Abstract
Previous studies demonstrate that soluble HIV proteins impact both hepatocyte function and HCV replication in vitro. It has also been reported that HIV can productively infect hepatocytes. We therefore investigated the impact of HIV infection of hepatocytes on HCV expression. The Huh7.5JFH1 cell line that constitutively expresses infectious HCV was infected with the lab-adapted strains HIVNL4-3 or HIVYK-JRCSF. HCV expression was quantified via HCV core antigen ELISA, Western blot, and strand-specific real-time PCR for positive-sense and negative-sense HCV RNA. After HIVNL4-3 infection of Huh7.5JFH1 cells, positive-sense and negative-sense HCV RNA levels were elevated compared to HIV uninfected cells. Increased HCV RNA synthesis was also observed after infection of Huh7.5JFH1 cells with HIVYK-JRCSF. HIV-induced HCV core production was decreased in the presence of the anti-HIV drugs AZT, T20, and raltegravir, although these medications had a minimal effect on HCV expression in the absence of HIV. HCV core, NS3, and NS5A protein expression were increased after HIV infection of Huh7.5JFH1 cells. Chemically inactivated HIV had a minimal effect on HCV expression in Huh7.5JFH1 cells suggesting that ongoing viral replication was critical. These data demonstrate that HIV induces HCV RNA synthesis and protein production in vitro and complement previous in vivo reports that HCV RNA levels are elevated in individuals with HIV/HCV co-infection compared to those with HCV mono-infection. These findings suggest that HIV suppression may be a critical factor in controlling liver disease, particularly if the underlying liver disease is not treated.
Introduction
Since the introduction of highly active antiretroviral therapy (HAART), liver disease – frequently caused by hepatitis C virus (HCV) co-infection – has surpassed AIDS-defining illnesses as a major cause of morbidity and mortality in HIV-positive persons [1], [2]. Multiple clinical studies have clearly demonstrated that HIV co-infection results in increased HCV RNA levels, progressive liver disease, and decreased HCV treatment response rates (reviewed in [3], [4]). HIV infection is associated with a number of hepatic and biliary tract disorders, hepatomegaly, hepatic steatosis, and elevated serum liver enzymes [5]–[8]. HIV-positive patients with no evidence of viral hepatitis co-infection also exhibit mild-to-moderate increases in liver enzyme levels [9]–[11]. Several groups, including our own, have also reported that plasma HIV RNA levels were associated with liver disease during HIV mono-infection, suggesting a direct involvement of HIV in liver disease [12]–[15].
The influence of HIV on HCV disease is not solely immune mediated, as HCV RNA levels are strongly associated with HIV RNA levels [16]. Thus, virus-virus interactions are likely. Such interactions may be mediated via 1) secreted HIV proteins that interact with HCV-infected hepatocytes, and/or 2) direct HIV infection of HCV-infected hepatocytes. However, until recently, in vitro systems to study the complete HCV life cycle were unavailable; therefore, our ability to explore HIV/HCV interactions were also limited. Recently, the advent of infectious HCV cell culture systems has spawned renewed interest in exploring the cellular pathways contributing to HIV/HCV pathogenesis. Moreover, the availability of distinct classes of anti-HIV agents that target viral entry, reverse transcription, integration, or infectious virion production allows for more detailed mechanistic studies of HIV-mediated HCV replication.
Several lines of evidence highlight the potential for HIV to interact directly with multiple liver cell populations (reviewed in [17], [18]). For example, HIV proviral DNA was detected in liver biopsies from patients with AIDS [19], while HIV proteins and viral RNA have been detected in hepatocytes, Kupffer cells, inflammatory mononuclear cells, and sinusoidal cells using liver samples from HIV-infected patients [19], [20]. Similarly, intracellular expression of HIV proteins has been reported in Kupffer cells, endothelial cells, and hepatocytes [21]–[23]. Once considered controversial because of a lack of data, several recent reports have convincingly demonstrated productive, low-level HIV infection of hepatocytes. Xiao et al. isolated an HIV strain from a patient with advanced disease that was able to infect hepatocytes [24]. Primary human hepatocytes were also susceptible to HIV infection. Fromentin et al. demonstrated that a hepatocyte-derived cell line binds to and internalizes HIV particles [25]. Furthermore, HIV infection of CD4+ T cells was enhanced after interactions with virus-loaded hepatocytes compared to cell-free virus. Iser et al. observed increased HIV reverse transcriptase activity following infection of hepatocyte cell lines with X4 or R5 HIV [26]. We recently demonstrated integrated HIV DNA in the Huh7.5 and Huh7.5JFH1 cell lines and primary hepatocytes that was inhibited by raltegravir in a dose-dependent manner [27]. HIV p24 protein was also detected in cell culture supernatants and was inhibited by AZT, although levels were modest compared to those in a lymphocyte cell line. The detection of HIV proteins and nucleic acids in hepatocytes is particularly interesting given that hepatocytes are the major site of HBV/HCV replication. Given these findings, we investigated whether HIV could regulate HCV expression in hepatocytes in vitro.
Materials and Methods
Cell lines and reagents
The Huh7.5JFH1 cell line – which produces infectious HCV genotype 2a virions – was provided by Dr. Guangxiang Luo [28] and maintained in DMEM high glucose medium supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 mg/mL). The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: AZT, T20 from Roche, TZM-bl cells [29] from Drs. John Kappes and Xiaoyun Wu and Tranzyme Inc., plasmids pNL4-3 [30] from Dr. Malcolm Martin and pYK-JRCSF [31] from Drs. Irvin Chen and Yoshio Koyanagi, and raltegravir from Merck & Company, Inc. 293T cells were obtained from ATCC.
Virus preparation
HIVNL4-3 (X4-tropic) and HIVYK-JRCSF (R5-tropic) were prepared by transfection of 1×106 293T cells per well in a 24-well plate with 1 ug of the appropriate full-length infectious HIV plasmid using the FuGene6 transfection reagent (Roche). Transfected cells were incubated at 37°C for an additional 48–72 hours. Virus-containing supernatants were passed through a 0.20 um filter to remove cellular debris and precipitated in polyethylene glycol at 4°C. Precipitated virus was then centrifuged at 14,000 g for 20 minutes, resuspended in PBS, and frozen at −80°C until use. The level of p24 protein in cell culture supernatants was determined by p24 ELISA (Perkin-Elmer; Boston, MA; lower limit of detection = 4.3 pg/mL) or by titering on TZM-bl cells.
AT-2 treatment of HIV
For preparation of non-infectious HIV with functionally intact envelope glycoproteins, HIVNL4-3 and HIVYK-JRCSF were first prepared by transfection of 293T cells as described above. After filter sterilization, virus was inactivated by adding 250 uM of aldrithiol-2 (AT-2; Sigma-Aldrich) to the filtered supernatants as described elsewhere [32]. AT-2-treated viruses were then incubated with the TZM indicator cell line to confirm that they were not infectious (data not shown). HIV-1 p24 ELISA was measured in treated virus preparations.
HIV infections
1×105 Huh7.5JFH1 cells were seeded per well of a 96-well, collagen-coated plate and incubated at 37°C in 5% CO2 with infectious HIV at a multiplicity of infection [MOI] of 1.0 in a volume of ∼100 uL. After 2 hours, unbound viruses were removed by washing cells five times with phosphate buffered saline, and fresh medium was added. Cells and cell culture supernatants were removed at various time points post-infection to measure HCV RNA or protein as described below. For experiments with antiretroviral agents, cells were incubated with 100 uM of AZT, 1 ug/mL of T20, or 1–100 nM raltegravir for one hour before and during HIV infection. For experiments with AT-2-treated HIV, Huh7.5JFH1 cells were incubated as described above in the presence of 0–200 ng of HIV as measured by HIV p24 ELISA.
Quantification of HCV RNA and protein expression
Real-time PCR conditions to quantify strand-specific HCV RNA levels have been described in detail previously [33]. Briefly, RNA was extracted from 140 uL of culture supernatant using the QIAamp Viral RNA Kit (Qiagen; Valencia, CA), and eluted in 60 uL of DEPC-treated dH2O. For cell lysates, HCV RNA values (diluted 1∶1000 for positive-sense RNA and 1∶100 for negative-sense RNA) were normalized to the copy number of a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and presented as fold change. For Western Blots, 100,000 cells were harvested at days 3, 5, 7, and 14 post-infection and lysed in 100 uL of buffer; 10 uL was loaded per well. HCV NS3 and NS5A proteins were detected using a mouse monoclonal antibody (Ab) against HCV NS3 (ab18671; Abcam; Cambridge, MA) or a rabbit polyclonal Ab against HCV NS5A (ab2594; Abcam) as the primary Ab and a rabbit polyclonal IgG Ab as the secondary Ab (ab6921; Abcam). As an additional loading control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was detected using a rabbit polyclonal Ab from Santa Cruz Biotechnology (SC-25778; Santa Cruz, CA). HCV core antigen was quantified in cell culture supernatants by the QuikTiter HCV Core Antigen ELISA Kit (Cell Biolabs, Inc.; San Diego, CA) or the Ortho HCV Antigen ELISA Test Kit (Ortho Clinical Diagnostics; Tokyo, Japan).
Statistical analyses
Graphical inspection and basic descriptive statistics were used to check for potential outlying observations or violations of assumptions for parametric statistical models. Analysis of variance (ANOVA) was used to assess variation among experimental conditions. The choice between heterogeneous or homogeneous residual variance among experimental conditions was made in each experiment on the basis of the Akaike Information Criterion. In order to balance Type I errors against the relatively low power afforded by the small sample size, P values≤0.10 are reported. SAS PROC MIXED version 9.2 was used for all inferential analyses.
Results
HIV infection of Huh7.5JFH1 cells increases HCV RNA synthesis
We previously reported that integrated HIV DNA was detected in Huh7.5 and Huh7.5JFH1 cells, as well as in primary hepatocytes, after in vitro infection [27]. Several antiretroviral agents inhibited HIV infection of hepatocytes, although HIV levels were modest compared to those in a lymphocyte cell line. Culture supernatants from HIV-infected hepatocytes were capable of infecting a non-hepatic HIV indicator cell line suggesting production of infectious HIV from hepatocytes. In the current study, we examined the effects of HIV infection on HCV replication in Huh7.5JFH1 hepatocytes.
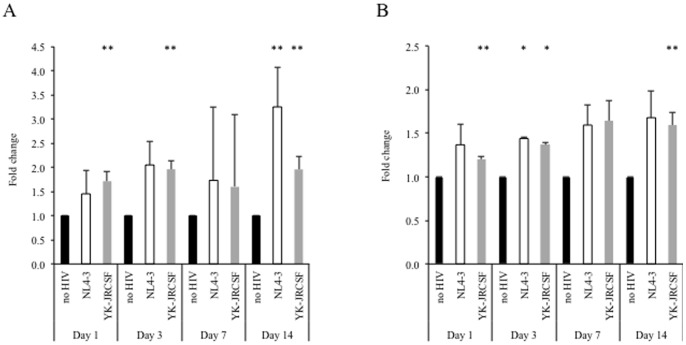
HCV RNA was quantified at days 1, 3, 7, and 14 post-infection with HIVNL4-3 or HIVYK-JRCSF by strand-specific real-time PCR. As shown in Figure 1A–B, both positive-sense and negative-sense HCV RNA were elevated in the presence of the HIV. Increased HCV RNA expression showed a trend towards statistical significance for positive-sense RNA in the presence of HIVNL4-3 at day 14 and in the presence of HIVYK-JRCSF at days 1, 3, and 14. Similarly, increased negative-sense RNA was statistically significant in the presence of HIVNL4-3 at day 3 and in the presence of HIVYK-JRCSF at day 3. As well, there was a trend towards statistical significance in the presence of HIVYK-JRCSF at days 1 and 14.
Figure 1. Infection of Huh7.5JFH1 cells with HIV results in increased HCV RNA synthesis.
100,000 Huh7.5JFH1 cells were incubated with DNase-treated HIVNL4-3 (white bars) or HIVYK-JRCSF (grey bars) at an MOI = 1 for two hours. At days 1, 3, 7, and 14 post-infection, positive-sense HCV RNA (A) and negative-sense HCV RNA (B) were quantified in cell lysates, compared to the no HIV condition (black bars) by real-time, strand-specific PCR, and normalized to cellular GAPDH levels as described previously [33]. * p≤0.05; ** p≤0.10 compared to HIV-uninfected Huh7.5JFH1 cells at each time point.
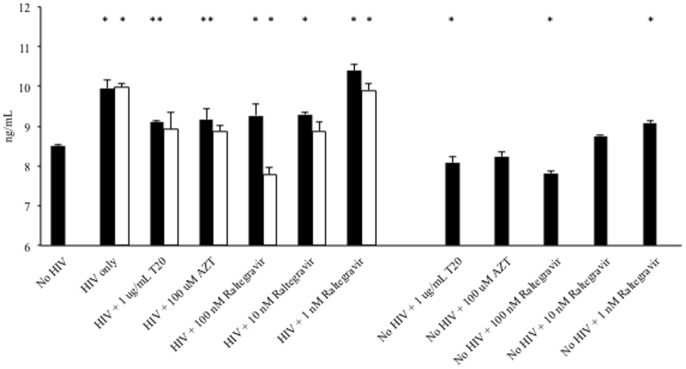
Pre-treatment of cells with the reverse transcriptase inhibitor AZT (100 uM), the entry inhibitor T20 (1 ug/mL), or the integrase inhibitor raltegravir (1–100 nM) resulted in decreased HCV core antigen production in the presence of HIVNl4-3 and/or HIVYK-JRCSF (Figure 2). In the absence of HIV infection, a modest but statistically significant impact of certain HIV medications on HCV expression was observed.
Figure 2. HIV infection of Huh7.5JFH1 cells is inhibited by anti-HIV medications.
100,000 Huh7.5JFH1 cells were incubated with DNase-treated HIVNL4-3 (black bars) or HIVYK-JRCSF (white bars) an MOI = 1 for two hours. Cells were pre-treated with AZT (100 uM), T20 (1 ug/mL), or raltegravir (1–100 nM) for one hour and then during HIV infection and incubation. At day 3 post-infection, HCV core antigen was quantified in cell lysates. * p≤0.05; ** p≤0.10 compared to HIV-uninfected Huh7.5JFH1 cells under each treatment condition.
HIV infection of Huh7.5JFH1 cells increases HCV protein expression
At days 1, 3, 7, and 14 post-infection with infection with HIVNL4-3, HCV core antigen expression was quantified in cell lysates and shown to be elevated in the presence of HIV (Figure 3). Additionally, increased HCV NS3 and NS5A protein expression were observed via Western blot in Huh7.5JFH1 cells infected with HIVNL4-3 at days 1, 3, 7, and 14 post-infection compared to uninfected control levels after normalization to GAPDH protein levels (Figure 4). Relative HCV NS3 and NS5A protein levels were significantly reduced at day 3 when HIVNL4-3 infection was performed in the presence of the antiretroviral drug AZT (0.84 versus 0.35 and 0.71 versus 0.28, respectively).
Figure 3. Infection of Huh7.5JFH1 cells with HIV results in increased HCV core protein production.
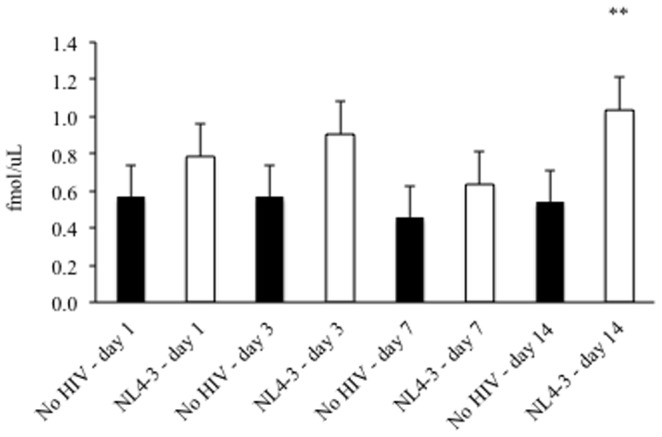
100,000 Huh7.5JFH1 cells were incubated with DNase-treated HIVNL4-3 at an MOI = 1 for two hours. At days 1, 3, 7, and 14 post-infection, HCV core antigen was quantified in cell lysates in the absence (black bars) or presence (white bars) of HIV. * p≤0.05; ** p≤0.10 compared to HIV-uninfected Huh7.5JFH1 cells at each time point.
Figure 4. Infection of Huh7.5JFH1 cells with HIV results in increased HCV NS3 and NS5A protein production.
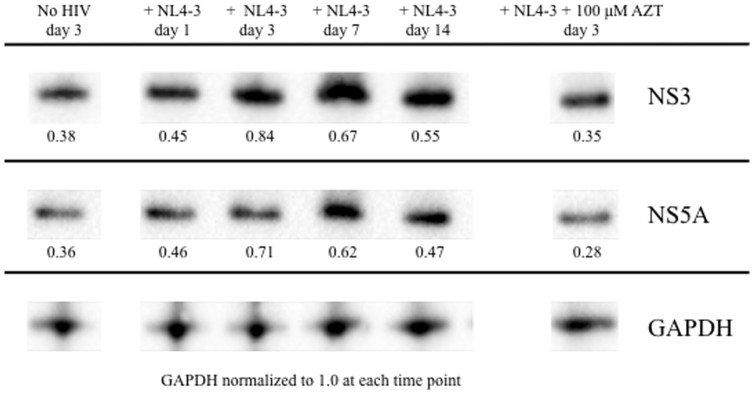
100,000 Huh7.5JFH1 cells were incubated with DNase-treated HIVNL4-3 at an MOI = 1 for two hours. Cell lysates were harvested at days 1, 3, 7, and 14 post-infection for the detection of HCV NS3 and NS5A proteins by Western Blot. Equivalent total cell protein concentrations were loaded, and all data were normalized to cellular GAPDH levels at each time point. The uninfected (no HIV) condition, as well as infection with HIVNL4-3 in the presence of 100 uM AZT, are shown as additional controls.
AT-2-treated HIV does not alter HCV protein expression
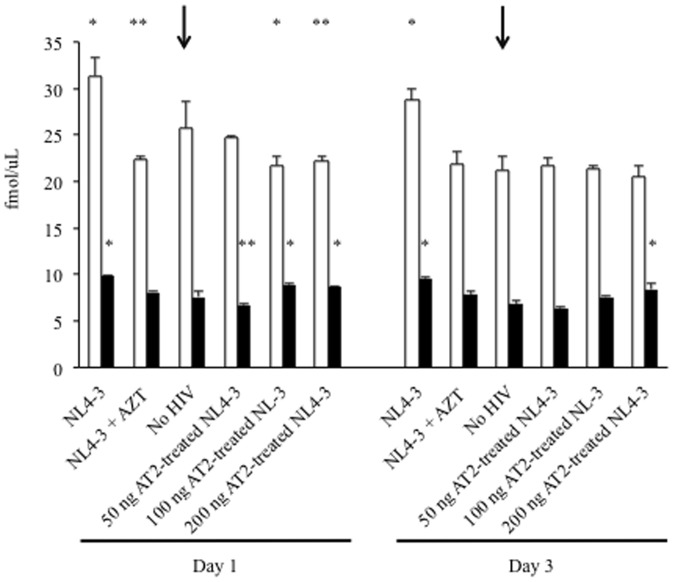
To examine whether HIV replication was necessary to induce HCV expression, Huh7.5JFH1 cells were exposed to 0 ng, 50 ng, 100 ng, or 200 ng of AT2-treated HIVNL4-3. As shown in Figure 5, the impact of AT-2-treated HIVNL4-3 on cell lysate levels of HCV core protein was not markedly increased from Huh7.5JFH1 cells grown in the absence of HIVNL4-3. Thus, the effects of HIV on HCV protein expression may require ongoing HIV replication in hepatocytes.
Figure 5. Chemically inactivated HIV does not increase HCV protein expression.
100,000 Huh7.5JFH1 cells were incubated with infectious HIVNL4-3 alone, infectious HIVNL4-3 + 100 uM AZT, no HIV, or AT-2-treated HIVNL4-3 (50 ng, 100 ng, or 200 ng) at an MOI = 1 for two hours. At days 1 and 3 post-infection, HCV core antigen was quantified in cell lysates (white bars) and culture supernatants (black bars). * p≤0.05; ** p≤0.10 compared to HIV-uninfected Huh7.5JFH1 cells at each time point. Arrows highlight the control – no infectious HIV added – condition.
Discussion
HIV is a critical regulator of viral hepatitis in vivo. Emerging data also suggests that HIV can directly impact liver disease. Moreover, several recent reports have convincingly demonstrated productive, low-level HIV infection of hepatocytes [24]–[27], [34]. A number of mechanisms have been postulated to explain the adverse effect of HIV on HCV replication (reviewed in [17], [18]). At the cellular level, HIV-HCV interactions may be mediated via secreted HIV proteins that interact with HCV-infected hepatocytes, and/or direct HIV infection of HCV-infected hepatocytes. For instance, IL-8 attenuates the anti-HCV actions of IFN in vivo and in vitro [35]–[37], and recombinant, monomeric HIV gp120 protein induces IL-8 production in uninfected hepatocytes [38]. Additionally, STAT1 – a critical element of the Jak-Stat signaling pathway – is antagonized by the HCV core protein, resulting in increased HCV expression [39], [40]. Interestingly, recombinant HIV and HCV envelope proteins cooperatively enhance STAT1-mediated apoptosis in uninfected hepatocytes [41]. Similarly, recombinant HIV gp120 can induce apoptosis in hepatocytes by several distinct pathways that involve Fas ligand, the anti-apoptotic molecule AKT, and caspase expression [42], [43]. Subsequent studies have further demonstrated that gp120 also induces TRAIL-mediated apoptosis [44], as well as enhanced STAT1-mediated apoptosis in uninfected hepatocytes [41]. Recombinant gp120 also increases expression of transforming growth factor b1 (TGFb1) – a key mediator of liver fibrosis – and HCV replication in vitro [45]. However, these prior studies were limited in one or more ways as they did not utilize infectious HIV or HIV proteins in their native confirmation, or used hepatocyte-derived cell lines that are not HCV permissive and therefore did not examine the impact on HCV replication. Thus, the impact of infectious HIV on HCV replication has not been evaluated in vitro.
In the current study, increased HCV replication in the presence of infectious HIV was demonstrated in several ways. First, infectious HIV resulted in elevated levels of HCV positive- and negative-sense RNA at several time points post-infection. This increased expression of HCV replication occurred in the presence of an R5- or X4-utilizing HIV isolate. Replication of HIV was required for this effect as various antiretroviral medications blocked this pro-HCV effect. HCV protein expression was also elevated in the presence of infectious HIV. While HCV core protein levels were elevated at several time points post-infection with infectious HIV, these differences were not statistically significant. Nonetheless, NS3 and NS5A levels were elevated in the presence of infectious HIV as measured by Western Blot, collectively suggesting that HIV infection results in increased production of several HCV proteins. Moreover, increased HCV replication appeared to require replication-competent HIV, as there was a nominal effect on HCV protein levels when HIV was pretreated with AT-2 to make it replication defective. During the preparation of this manuscript, Zhang et al. reported similar findings using Huh7.5 cells that were transduced with CD4 prior to infection with HCV [46]. However, our two studies differ as Zhang et al. 1) transduced the Huh7.5 hepatocytes with CD4, rather than relying on Huh7.5 hepatocytes in their “natural” state with a low level of CD4 expression, and 2) utilized Huh7.5 hepatocytes (not constitutively expressing HCV RNA and protein) rather than the Huh7.5JFH1 hepatocyte cell line that constitutively expresses HCV RNA and protein). Moreover, we also evaluated HCV protein expression and/or HCV RNA synthesis in the presence of infectious HIV or AT-2 treated virus. Thus, we feel that our model more accurately reflects the hepatic environment in vivo.
It is well documented that HCV RNA levels are higher in the presence of HIV co-infection in vivo [47]–[49]. While this may be due in part to immune-mediated effects, direct virus-virus interactions are also likely. Thus, HIV suppression may be a critical factor in controlling liver disease, particularly if the underlying liver disease is not treated. Moreover, characterization of the intracellular pathways by which infectious HIV proteins impact liver cell function and/or replication of hepatitis viruses will significantly improve our understanding of HIV pathogenesis and may ultimately improve treatment modalities for HIV-mediated liver disease.
Acknowledgments
These data were presented at the 18th Conference on Retroviruses and Opportunistic Infections held in Boston, MA from February 27 to March 2, 2011. The authors would like to thank Drs. M. Tarek Shata and Kenneth Sherman for their reviews of this manuscript.
Funding Statement
This work was supported by Merck, Inc. and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR000077-04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tedaldi E, Baker R, Moorman A, Alzola C, Furhrer J, et al. (2003) Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clinical Infectious Diseases 36: 363–367. [DOI] [PubMed] [Google Scholar]
- 2. Bica I, McGovern B, Dhar R, Stone D, McGowan K, et al. (2001) Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clinical Infectious Diseases 32: 492–497. [DOI] [PubMed] [Google Scholar]
- 3. Kim AY, Chung R (2009) Coinfection with HIV-1 and HCV - a one-two punch. Gastroenterology 137: 795–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rotman Y, Liang T (2009) Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. Journal of Virology 83: 7366–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keaveny AP, Karasik M (1998) Hepatobiliary and pancreatic infections in AIDS: Part one. AIDS Patient Care and STDs 12: 347–357. [DOI] [PubMed] [Google Scholar]
- 6. Kahn J, Walker B (1998) Acute human immunodeficiency virus type 1 infection. New England Journal of Medicine 339: 33–39. [DOI] [PubMed] [Google Scholar]
- 7. Bonacini M (1992) Hepatobiliary complications in patients with human immunodeficiency virus infection. American Journal of Medicine 92: 404–411. [DOI] [PubMed] [Google Scholar]
- 8. Ingiliz P, Valantin MA, Duvivier C, Medja F, Dominguez S, et al. (2009) Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology 49: 436–442. [DOI] [PubMed] [Google Scholar]
- 9. Sterling R, Wilson M, Sanyal A, Luketic V, Stravitz R, et al. (2004) Impact of highly active antiretroviral therapy on the spectrum of liver disease in HCV-HIV coinfection. Clinical Gastroenterology and Hepatology 2: 432–439. [DOI] [PubMed] [Google Scholar]
- 10. Mata-Marin JA, Gaytan-Martinez JE, Grados-Chavarria BH, Fuentes-Allen JL, Arroyo-Anduiza CI, et al. (2009) Correlation between HIV viral and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross-sectional study. Virology Journal 6: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen YJ, Tsai HC, Cheng MF, Lee SS, Chen Y (2010) Primary human immunodeficiency virus infection presenting as elevated aminotransferases. Journal of Microbiology, Immunology, and Infection 43: 175–179. [DOI] [PubMed] [Google Scholar]
- 12. Blackard JT, Welge JA, Taylor LE, Mayer KE, Klein RS, et al. (2011) HIV mono-infection is associated with FIB-4 - a noninvasive index of liver fibrosis - in women. Clinical Infectious Diseases 52: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendeni M, Focà E, Gotti D, Ladisa N, Angarano G, et al. (2011) Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clinical Infectious Diseases 52: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 14. Price JC, Seaberg EC, Badri S, Witt MD, D'Acunto K, et al. (2012) HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. Journal of Infectious Diseases 205: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Towner WJ, Xu L, Leyden WA, Horberg MA, Chao CR, et al. (2012) The effect of HIV infection, immunodeficiency, and antiretroviral therapy on the risk of hepatic dysfunction. Journal of Acquired Immune Deficiency Syndromes 60: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas DL, Rich JD, Schuman P, Smith DK, Astemborski JA, et al. (2001) Multicenter evaluation of hepatitis C RNA levels among female injection drug users. Journal of Infectious Diseases 183: 973–976. [DOI] [PubMed] [Google Scholar]
- 17. Blackard JT, Sherman K (2008) HCV/HIV co-infection: time to re-evaluate the role of HIV in the liver? Journal of Viral Hepatitis 15: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal M, Blackard J (2012) Effects of HIV on liver cell populations; Sherman K, editor. New York: Springer Science+Business Media [Google Scholar]
- 19. Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, et al. (1992) Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS 6: 65–70. [DOI] [PubMed] [Google Scholar]
- 20. Housset C, Lamas E, Brechot C (1990) Detection of HIV1 RNA and p24 antigen in HIV-1-infected human liver. Research in Virology 141: 153–159. [DOI] [PubMed] [Google Scholar]
- 21. Jiang TJ, Zhao M, Zhao JM, Zhou GD, Pan D, et al. (2005) Immunohistochemical evidence for HIV-1 infection in the liver of HIV-infected patients. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 19: 152–154. [PubMed] [Google Scholar]
- 22. Hoda SA, White JE, Gerber M (1991) Immunohistochemical studies of human immunodeficiency virus-1 in liver tissues of patients with AIDS. Modern Pathology 4: 578–581. [PubMed] [Google Scholar]
- 23. Lang ZW, Dao WB, Zhang FJ, Shi XH, Ma ZC, et al. (2005) A pathological study on liver tissues of patients with HIV infection. Zhonghua Gan Zang Bing Za Zhi 13: 930–932. [PubMed] [Google Scholar]
- 24. Xiao P, Usami O, Suzuki Y, Ling H, Shimizu N, et al. (2008) Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. AIDS 22: 1749–1757. [DOI] [PubMed] [Google Scholar]
- 25. Fromentin R, Tardif MR, Tremblay M (2010) Human hepatoma cells transmit surface bound HIV-1 to CD4+ T cells through an ICAM-1/LFA-1-dependent mechanism. Virology 398: 168–175. [DOI] [PubMed] [Google Scholar]
- 26. Iser DM, Warner N, Revill PA, Solomon A, Wightman F, et al. (2010) Coinfection of hepatic cell lines with human immunodeficiency virus and hepatitis B virus leads to an increase in intracellular hepatitis B surface antigen. Journal of Virology 84: 5860–5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kong L, Cardona Maya W, Moreno-Fernandez ME, Ma G SM, Sherman KE, et al. (2012) Low-level HIV infection of hepatocytes. Virology Journal 9: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai Z, Zhang C, Chang KS, Jiang J, Ahn BC, et al. (2005) Robust production of infectious hepatitis C virus (HCV) from stably HCV cDNA-transfected human hepatoma cells. Journal of Virology 79: 13963–13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D (1998) Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage tropic isolates of human immunodeficiency virus type 1. Journal of Virology 72: 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adachi A, Gendelman H, Koenig S, Folks T, Willey R, et al. (1986) Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. Journal of Virology 59: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haltiner M, Kempe T, Tjian R (1985) A novel strategy for constructing clustered point mutations. Nucleic Acids Research 13: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW Jr, et al. (1998) Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. Journal of Virology 72: 7992–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackard JT, Smeaton L, Hiasa Y, Horiike N, Onji M, et al. (2005) Detection of hepatitis C virus (HCV) in serum and peripheral blood mononuclear cells of HCV-monoinfected and HIV/HCV-coinfected persons. Journal of Infectious Diseases 192: 258–265. [DOI] [PubMed] [Google Scholar]
- 34. Cao YZ, Friedman-Kein AE, Huang YX, Li XL, Mirabile M, et al. (1990) CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. Journal of Virology 64: 2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jia Y, Wei L, Jiang D, Wang J, Cong X, et al. (2007) Antiviral action of interferon-alpha against hepatitis C virus replicon and its modulation by interferon-gamma and interleukin-8. Journal of Gastroenterology and Hepatology 22: 1278–1285. [DOI] [PubMed] [Google Scholar]
- 36. Polyak SJ, Khabar KS, Rezeiq M, Gretch D (2001) Elevated levels of interleukin-8 in serum are associated with hepatitis C infection and resistance to interferon therapy. Journal of Virology 75: 6209–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polyak SJ, Khabar KS, Paschal DM, Ezelle HJ, Duverlie G, et al. (2001) Hepatitis C virus nonstructural 5A protein induces interleukin-8, leading to partial inhibition of the interferon-induced antiviral response. Journal of Virology 75: 6095–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balasubramanian A, Ganju R, Groopman J (2003) HCV and HIV envelope proteins collaboratively mediate IL-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. Journal of Biological Chemistry 278: 35755–35766. [DOI] [PubMed] [Google Scholar]
- 39. Lin W, Choe W, Hiasa Y, Kamegaya Y, Blackard J, et al. (2005) Hepatitis C virus expression suppresses interferon signaling by degrading STAT1. Gastroenterology 128: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 40. Lin W, Kim SS, Yeung E, Kamegaya Y, Blackard JT, et al. (2006) Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. Journal of Virology 80: 9226–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balasubramanian A, Ganju RK, Groopman JE (2006) Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. Journal of Infectious Diseases 194: 670–681. [DOI] [PubMed] [Google Scholar]
- 42. Munshi N, Balasubramanian A, Koziel M, Ganju R, Groopman J (2003) Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. Journal of Infectious Diseases 188: 1192–1204. [DOI] [PubMed] [Google Scholar]
- 43. Balasubramanian A, Koziel M, Groopman J, Ganju R (2005) Molecular mechanism of hepatic injury in coinfection with hepatitis C virus and HIV. Clinical Infectious Diseases 41: S32–S37. [DOI] [PubMed] [Google Scholar]
- 44. Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza S (2009) HIV induces TRAIL sensitivity in hepatocytes. PLoS One 4: e4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, et al. (2008) HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology 164: 803–811. [DOI] [PubMed] [Google Scholar]
- 46. Zhang X, Daucher M, Baeza J, Kim CW, Russell R, et al. (2012) Human immunodeficiency virus enhances hepatitis C virus replication by differential regulation of IFN and TGF family genes. Journal of Medical Virology 84: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 47. Yokozaki S, Takamatsu J, Nakano I, Katano Y, Toyoda H, et al. (2000) Immunological dynamics in hemophiliac patients infected with hepatitis C and human immunodeficiency virus: influence of antiretroviral therapy. Blood 96: 4293–4299. [PubMed] [Google Scholar]
- 48. Sherman KE, Andreatta C, O'Brien J, Gutierrez A, Harris R (1996) Hepatitis C in human immunodeficiency virus-coinfected patients: increased variability in the hypervariable envelope coding domain. Hepatology 23: 688–694. [DOI] [PubMed] [Google Scholar]
- 49. Beld M, Penning M, Lukashov V, McMorrow M, Roos M, et al. (1998) Evidence that both HIV and HIV-induced immunodeficiency enhance HCV replication among HCV seroconverters. Virology 244: 504–512. [DOI] [PubMed] [Google Scholar]