, Blood. 2010, 115: 1319–30.© the American Society of Hematology.
In addition to mammals, the DNA sequences of xenopus, the green lizard and zebrafish also encode a C-terminal lysine. The Plg-RKT sequence also encodes a putative conserved DUF2368 domain (encompassing amino acids 1–135), an uncharacterized protein with unknown function conserved from nematodes to humans. Notably, the DNA sequences of Plg-RKT orthologs of lower organisms (e.g. the sea urchin, Strongylocentrotus purpuratus, Drosophila and Paramecium) predicted proteins of different lengths and did not consistently predict C-terminal lysines. It is interesting to note that the evolutionary origin of plasminogen is currently believed to originate with protochordates (103), so that lower organisms without plasminogen would not need the C-terminal lysine of Plg-RKT to bind plasminogen.
It is noteworthy that the primary sequence of C9orf46/Plg-RKT is apparently tightly conserved in humans, with no validated coding polymorphisms (cSNPs) thus far identified within the 6 exons encoded by the gene (on chromosome 9p24.1) in the NCBI human genome sequence variation database (dbSNP, http://www.ncbi.nlm.nih.gov/SNP).
We analyzed the C9orf46 homolog/Plg-RKT sequence in the TMpred site (www.ch.embnet.org/cgi-bin/TMPRED). The strongly preferred model included two transmembrane helices extending from F53-L73 (secondary helix, oriented from outside the cell to inside the cell) and P78-Y99 (primary helix, oriented from inside the cell to outside the cell) (Figure 1). Thus, a 52 amino acid N-terminal region and a 48 amino acid C-terminal tail with a C-terminal lysine were predicted to be exposed on the cell surface. The predictions of the topology model were supported using several experimental approaches. 1) In Triton X-114 phase separation experiments Plg-RKT partitioned to the detergent phase, thus behaving as an integral membrane protein (79;104). 2) When we treated intact cells with CpB, prior to performing our proteomic analysis, Plg-RKT was not recovered, consistent with cell surface exposure of the C-terminus of Plg-RKT (79). 3) A mAb raised against the C-terminal peptide of Plg-RKT reacted with the cell surface (79;104). 4) Extracellular exposure of both N-and C-termini of Plg-RKT was supported by protease accessibility experiments (102;105).
Plg-RKT exhibited key properties of a plasminogen receptor. First, plasminogen bound directly to the C-terminal peptide of Plg-RKT and a mAb raised against the C-terminal peptide of Plg-RKT recognized plasminogen binding sites on cells (79). Second, Plg-RKT promoted plasminogen activation by both t-PA (79) and uPA (106). Third, over-expression of Plg-RKT increased the ability of cells to promote plasminogen activation (104). In our first evaluations of potential physiological functions of Plg-RKT, we found that Plg-RKT regulated cellular invasion through an extracellular matrix, promoted chemotaxis/cell migration in vitro and regulated plasminogen-dependent macrophage recruitment in the inflammatory response in vivo (106). In addition, Plg-RKT regulates plasmin-dependent modulation of catecholamine release from catecholaminergic cells (104).
Because the murine genome has been sequenced we searched for C9orf46 homolog/Plg-RKT mRNA microarray expression data at http://www.ebi.ac.uk/microarray-as/aew/. Plg-RKT mRNA is present in monocytes, leukocytes, NK cells, T cells, myeloid, dendritic, and plasmacytoid cells, breast cancer, acute lymphoblastic leukemia and Molt-4 acute lymphoblastic leukemia cells. These data are consistent with previous reports documenting expression of plasminogen binding sites on peripheral blood leukocytes (13), breast cancer cells (32;107) and other tissues [reviewed in (108)]. The broad distribution in tissues that express plasminogen binding sites, suggest that Plg-RKT provides plasminogen receptor function that may serve to modulate plasmin proteolytic functions in these tissues, as well.
Interplay Among Plasminogen Receptors in Cellular Functions
The high number of plasminogen binding sites/cell taken together with the diversity of cell types that bind plasminogen (15) suggests that the plasminogen binding capacity of a given cell may be composed of contributions from a set of distinct cell surface proteins and that different cell types may utilize a different panel of plasminogen receptors [recently reviewed by Plow and colleagues in (109)]. The results of our proteomic analysis of differentiated monocyte progenitor cells were consistent with this concept: In addition to peptides corresponding to Plg-RKT, peptides corresponding to other proteins previously identified as plasminogen binding proteins on monocytes were also detected in the membrane preparations: α-enolase, gamma actin, S100A10, annexin 2 (that most likely bound to the plasminogen-Sepharose column via S100A10 in the annexin 2 heterotetramer), histone H2B, and β2 integrin.
The thioglycollate-induced model of macrophage recruitment originally demonstrated the key role of plasminogen receptors with C-terminal lysines in cell migration in the inflammatory response in vivo (82). Recent studies utilizing this model illustrate the interplay among distinct plasminogen receptors in vivo because the sum of the effects of functional blockade of specific plasminogen receptors, is greater than a 100% reduction in plasminogen-dependent macrophage recruitment. Intravenous injection of specific antibodies to histone H2B results in 48% less macrophage recruitment (110), injection of specific antibodies to α-enolase results in 24% less recruitment (110) and injection of mice with anti- Plg-RKT mAb results in 49% less macrophage recruitment (106) (compared to injection of nonimmune control). In S100A10−/− mice, macrophage recruitment in response to thioglycollate is 53% less in S100A10 −/− mice, compared to wild type mice (111). Thus, it is likely that each specific plasminogen receptor may be required at different steps in the inflammatory response, for example chemotactic migration to the peritoneum, or, perhaps, crossing different layers of peritoneal tissue at which different contributions of direct plasmic cleavage of the extracellular matrix or activation of MMP-9 for collagen degradation (112) may predominate. It is noteworthy that a reduction in pro-MMP-9 activation has been demonstrated in S100A10−/− peritoneal macrophages in culture (111) and by treatment of mice with anti-Plg-RKT mAb in vivo and there may be overlap in this function, as well. Although each of these receptors regulates Matrigel invasion in vitro (106;110;111;113), only Plg-RKT has been demonstrated to contribute to directed chemotaxis/chemokinesis (106). Thus, Plg-RKT may be the predominant modulator of this component of macrophage recruitment.
It should also be recognized that the annexin A2/S100A10 complex binds to anionic phospholipids in a Ca+2-dependent manner (31;46;114;115) and it has recently been shown that histone H2B is tethered to the cell surface via an electrostatic interaction with phosphatidyl serine (116). Phosphatidyl serine exposure is a well established marker of apoptosis. When apoptosis is induced, cell surface expression of both histone H2B and S100A10 is increased (109;116). In addition, differentiation of monocytes to macrophages (117;118) and cellular activation (119) are associated with phosphatidyl serine exposure and when cells are induced to differentiate, phosphatidyl serine exposure is increased on non-apoptotic cells (116). Thus, differential exposure of phosphatidyl serine may play a role in determining which plasminogen receptors are utilized at a given stage in cell maturation and activation.
The contribution of distinct plasminogen receptors to macrophage recruitment may also be tissue- and stimulus- specific. For example in a model of monocyte recruitment to the alveolar compartment, α-enolase appears to play a predominant role (113). α-enolase also plays a role in inflammatory cell infiltration required for muscle repair after injury (120).
The Role of Co-localization of Plasminogen and Plasminogen Activators on the Cell Surface
Both Plg-RKT and S100A1 co-localize with uPAR on the cell surface (46;79) and uPAR is also detected in immunopreciptates of Plg-RKT, of S100A10 and of αMβ2 (46;47;104;121). This physical association supports a mechanism for enhancement of plasminogen activation when plasminogen is bound to Plg-RKT or S100A10 and uPA is bound to the uPAR. In addition, the group of Angles-Cano has recently suggested a new mechanism for cell surface plasmin generation, which bypasses the requirement for molecular coassembly of plasminogen and uPA on the same surface, via proteolytic crosstalk between plasminogen and uPA bound to uPAR on adjacent cells, rather than simultaneously to the same cell (59). Alternatively, a uPAR-independent mechanism requiring simultaneous binding of both plasminogen and uPA (via its amino terminal domain) for stimulation of plasminogen activation has been demonstrated using leukemic cell lines (9). This involves a low affinity/high capacity interaction of uPA with the cell surface (9) and may be similar to the mechanism by which plasminogen activation by uPA is enhanced on the platelet surface (1;122) as platelets do not express the uPAR. Nonetheless, all of the foregoing mechanisms require that plasminogen be directly bound to the cell surface (5;9) and the relative importance of each mechanism may depend on the specific plasminogen receptor(s) utilized by a given cell type.
In contrast to the results with uPA, molecular cross-talk between t-PA and plasminogen bound to adjacent cells was not observed (59), and, thus, may require their simultaneous colocalization on the same cell surface. It is noteworthy that plasminogen and t-PA share binding sites on the cell surface, based on cross-competition studies (123). Both Plg-RKT and S100A10 have been demonstrated to directly bind t-PA (79;124–126). Despite potentially sharing binding sites on Plg-RKT and S10010, the relative concentrations of t-PA and plasminogen in the circulation should permit simultaneous binding of both ligands to the cell surface, and each t-PA molecule should be bound proximally to several plasminogen molecules (123).
Conclusions
Over the past 25 years, a broad spectrum of experimental approaches and areas of investigation have demonstrated the wide array of cellular events and functions that are mediated by the interaction of plasminogen with it cellular receptors. Plasminogen receptors play a fundamentally important role in physiological and pathological processes. Future studies in vivo with antibody blockade of specific receptors and with transgenic mice will further elucidate mechanisms by which these processes are regulated.
Acknowledgments
Supported by National Institutes of Health Grants (HL 081046 and CA 166473 to L.A.M., HL50398 to R.J.P.) and Department of Veterans Affairs to R.J.P. This is manuscript # 22040 from The Scripps Research Institute.
Abbreviations
- CpB
Carboxypeptidase B
- EACA
ε-aminocaproic acid
- FACS
fluorescence activated cell sorting
- M-CSF
macrophage colony stimulating factor
- MudPIT
multidimensional protein identification technology
- RIBS
receptor-induced binding sites
- t-PA
tissue type plasminogen activator
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase plasminogen activator receptor
Reference List
- 1.Miles LA, Plow EF. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985;260(7):4303–4311. [PubMed] [Google Scholar]
- 2.Stricker RB, Wong D, Tak Shiu D, Reyes PT, Shuman MA. Activation of plasminogen by tissue plasminoagen activator on normal and thrombasthenic platelets: Effects on surface proteins and platelet aggregation. Blood. 1986;68(1):275–280. [PubMed] [Google Scholar]
- 3.Hajjar KA, Harpel PC, Jaffe EA, Nachman RL. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986;261(25):11656–11662. [PubMed] [Google Scholar]
- 4.Loscalzo J, Vaughan DE. Tissue plasminogen activator promotes platelet disaggregation in plasma. J Clin Invest. 1987;79(6):1749–1755. doi: 10.1172/JCI113015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis V, Behrendt N, Dano K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266(19):12752–12758. [PubMed] [Google Scholar]
- 6.Gonias SL, Braud LL, Geary WA, VandenBerg SR. Plasminogen binding to rat hepatocytes in primary culture and to thin slices of rat liver. Blood. 1989;74(2):729–736. [PubMed] [Google Scholar]
- 7.Duval-Jobe C, Parmely MJ. Regulation of plasminogen activation by human U937 promonocytic cells. J Biol Chem. 1994;269(33):21353–21357. [PubMed] [Google Scholar]
- 8.Félez J, Miles LA, Fábregas P, Jardi M, Plow EF, Lijnen RH. Characterization of cellular binding sites and interactive regions within reactants required for enhancement of plasminogen activation by tPA on the surface of leukocytic cells. Thromb Haemost. 1996;76(4):577–584. [PubMed] [Google Scholar]
- 9.Longstaff C, Merton RE, Fabregas P, Felez J. Characterization of cell-associated plasminogen activation catalyzed by urokinase-type plasminogen activator, but independent of urokinase receptor (uPAR, CD87) Blood. 1999;93(11):3839–3846. [PubMed] [Google Scholar]
- 10.Sinniger V, Merton RE, Fabregas P, Felez J, Longstaff C. Regulation of tissue plasminogen activator activity by cells. J Biol Chem. 1999;274(18):12414–12422. doi: 10.1074/jbc.274.18.12414. [DOI] [PubMed] [Google Scholar]
- 11.Plow EF, Freaney DE, Plescia J, Miles LA. The plasminogen system and cell surfaces: Evidence for plasminogen and urokinase receptors on the same cell type. J Cell Biol. 1986;103(6 Pt 1):2411–2420. doi: 10.1083/jcb.103.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall SW, Humphries JE, Gonias SL. Inhibition of cell surface receptor-bound plasmin by a2-antiplasmin and a2-macroglobulin. J Biol Chem. 1991;266(19):12329–12336. [PubMed] [Google Scholar]
- 13.Miles LA, Plow EF. Receptor mediated binding of the fibrinolytic components, plasminogen and urokinase, to peripheral blood cells. Thromb Haemost. 1987;58(3):936–942. [PubMed] [Google Scholar]
- 14.Silverstein RL, Friedlander RJ, Jr, Nicholas RL, Nachman RL. Binding of lys-plasminogen to monocytes/macrophages. J Clin Invest. 1988;82(6):1948–1955. doi: 10.1172/JCI113814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles LA, Levin EG, Plescia J, Collen D, Plow EF. Plasminogen receptors, urokinase receptors and their modulation on human endothelial cells. Blood. 1988;72(2):628–635. [PubMed] [Google Scholar]
- 16.Gonzalez-Gronow M, Gawdi G, Pizzo SV. Characterization of the plasminogen receptors of normal and rheumatoid arthritis human synovial fibroblasts. J Biol Chem. 1994;269(6):4360–4366. [PubMed] [Google Scholar]
- 17.Parmer RJ, Mahata M, Gong Y, Mahata SK, Jiang Q, O'Connor DT, et al. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest. 2000;106(7):907–915. doi: 10.1172/JCI7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Yasothornsrikul S, Taupenot L, Miles LA, Parmer RJ. The local chromaffin cell plasminogen/plasmin system and the regulation of catecholamine secretion. Ann N Y Acad Sci. 2002;971:445–449. doi: 10.1111/j.1749-6632.2002.tb04506.x. [DOI] [PubMed] [Google Scholar]
- 19.Durliat M, Komano O, Correc P, Bertrand O, Cochet S, Caignard A, et al. Plasminogen receptors on rat colon carcinoma cells. Br J Cancer. 1992;66(1):51–56. doi: 10.1038/bjc.1992.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hembrough TA, Vasudevan J, Allietta MM, Glass WF, Gonias SL. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J Cell Sci. 1995;108(Pt 3):1071–1082. doi: 10.1242/jcs.108.3.1071. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110(7):2432–2439. doi: 10.1182/blood-2007-02-069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez dL, et al. Leukocyte-and endothelial-derived microparticles: a circulating source for fibrinolysis. Haematologica. 2012 doi: 10.3324/haematol.2012.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya S, Ploplis VA, Castellino FJ. Bacterial plasminogen receptors utilize host plasminogen system for effective invasion and dissemination. J Biomed Biotechnol. 2012;2012:482096. doi: 10.1155/2012/482096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanderson-Smith ML, De Oliveira DM, Ranson M, McArthur JD. Bacterial plasminogen receptors: mediators of a multifaceted relationship. J Biomed Biotechnol. 2012;2012:272148. doi: 10.1155/2012/272148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91(6):2005–2009. [PubMed] [Google Scholar]
- 26.Plow EF, Ploplis VA, Busuttil S, Carmeliet P, Collen D. A role of plasminogen in atherosclerosis and restenosis models in mice. Thromb Haemost. 1999;82(Suppl 1):4–7. [PubMed] [Google Scholar]
- 27.Busuttil SJ, Ploplis VA, Castellino FJ, Tang L, Eaton JW, Plow EF. A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost. 2004;2(10):1798–1805. doi: 10.1111/j.1538-7836.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 28.Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, et al. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996;2(3):287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 29.Creemers E, Cleutjens J, Smits J, Heymans S, Moons L, Collen D, et al. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am J Pathol. 2000;156(6):1865–1873. doi: 10.1016/S0002-9440(10)65060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madureira PA, Hill R, Miller VA, Giacomantonio C, Lee PW, Waisman DM. Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget. 2011;2(12):1075–1093. doi: 10.18632/oncotarget.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madureira PA, O'Connell PA, Surette AP, Miller VA, Waisman DM. The Biochemistry and Regulation of S100A10: A Multifunctional Plasminogen Receptor Involved in Oncogenesis. J Biomed Biotechnol. 2012;2012:353687. doi: 10.1155/2012/353687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranson M, Andronicos NM, O'Mullane MJ, Baker MS. Increased plasminogen binding is associated with metastatic breast cancer cells: differential expression of plasminogen binding proteins. Br J Cancer. 1998;77(10):1586–1597. doi: 10.1038/bjc.1998.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palumbo JS, Talmage KE, Liu H, La Jeunesse CM, Witte DP, Degen JL. Plasminogen supports tumor growth through a fibrinogen-dependent mechanism linked to vascular patency. Blood. 2003;102(8):2819–2827. doi: 10.1182/blood-2003-03-0881. [DOI] [PubMed] [Google Scholar]
- 34.Suelves M, Lopez-Alemany R, Lluis F, Aniorte G, Serrano E, Parra M, et al. Plasmin activity is required for myogenesis in vitro and skeletal muscle regeneration in vivo. Blood. 2002;99(8):2835–2844. doi: 10.1182/blood.v99.8.2835. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Alemany R, Suelves M, Munoz-Canoves P. Plasmin generation dependent on alpha-enolase-type plasminogen receptor is required for myogenesis. Thromb Haemost. 2003;90(4):724–733. doi: 10.1160/TH03-04-0291. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Alemany R, Suelves M, Diaz-Ramos A, Vidal B, Munoz-Canoves P. Alpha-enolase plasminogen receptor in myogenesis. Front Biosci. 2005;10:30–36. doi: 10.2741/1503. [DOI] [PubMed] [Google Scholar]
- 37.Jacovina AT, Zhong F, Khazanova E, Lev E, Deora AB, Hajjar KA. Neuritogenesis and the nerve growth factor-induced differentiation of PC-12 cells requires annexin II-mediated plasmin generation. J Biol Chem. 2001;276(52):49350–49358. doi: 10.1074/jbc.M106289200. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez-Fernandez A, Gingles NA, Bai H, Castellino FJ, Parmer RJ, Miles LA. Plasminogen enhances neuritogenesis on laminin-1. J Neurosci. 2009;29(40):12393–12400. doi: 10.1523/JNEUROSCI.3553-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Q, Taupenot L, Mahata SK, Mahata M, O'Connor DT, Miles LA, et al. Proteolytic cleavage of chromogranin A (CgA) by plasmin. Selective liberation of a specific bioactive CgA fragment that regulates catecholamine release. J Biol Chem. 2001;276(27):25022–25029. doi: 10.1074/jbc.M101545200. [DOI] [PubMed] [Google Scholar]
- 40.Bai H, Nangia S, Parmer RJ. The plasminogen activation system and the regulation of catecholaminergic function. J Biomed Biotechnol. 2012;2012:721657. doi: 10.1155/2012/721657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92(3):509–519. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Syrovets T, Simmet T. The Serine Protease Plasmin Triggers Expression of the CC-Chemokine Ligand 20 in Dendritic Cells via Akt/NF-kappaB-Dependent Pathways. J Biomed Biotechnol. 2012;2012:186710. doi: 10.1155/2012/186710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, et al. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest. 2004;113(1):38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dassah M, Deora AB, He K, Hajjar KA. The endothelial cell annexin A2 system and vascular fibrinolysis. Gen Physiol Biophys. 2009;28(Spec No Focus):F20–F28. [PMC free article] [PubMed] [Google Scholar]
- 45.Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011;118(11):3172–3181. doi: 10.1182/blood-2011-05-353482. [DOI] [PubMed] [Google Scholar]
- 46.Madureira PA, Surette AP, Phipps KD, Taboski MA, Miller VA, Waisman DM. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood. 2011;118(18):4789–4797. doi: 10.1182/blood-2011-06-334672. [DOI] [PubMed] [Google Scholar]
- 47.Pluskota E, Soloviev DA, Bdeir K, Cines DB, Plow EF. Integrin alphaMbeta2 orchestrates and accelerates plasminogen activation and fibrinolysis by neutrophils. J Biol Chem. 2004;279(17):18063–18072. doi: 10.1074/jbc.M310462200. [DOI] [PubMed] [Google Scholar]
- 48.Law RH, Caradoc-Davies T, Cowieson N, Horvath AJ, Quek AJ, Encarnacao JA, et al. The X-ray crystal structure of full-length human plasminogen. Cell Rep. 2012;1(3):185–190. doi: 10.1016/j.celrep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y, Bodin C, Olsson K. Crystal structure of the native plasminogen reveals an activation-resistant compact conformation. J Thromb Haemost. 2012;10(7):1385–1396. doi: 10.1111/j.1538-7836.2012.04765.x. [DOI] [PubMed] [Google Scholar]
- 50.Miles LA, Dahlberg CM, Plow EF. The cell-binding domains of plasminogen and their function in plasma. J Biol Chem. 1988;263(24):11928–11934. [PubMed] [Google Scholar]
- 51.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93(4):647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 52.Violand BN, Sodetz JM, Castellino FJ. The effect of epsilon-amino caproic acid on the gross conformation of plasminogen and plasmin. Arch Biochem Biophys. 1975;170(1):300–305. doi: 10.1016/0003-9861(75)90121-6. [DOI] [PubMed] [Google Scholar]
- 53.Wiman B. Primary structure of peptides released during activation of human plasminogen by urokinase. Eur J Biochem. 1973;39(1):1–9. doi: 10.1111/j.1432-1033.1973.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 54.Wiman B, Wallen P. Activation of human plasminogen by an insoluble derivative or urokinase. Structural changes of plasminogen in the course of activation to plamsin and demonstration of a possible intermediate complex. Eur J Biochem. 1973;36(1):25–31. doi: 10.1111/j.1432-1033.1973.tb02880.x. [DOI] [PubMed] [Google Scholar]
- 55.Violand BN, Castellino FJ. Mechanism of the urokinase-catalyzed activation of human plasminogen. J Biol Chem. 1976;251(13):3906–3912. [PubMed] [Google Scholar]
- 56.Han J, Baik N, Kim KH, Yang JM, Han GW, Gong Y, et al. Monoclonal antibodies detect receptor-induced binding sites in Glu-plasminogen. Blood. 2011;118(6):1653–1662. doi: 10.1182/blood-2010-11-316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felez J, Jardi M, Fabregas P, Parmer RJ, Miles LA. Monoclonal antibodies against receptor-induced binding sites detect cell-bound plasminogen in blood. Blood. 2012;120(3):678–681. doi: 10.1182/blood-2012-02-410480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jardi M, Fabregas P, Sagarra-Tio M, Perez-Lucena MJ, Felez J. Characterization of Plasminogen Binding to NB4 Promyelocytic Cells Using Monoclonal Antibodies against Receptor-Induced Binding Sites in Cell-Bound Plasminogen. J Biomed Biotechnol. 2012;2012:984589. doi: 10.1155/2012/984589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dejouvencel T, Doeuvre L, Lacroix R, Plawinski L, Dignat-George F, Lijnen HR, et al. Fibrinolytic cross-talk: a new mechanism for plasmin formation. Blood. 2010;115(10):2048–2056. doi: 10.1182/blood-2009-06-228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong Y, Kim S-O, Felez J, Grella DK, Castellino FJ, Miles LA. Conversion of glu-plasminogen to lys-plasminogen is necessary for optimal stimulation of plasminogen activation on the endothelial cell surface. J Biol Chem. 2001;276(22):19078–19083. doi: 10.1074/jbc.M101387200. [DOI] [PubMed] [Google Scholar]
- 61.Miles LA, Castellino FJ, Gong Y. Critical role for conversion of glu-plasminogen to Lys-plasminogen for optimal stimulation of plasminogen activation on cell surfaces. Trends Cardiovasc Med. 2003;13(1):21–30. doi: 10.1016/s1050-1738(02)00190-1. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Gong Y, Grella DK, Castellino FJ, Miles LA. Endogenous plasmin converts Glu-plasminogen to Lys-plasminogen on the monocytoid cell surface. J Thromb Haemost. 2003;1(6):1264–1270. doi: 10.1046/j.1538-7836.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 63.Thorsen S. The mechanism of plasminogen activation and the variability of the fibrin effector during tissue-type plasminogen activator-mediated fibrinolysis. Ann N Y Acad Sci. 1992;667:52–63. doi: 10.1111/j.1749-6632.1992.tb51597.x. [DOI] [PubMed] [Google Scholar]
- 64.Marshall JM, Brown AJ, Ponting CP. Conformational studies of human plasminogen and plasminogen fragments: Evidence for a novel third conformation of plasminogen. Biochemistry. 1994;33(12):3599–3606. doi: 10.1021/bi00178a017. [DOI] [PubMed] [Google Scholar]
- 65.Hasumi K, Yamamichi S, Harada T. Small-molecule modulators of zymogen activation in the fibrinolytic and coagulation systems. FEBS J. 2010;277(18):3675–3687. doi: 10.1111/j.1742-4658.2010.07783.x. [DOI] [PubMed] [Google Scholar]
- 66.Tsirka SE, Bugge TH, Degen JL, Strickland S. Neuronal death in the central nervous system demonstrates a non-fibrin substrate for plasmin. Proc Natl Acad Sci. 1997;94(26):9779–9781. doi: 10.1073/pnas.94.18.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91(7):917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 68.Houard X, Monnot C, Dive V, Corvol P, Pagano M. Vascular smooth muscle cells efficiently activate a new proteinase cascade involving plasminogen and fibronectin. J Cell Biochem. 2003;88(6):1188–1201. doi: 10.1002/jcb.10460. [DOI] [PubMed] [Google Scholar]
- 69.Meilhac O, Ho-Tin-Noe B, Houard X, Philippe M, Michel JB, Angles-Cano E. Pericellular plasmin induces smooth muscle cell anoikis. FASEB J. 2003;17(10):1301–1303. doi: 10.1096/fj.02-0687fje. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Chaudhry A, Chintala SK. Inhibition of plasminogen activation protects against ganglion cell loss in a mouse model of retinal damage. Mol Vis. 2003;9:238–248. [PubMed] [Google Scholar]
- 71.Rossignol P, Ho-Tin-Noe B, Vranckx R, Bouton MC, Meilhac O, Lijnen HR, et al. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J Biol Chem. 2004;279(11):10346–10356. doi: 10.1074/jbc.M310964200. [DOI] [PubMed] [Google Scholar]
- 72.O'Mullane MJ, Baker MS. Loss of cell viability dramatically elevates cell surface plasminogen binding and activation. Exp Cell Res. 1998;242(1):153–164. doi: 10.1006/excr.1998.4067. [DOI] [PubMed] [Google Scholar]
- 73.O'Mullane MJ, Baker MS. Elevated plasminogen receptor expression occurs as a degradative phase even in cellular apoptosis. Immunol Cell Biol. 1999;77(3):249–255. doi: 10.1046/j.1440-1711.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell JW, Baik N, Castellino FJ, Miles LA. Plasminogen inhibits TNF{alpha}-induced apoptosis in monocytes. Blood. 2006;107(11):4383–4390. doi: 10.1182/blood-2005-07-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pluskota E, Soloviev DA, Szpak D, Weber C, Plow EF. Neutrophil apoptosis: selective regulation by different ligands of integrin alphaMbeta2. J Immunol. 2008;181(5):3609–3619. doi: 10.4049/jimmunol.181.5.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Casar B, He Y, Iconomou M, Hooper JD, Quigley JP, Deryugina EI. Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells. Oncogene. 2012;31(35):3924–3938. doi: 10.1038/onc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deryugina EI, Quigley JP. Cell surface remodeling by plasmin: a new function for an old enzyme. J Biomed Biotechnol. 2012;2012:564259. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ranson M, Andronicos NM. Plasminogen binding and cancer: promises and pitfalls. Front Biosci. 2003;8:s294–s304. doi: 10.2741/1044. [DOI] [PubMed] [Google Scholar]
- 79.Andronicos NM, Chen EI, Baik N, Bai H, Parmer CM, Kiosses WB, et al. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010;115(7):1319–1330. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang GG, Calvo KR, Pasillas MP, Sykes DB, Hacker H, Kamps MP. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat Methods. 2006;3(4):287–293. doi: 10.1038/nmeth865. [DOI] [PubMed] [Google Scholar]
- 81.Felez J, Miles LA, Fabregas P, Jardi M, Plow EF, Lijnen RJ. Characterization of cellular binding sites and interactive regions within reactants required for enhancement of plasminogen activation by tPA on the surface of leukocytic cells. Thromb Haemost. 1996;76(4):577–584. [PubMed] [Google Scholar]
- 82.Swaisgood CM, Schmitt D, Eaton D, Plow EF. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J Clin Invest. 2002;110(9):1275–1282. doi: 10.1172/JCI15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: Identification of alpha-Enolase as a candidate plasminogen receptor. Biochemistry. 1991;30(6):1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 84.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227(1–2):407–415. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 85.Hembrough TA, Kralovich KR, Li L, Gonias SL. Cytokeratin 8 released by breast carcinoma cells in vitro binds plasminogen and tissue-type plasminogen activator and promotes plasminogen activation. Biochem J. 1996;317(Pt 3):763–769. doi: 10.1042/bj3170763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, et al. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37(48):16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- 87.Choi KS, Fogg DK, Yoon CS, Waisman DM. p11 regulates extracellular plasmin production and invasiveness of HT1080 fibrosarcoma cells. FASEB J. 2003;17(2):235–246. doi: 10.1096/fj.02-0697com. [DOI] [PubMed] [Google Scholar]
- 88.Hawley SB, Tamura T, Miles LA. Purification, cloning, and characterization of a profibrinolytic plasminogen-binding protein, TIP49a. J Biol Chem. 2001;276(1):179–186. doi: 10.1074/jbc.M004919200. [DOI] [PubMed] [Google Scholar]
- 89.Herren T, Burke TA, Das R, Plow EF. Identification of histone H2B as a regulated plasminogen receptor. Biochemistry. 2006;45(31):9463–9474. doi: 10.1021/bi060756w. [DOI] [PubMed] [Google Scholar]
- 90.Dudani AK, Ganz PR. Endothelial cell surface actin serves as a binding site for plasminogen, tissue plasminogen activator and lipoprotein(a) Br J Haematol. 1996;95(1):168–178. doi: 10.1046/j.1365-2141.1996.7482367.x. [DOI] [PubMed] [Google Scholar]
- 91.Miles LA, Andronicos NM, Baik N, Parmer RJ. Cell-surface actin binds plasminogen and modulates neurotransmitter release from catecholaminergic cells. J Neurosci. 2006;26(50):13017–13024. doi: 10.1523/JNEUROSCI.2070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem. 1994;269(33):21191–21197. [PubMed] [Google Scholar]
- 93.Fan ZQ, Larson PJ, Bognacki J, Raghunath PN, Tomaszewski JE, Kuo A, et al. Tissue factor regulates plasminogen binding and activation. Blood. 1998;91(6):1987–1998. [PubMed] [Google Scholar]
- 94.Miles LA, Dahlberg CM, Levin EG, Plow EF. Gangliosides interact directly with plasminogen and urokinase and may mediate binding of these components to cells. Biochemistry. 1989;28(24):9337–9343. doi: 10.1021/bi00450a014. [DOI] [PubMed] [Google Scholar]
- 95.Miles LA, Ginsberg MH, White JG, Plow EF. Plasminogen interacts with human platelets through two distinct mechanisms. J Clin Invest. 1986;77(6):2001–2009. doi: 10.1172/JCI112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez-Gronow M, Gawdi G, Pizzo SV. Plasminogen activation stimulates an increase in intracellular calcium in human synovial fibroblasts. J Biol Chem. 1993;268(28):20791–20795. [PubMed] [Google Scholar]
- 97.Lishko VK, Novokhatny VV, Yakubenko VP, Skomorovska-Prokvolit HV, Ugarova TP. Characterization of plasminogen as an adhesive ligand for integrins {alpha}M{beta}2 (Mac-1) and {alpha}5{beta}1(VLA-5) Blood. 2004;104(3):719–726. doi: 10.1182/blood-2003-09-3016. [DOI] [PubMed] [Google Scholar]
- 98.Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander EO, Baumann M, et al. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268(26):19726–19738. [PubMed] [Google Scholar]
- 99.Kanalas JJ, Makker SP. Identification of the rat Heymann nephritis autoantigen (GP330) as a receptor site for plasminogen. J Biol Chem. 1991;266(17):10825–10829. [PubMed] [Google Scholar]
- 100.Kanalas JJ. Analysis of plasmin binding and urokinase activation of plasminogen bound to the Heymann nephritis autoantigen, gp330. Arch Biochem Biophys. 1992;299(2):255–260. doi: 10.1016/0003-9861(92)90272-x. [DOI] [PubMed] [Google Scholar]
- 101.Eng JK, McCormick AL, Yates JRI. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 102.Miles LA, Lighvani S, Baik N, Andronicos NM, Chen EI, Parmer CM, et al. The Plasminogen Receptor, Plg-R(KT), and Macrophage Function. J Biomed Biotechnol. 2012;2012:250464. doi: 10.1155/2012/250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu M, Zhang S. A kringle-containing protease with plasminogen-like activity in the basal chordate Branchiostoma belcheri. Biosci Rep. 2009;29(6):385–395. doi: 10.1042/BSR20080173. [DOI] [PubMed] [Google Scholar]
- 104.Bai H, Baik N, Kiosses WB, Krajewski S, Miles LA, Parmer RJ. The novel plasminogen receptor, plasminogen receptor(KT) (Plg-R(KT)), regulates catecholamine release. J Biol Chem. 2011;286(38):33125–33133. doi: 10.1074/jbc.M111.218693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miles LA, Andronicos NM, Chen EI, Baik N, Bai H, Parmer CM, et al. Man TK, Flores RJ, editors. Identification of the novel plasminogen receptor, Plg-RKT. Proteomics/Book 1: Human Diseases and Protein Functions. Intech. 2012:219–238. [Google Scholar]
- 106.Lighvani S, Baik N, Diggs JE, Khaldoyanidi S, Parmer RJ, Miles LA. Regulation of macrophage migration by a novel plasminogen receptor Plg-R KT. Blood. 2011;118(20):5622–5630. doi: 10.1182/blood-2011-03-344242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Correc P, Fondanèche M-C, Bracke M, Burtin P. The presence of plasmin receptors on three mammary carcinoma MCF-7 sublines. Int J Cancer. 1990;46(4):745–750. doi: 10.1002/ijc.2910460432. [DOI] [PubMed] [Google Scholar]
- 108.Miles LA, Hawley SB, Baik N, Andronicos NM, Castellino FJ, Parmer RJ. Plasminogen receptors: the sine qua non of cell surface plasminogen activation. Front Biosci. 2005;10:1754–1762. doi: 10.2741/1658. [DOI] [PubMed] [Google Scholar]
- 109.Plow EF, Doeuvre L, Das R. So many plasminogen receptors: why? J Biomed Biotechnol. 2012;2012:141806. doi: 10.1155/2012/141806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Das R, Burke T, Plow EF. Histone H2B as a functionally important plasminogen receptor on macrophages. Blood. 2007;110(10):3763–3772. doi: 10.1182/blood-2007-03-079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O'Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116(7):1136–1146. doi: 10.1182/blood-2010-01-264754. [DOI] [PubMed] [Google Scholar]
- 112.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118(9):3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, et al. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113(22):5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 114.Rattner JB, Martin L, Waisman DM, Johnstone SA, Fritzler MJ. Autoantibodies to the centrosome (centriole) react with determinants present in the glycolytic enzyme enolase. J Immunol. 1991;146(7):2341–2344. [PubMed] [Google Scholar]
- 115.Ross M, Gerke V, Steinem C. Membrane composition affects the reversibility of annexin A2t binding to solid supported membranes: a QCM study. Biochemistry. 2003;42(10):3131–3141. doi: 10.1021/bi027069z. [DOI] [PubMed] [Google Scholar]
- 116.Das R, Plow EF. Phosphatidylserine as an anchor for plasminogen and its plasminogen receptor, histone H2B, to the macrophage surface. J Thromb Haemost. 2011;9(2):339–349. doi: 10.1111/j.1538-7836.2010.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marguet D, Luciani MF, Moynault A, Williamson P, Chimini G. Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey. Nat Cell Biol. 1999;1(7):454–456. doi: 10.1038/15690. [DOI] [PubMed] [Google Scholar]
- 118.Callahan MK, Halleck MS, Krahling S, Henderson AJ, Williamson P, Schlegel RA. Phosphatidylserine expression and phagocytosis of apoptotic thymocytes during differentiation of monocytic cells. J Leukoc Biol. 2003;74(5):846–856. doi: 10.1189/jlb.0902433. [DOI] [PubMed] [Google Scholar]
- 119.Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62(9):971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PubMed] [Google Scholar]
- 120.Diaz-Ramos A, Roig-Borrellas A, Garcia-Melero A, Lopez-Alemany R. alpha-Enolase, a Multifunctional Protein: Its Role on Pathophysiological Situations. J Biomed Biotechnol. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simon DI, Rao NK, Xu H, Wei Y, Majdic O, Ronne E, et al. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88(8):3185–3194. [PubMed] [Google Scholar]
- 122.Jiang YP, Pannell R, Liu JN, Gurewich V. Evidence for a novel binding protein to urokinase-type plasminogen activator in platelet membranes. Blood. 1996;87(7):2775–2781. [PubMed] [Google Scholar]
- 123.Felez J, Chanquia CJ, Fabregas P, Plow EF, Miles LA. Competition between plasminogen and tissue plasminogen activator for cellular binding sites. Blood. 1993;82(8):2433–2441. [PubMed] [Google Scholar]
- 124.Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, et al. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37(48):16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- 125.Fogg DK, Bridges DE, Cheung KK, Kassam G, Filipenko NR, Choi KS, et al. The p11 subunit of annexin II heterotetramer is regulated by basic carboxypeptidase. Biochemistry. 2002;41(15):4953–4961. doi: 10.1021/bi012045y. [DOI] [PubMed] [Google Scholar]
- 126.MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J Biol Chem. 2003;278(28):25577–25584. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]