Abstract
In the 2nd NCI Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation, the Scientific/Educational Session on the Prevention and Treatment of Relapse after Allogeneic Transplantation highlighted progress in developing new therapeutic approaches since the 1st Relapse Workshop. Recent insights that might provide a basis for the development of novel, practical clinical trials were emphasized, including utilization of newer agents, optimization of donor lymphocyte infusion (DLI), and investigation of novel cellular therapies. Dr. de Lima discussed preemptive and maintenance strategies to prevent relapse after transplantation, e.g., recent promising results suggestive of enhanced graft-versus-tumor activity with hypomethylating agents. Dr. Schmid provided an overview of adjunctive strategies to improve cell therapy for relapse, including cytoreduction prior to DLI, combination of targeted agents with DLI, and considerations in use of second transplants. Dr. Porter addressed strategies to enhance T-cell function, including ex-vivo activated T cells and T-cell engineering, and immunomodulatory approaches to enhance T-cell function in vivo, including exogenous cytokines and modulation of costimulatory pathways.
INTRODUCTION
Cancer relapse remains the major cause of treatment failure after allogeneic hematopoietic stem cell transplantation (AlloSCT). For the 1st NCI-sponsored workshop on the Biology, Prevention and Treatment of Relapse in 2009, extensive reviews of disease-specific prevention and treatment strategies were published in the Workshop Proceedings (1, 2). Progress in prevention and treatment was emphasized in the 2nd workshop as well, and focused on ideas that might provide a basis for the development of novel, practical clinical trials. Employment of new agents, optimal utilization of donor lymphocyte infusion (DLI) and immunomodulatory therapeutics, and investigation of targeted interventions, e.g., genetically modified donor cells, and of novel cellular therapies are areas of ongoing study in the field; promising advances reported since the 1st Workshop are discussed here.
I. PREVENTION
Prevention will likely be the most feasible and effective means of managing relapse after AlloSCT. In the case of acute leukemias, since even extraordinarily low-level minimal residual disease (MRD) is associated with a high risk of relapse, the goal of prevention should be to achieve an MRD-negative state (3). While most clearly defined for leukemias, the goal of MRD-negative remission is also relevant to relapse prevention for indolent malignancies and after reduced-intensity AlloSCT, i.e., in settings where remission is established some time after AlloSCT. Our ability to target prevention interventions at individuals whose cancers have the highest risk of relapse is improvingly rapidly, with emerging data from molecular, proteomic and genomic tumor investigations leading to better-informed relapse risk stratification (4) and increasingly sensitive means of detecting residual disease (5–7). Precise application of preemptive strategies that permit intervention when the burden of disease is minimal could improve our ability to eradicate malignancy before overt relapse. Indeed, many investigational treatments — even with modest efficacy in established relapse — might significantly improve AlloSCT outcomes if applied in the preventive setting. Preventive therapy decisions pose a dilemma: withholding potentially efficacious therapy until relapse is detected compromises the patient’s chance of cure, yet administering potentially toxic therapy without evidence of relapse will result in overtreatment for some. Toxicity is a major concern in preventive therapy, particularly in the early months following AlloSCT, when side effects (e.g., myelosuppression, rash, diarrhea) and drug interactions would present significant management challenges, yet also when relapse often occurs and intervention might be most effective (8).
Strategic aims of prevention include: 1) improving disease control before AlloSCT; 2) increasing graft-versus-tumor (GVT) potency of the transplant; 3) maintaining disease control while the allograft matures; and 4) detecting and preempting an impending relapse (Table 1). Preventing relapse in individuals whose cancers are active or demonstrate high-risk biology may require employment of multiple strategies.
Table 1.
Strategies for Relapse Prevention
Improved Preparative Therapy
|
Graft Engineering
|
Preemptive Treatment
|
Early Withdrawal of Immunosuppression
|
Maintenance
|
Ideal Maintenance Agent
Caveats to Maintenance Strategies |
Pre-transplant approaches may permit use of agents with significant hematologic toxicity, but require pharmacokinetic consideration of potential effects upon donor stem cell and lymphocyte populations. Use of novel agents (targeting signaling pathways, growth factors, cell surface antigens, etc.) may deepen remissions through effects on cancer cells or the tumor microenvironment and thus improve outcomes. A role for novel agents in the pre-transplant setting is suggested by observations of improved AlloSCT outcomes following their use in “bridge” therapy, such as with tyrosine kinase inhibitors in Philadelphia chromosome-positive acute lymphoblastic leukemia (ALL) (9) and brentuximab vedotin in Hodgkin’s lymphoma (10); distinct toxicity profiles and unique mechanisms of action have led to investigation of incorporating monoclonal antibodies into reduced-intensity conditioning (RIC) regimens, resulting in immunomodulatory as well as direct antitumor effects (11). New cancer drugs with novel targets and innovative methods of drug delivery are entering the clinic at a phenomenal rate; their potential to permit or augment GVT is an important research opportunity.
Transplant modifications to potentiate GVT effects may incorporate donor selection tactics, immunotherapeutic maneuvers and tumor-specific immunotherapies. Recent advances in our understanding of NK immunogenetic influences on transplant outcome, including relapse risk (particularly in acute myelogenous leukemia (AML)) may yield opportunities to prevent relapse through donor selection based on killer-cell immunoglobulin-like receptor (KIR) genotyping in the context of HLA-mismatch (#REF:SessIIManu)(12). Early withdrawal of immune suppression (WIS), with or without prophylactic donor lymphocyte infusion (DLI), is another consideration in patients at very high risk of relapse but randomized trial data are lacking and there is significant risk of graft-versus-host disease (GVHD) (13). Furthermore, when employed to preempt impending or early leukemia relapse, these immunotherapeutic maneuvers appear to have limited activity outside of chronic myelogenous leukemia and result in considerable GVHD morbidity (14). The morbidity of prophylactic DLI may be reduced in the setting of T-cell depleted allografts or mixed chimerism (15, 16). Interestingly, preliminary results of administering ex-vivo activated DLI prophylaxis suggest fairly modest GVHD toxicity following RIC AlloSCT with alemtuzumab (17). Efforts to optimize selective subset depletion of DLI (or allograft) continue, attempting to reduce risk of GVHD while maintaining protection from relapse (18).
There has been significant progress in developing tumor-targeted immunotherapies, including tumor vaccines (62), genetically modified T cells (discussed in Section III) and selectively expanded antigen-specific T cells (19). The early post-transplant period may be an ideal time for their administration, when minimal tumor burden coincides with lymphopenia-induced homeostatic cytokine abundance and increased efficiency of antigen-specific lymphocyte proliferation (63). The use of novel, e.g., targeted agents in maintenance therapy will require phase-1 evaluation of cumulative and overlapping toxicities (e.g., with conditioning and immunoprophylaxis agents), with particular attention to effects upon rapidly expanding progenitor and lymphocyte populations.
Maintenance therapeutics may be effective in relapse prevention, providing early tumor control and, potentially, immunomodulatory support for the development of an allogeneic immune response. Acute leukemia relapse poses a particularly great management challenge after AlloSCT due to rapid cell growth and disease progression once recurrence is detected; as such, maintenance approaches for acute leukemia may be informative in indolent malignancies as well. A phase 1 trial at MD Anderson defined a safe, low-dose azacitidine maintenance regimen (32 mg/m2/day, Days 1 – 5 of 30, beginning Day +40 after AlloSCT), with preliminary results suggesting improved event-free and overall survival and less chronic GVHD (20); an ongoing trial is examining one year of maintenance with this regimen (NCT00887068). Others have confirmed the favorable toxicity profile of low-dose azacitidine maintenance, with indirect evidence suggesting azacitidine may mediate enhanced GVT effects and modulate GVHD by increasing T cell tumor antigen responsiveness and numbers of circulating regulatory T cells (21, 22).
Preemptive treatment strategies are being investigated which, employing monitoring, initiate therapy upon detection of MRD or other biological surrogate of impending relapse. In the RELAZA trial (23), azacitidine was used to treat patients with imminent relapse as defined by decreasing CD34+ cell donor chimerism (“CD34 chimerism”) after AlloSCT. Twenty patients with decreasing CD34 chimerism while in complete hematologic remission received four cycles of standard-dose azacitidine (75 mg/m2/day for 7 days). Responses were observed in 16 patients during treatment, with CD34 chimerism either increasing (50%) or stabilizing (30%) without signs of hematologic relapse. Additional cycles were given to 11 patients. Although 13 of the 20 patients ultimately relapsed, the time to relapse was longer than expected in this very high-risk cohort, suggesting that a preemptive strategy may be effective, although alternative monitoring approaches and/or employment of more intensive preemptive therapy may be necessary.
II. STRATEGIES TO IMPROVE CELL THERAPY FOR RELAPSE
Donor cell therapy remains the foundation of most approaches to induce remission for AlloSCT relapse, attempting to restore or kindle a potentially curative GVT effect. However, except for chronic myelogenous leukemia and, to some extent, other indolent malignancies, responses to unmodified DLI or second transplantation in overt relapse after AlloSCT are disappointing. While data are limited, adjunctive therapies are now routinely used in conjunction with donor cells for their direct cytotoxic and/or immunomodulatory effects.
Remission Induction Prior to Cellular Therapy
In acute leukemias and other aggressive malignancies, rapid tumor growth kinetics, a high tumor burden at the time of relapse detection, and employment of immune escape mechanisms limit the clinical efficacy of DLI alone. Consistent with this, DLI (24) and second transplant (25) result in better outcomes if complete remission (CR) can be induced prior to cell therapy, affording time to establish a robust GVT effect (26) and, perhaps, increase tumor cell immunogenicity may contribute as well (27).
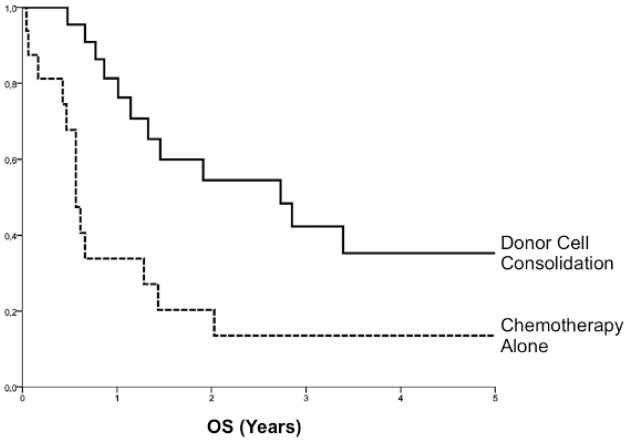
There are no standard cytotoxic regimens for any cancer relapse after AlloSCT, and an individualized approach to agents, doses and schedules is often driven by such factors as prior chemosensitivity, interval from AlloSCT to relapse, age and comorbidities, including GVHD. In AML, approximately 45% of patients will achieve CR following standard anthracycline/cytarabine-based combinations (28, 29). Importantly, remission induction alone is not sufficient for long-term disease control in most patients. In a recent EBMT analysis of patients with AML relapse after reduced-intensity conditioning AlloSCT, durable remissions were observed almost exclusively in patients whose chemotherapy-induced CR was consolidated with either DLI or a second SCT (29). (Figure 1) In addition to cytoreduction, chemotherapy given prior to donor cell infusion might have immunomodulatory effects that promote GVT activity, e.g., by lymphodepletion, suppression of regulatory T cells and/or release of activating cytokines (30)(#REF:SessIManu).
Figure 1. Donor-Cell Consolidation of Remission for AML Relapse after AlloSCT.
Analysis of EBTR data from 38 patients in CR after first-line cytoreductive therapy for relapsed AML after AlloSCT demonstrated improved OS with use of donor cells for consolidation: 55 ± 11% vs. 20 ± 10% (p=.038); DLI and second AlloSCT were considered as time-dependent variables. Adapted from: Schmid C., et al. (Blood 2012;119:1599-1606).
Employment of Novel Agents
Therapeutic agents with novel mechanisms of action are under investigation for their ability to control cancer cell proliferation, including progression after AlloSCT (Table 2). As compared to conventional chemotherapy, these drugs often have less systemic toxicity and might therefore be used in patients with a recent history of intensive treatment, including conditioning for SCT, with active GVHD or other comorbidities. In addition to direct cytotoxic activity, some drugs (e.g., the hypomethylating agents, thalidomide and its derivatives, etc.) are reported to increase antitumor immune responsiveness by increased tumor immunogenicity (31) and enhanced activation of T cells and NK cells (32). These and other immunomodulatory agents, e.g., bortezomib (33) and rituximab (34), may have prophylactic or therapeutic benefit in GVHD, hence be useful adjuncts to reduce the risk of GVHD with DLI.
Table 2.
New Drugs in Treatment of Relapse After Allogeneic Stem Cell Transplantation
| References | Study Design | Diagnosis | Number Treated | Dosage | Responses | DLI | Outcome | Remarks |
|---|---|---|---|---|---|---|---|---|
|
HYPOMETHYLATING AGENTS
| ||||||||
| Azacytidine | ||||||||
| Jabbour, Cancer 2009 | Retrospective, single-center | AML | 9 | 16,24,40 mg/m2 d1–5, median 8 courses | ORR: 56% (33% CR, 22% PR) | No | Not reported | Patients with “indolent” disease reccurrence |
| Lübbert, BMT 2010 | Prospective pilot | AML/CMML | 26 | 100 mg within 3 days | CR: 16%, temporary disease control: 50% | Day 10 of each course in 73% of patients | 2Y OS 16% | |
| Czibere, BMT 2010 | Retrospective, multicenter | AML/MDS | 22 | 100mg/m2 d1–5, q 4w, up to 8 courses | ORR 72% (23% CR) | In 82% of patients | Median OS: 144 days, 2Y OS: 23% | |
| Bolanos-Meade, BBMT 2011 | Retrospective, single-center | Myeloid malignancies | 10 | Various | CR: 60%, SD: 10% | No | Median OS: 422.5 days | Patients without circulating blasts only |
| Schröder, ASH (#656) 2011; ASH (#1964) 2012 | Propspective, single-arm, multicenter, phase 2 | AML/MDS | 30 | 100mg/m2 d1–5, q4w, up to 8 courses | ORR 47% (CR: 23%, PR: 7%, SD: 17%) | Increasing doses after alternating courses | Median OS: 117 days | Upregulation of regulatory T cells following 5-Aza |
|
| ||||||||
|
TYROSINE KINASE INHIBITORS (Exclusive of CML)
| ||||||||
| Imatinib | ||||||||
| Wassmann, Blood 2005 | Prospective, single-arm, multicenter | Ph+ ALL | 27 | 400–800 mg/d | Molecular CR: 54% | No | 2Y DFS: early-responders 54%; nonresponders 8% | |
| Nilotinib | ||||||||
| Tiribelli, Leuk Res 2009 | Case report | Imatinib-resistant Ph+ ALL | 1 | 400 mg b.i.d | Molecular CR | Monthly DLI | CCR | |
| Dasatinib | ||||||||
| Ishida, Int J Hematol 2010 | Case report | Ph+ ALL | 1 | Not reported | Molecular CR | No | CCR following SCT2 | Particular side effects after SCT2 |
| Czyz, Med Oncol. 2010 | Case report | Ph+ ALL, persistent MRD | 1 | Not reported | Molecular CR | No | CCR | |
| Conchon, BMT 2010 | Case report | Imatinib-resistant Ph+ ALL | 1 | 140 mg/d, initiallly combined with VCR and dexamethasone | Molecular CR | No | CMR after 8 months of dasatinib maintenance | |
| Tchibana, Leuk Lymphoma 2011 | Case report | Imatinib-resistant Ph+ ALL | 1 | 140 mg/d (salvage), 80 mg/d (maintenance) | Minor hematological response as salvage, molecular CR as maintenance after SCT2 | No | CMR after SCT2 and short dasatinib maintenance | |
| Sorafenib | ||||||||
| Metzeler, Blood 2009 | Compassionate-use results | FLT3-ITD + AML | 5 | 2 × 400 mg/d | CCR in 3/5 patients | No | 2 × durable remission | |
| Sharma BBMT 2011 | Retrospective, single- center | AML | 16 | 2 × 400–600 mg/d +/− chemotherapy | No CR, reduction of circulating blasts in 80% | No; 3 pts. received SCT2 | Median OS 83 days | Sorafenib regarded as not effective in early relapse |
|
| ||||||||
|
IMMUNOMODULATORS
| ||||||||
| Thalidomide | ||||||||
| Kröger, Blood 2004 | Prospective, single- center, phase 1/2 | Multiple Myeloma | 18 | 100 mg / d, escalation to 300 mg in case of no response | ORR: 67%, CR: 22 | Escalating dosages, Day 14 of every cycle | 2Y OS: 100%, DFS: 84% | Only patients refractory to or relapsing after prior DLI |
| Lenalidomide | ||||||||
| Minemma, Leukemia 2011 | Retrospective, single- center | Multiple Myeloma | 16 | 25 mg/d, + dexamethasone with progression | ORR lenalidomide only: 46%, + Dex: 87.5% | No ( some with prior DLI) | Not reported | Severe aGvHD after lenalidomide alone |
| Lioznov, BMT 2010 | Retrospective, single- center | Multiple Myeloma | 24 | 15–25 mg/d, +/− dexamethasone | ORR: 66%, (CR: 8%, VGPR: 8%, PR: 50%, SD: 13%) | No | Median OS: 19.9 months, median TTP: 9.7 months | Less GvHD, increase of activated NK and T cells |
| Spina, Leuk Lymphoma 2011 | Retrospective, multicenter | Multiple Myeloma | 13 | 10–25 mg/d + dexamethasone | ORR: 100%, 69% long term responders | No | Not reported | Increases the frequency of CD4+Foxp3+ T cells |
| Bortezomib | ||||||||
| Kröger, Exp Hematol 2006 | Prospective pilot | Multiple Myeloma | 11 | 1.3 mg/m m2 day 1,4,8 and 11 | CR: 30%, PR: 50%, MR: 20% | No | Not reported | Patients with measurable disease only, progressive disease excluded |
| El-Ceikh, Hematologica 2008 | Retrospective, multicenter | Multiple Myeloma | 37 | 1,0 – 1,3 mg/m2 day 1,4,8,11 +/− dexamethasone | ORR: 73% (CR: 19%, VGPR 19%, PR: 36%) | No | 18 mo OS 65% | |
|
| ||||||||
|
MONOCLONAL ANTIBODIES
| ||||||||
| Rituximab | ||||||||
| Wudhikarn, BBMT 2011 | Retrospective | NHL | 27 | Not reported; additional chemotherapy in 7 pts. | ORR 44% | Not reported | Median response duration: 23 months (1–68) | Response even in patients pretreated with rituximab |
| Bi 20 (FBTA05) | ||||||||
| Buhmann, BMT 2009 | Compassionate-use results | CLL/NHL | 6 | Escalating doses (10–2000μg) | CLL: 3 × transient response, NHL: 1 SD | DLI in escalating doses or SCT2 | ||
| Blinatumumab | ||||||||
| Handgretinger, Leukemia 2011 | Retrospective, single- center | Pediatric ALL | 3 (2 MRD) | 3 × CR | 2 of 3 relapsed quickly after stopping drug; 1 CCR after haplo SCT2 | Expansion of donor- derived T cells in all cases | ||
| Brentuximab Vedotin | ||||||||
| Gopal, Blood 2012 | Prospective, pooled data (3 studies) | Hodgkin’s Lymphoma | 25 | 1,2 – 1,8 mg/kg, 1–16 (median: 8) cycles | ORR: 50%, CR: 38% | No | Median PFS; 7,8 months, median OS: not reached | |
While the literature on the use of novel agents for relapse after AlloSCT is predominantly retrospective and/or anecdotal, monotherapy for overt relapse generally appears to yield modest responses of limited duration (Table 2). Further, immunomodulatory effects of even highly targeted agents can be heterogeneous, yielding unanticipated negative effects on the immune response, i.e., exacerbation of GVHD and/or interference with GVT. As an example of the latter, the multi-tyrosine kinase inhibitor dasatinib has been shown to potently inhibit effector T cell function in vivo (35) and could theoretically blunt any GVT response. Such unexpected “targets” of novel agents highlight the need for their evaluation in clinical trials, including assessment of their immunomodulatory properties in the allogeneic setting.
Cytokines to augment the efficacy of donor immune cells
Various cytokines have been investigated for their capacity to improve the efficacy of donor cells. An older approach with new interest is the combination of GM-CSF and/or interferon-alpha (IFN-α) and DLI. Both cytokines have been shown to increase the capacity of dendritic cells and leukemia cells to present target antigens, and provide co-stimulatory signals and adhesion molecules for improved donor T-cell stimulation. In murine models, CD-8 dependent GVHD and GVT effects are enhanced by IFN signaling through its ability to both sensitize the leukemia cells to killing and to augment donor CTL function (36). There are several reports successfully using DLI plus IFN-α in diseases with historically poor responses to DLI alone, however, small, heterogeneous cohorts make it difficult to determine the real contribution of IFN-α (37, 38).
Tang et al. recently reported on 16 patients with relapsed acute leukemia (AML, 7; ALL, 9), treated with IFN-α plus G-CSF mobilized donor leukocytes (39). IFN-α (3 MU/day) was given from day -5 before DLI until CR, toxicity or relapse (median, 17 days, range 5–50). Twelve of 16 patients achieved CR, including six of nine patients who received no additional cytotoxic therapy. At last follow-up, seven patients were alive in CR.
Compared to 14 similar patients treated with DLI alone, IFN-α/DLI resulted in a higher CR rate (75% vs. 14%, p=0.001) and improved leukemia-free survival (50% vs. 7%, p=0.05), albeit with increased acute GVHD (56% vs. 27%, p=0.05).
Another approach to increase the potency of DLI is to interrupt the counter-regulatory effect of CTLA-4 upon T-cell activation through administration of ipilimumab, a neutralizing monoclonal antibody against CTLA-4. In a phase 1 trial for post-AlloSCT relapse, some immune-mediated adverse events were observed, although there was no significant GVHD, even in patients subsequently receiving DLI for disease progression (40). Ipilimumab showed modest activity in lymphoid malignancies, particularly Hodgkin’s Lymphoma, with two prolonged CR in patients treated at the highest dose-level. Consistent with expected biological effects, a dose-dependent, T-cell activation and expansion was observed in-vivo.
Further, immune-mediated systemic (“abscopal”) antitumor effects of targeted radiation, mediated through novel tumor antigen expression and inflammation-induced recruitment of antigen-presenting cells, are well described, and synergy with cellular immunotherapy, including CTLA-4 blockade, has been demonstrated in murine models (41). In an ongoing clinical phase 1/2 trial, the NCI is studying radiation-targeted DLI for relapse after AlloSCT, looking at systemic effects of single-fraction radiation to isolated tumors, including safety, clinical responses outside the radiation field, and effects on allogeneic lymphocyte populations (NCT00984165).
Second Allogeneic Transplantation
Although there are no prospective trials, second AlloSCT is frequently used for relapse after AlloSCT, particularly in acute leukemia. Available evidence is based on retrospective registry data, mainly with second AlloSCT from the same HLA-identical related donor. With the important caveat that the recipients of second AlloSCT represented a highly selected minority of individuals with relapse(42), reported long-term overall survival (OS) was between 20–30%, with a respective cumulative incidence of relapse and NRM of around 40% each. Duration of remission after initial AlloSCT, disease status at initial and second AlloSCT and age were the most important factors for OS.
Second AlloSCT after unrelated-donor transplantation, donor selection for second AlloSCT and optimal second-transplant conditioning regimens remain open questions. Recently, national registry studies in Italy and Germany have examined these issues in relapsed acute leukemia (43, 44). Independent of donor selection at first AlloSCT, both groups found a trend for increased NRM after unrelated-donor second AlloSCT as compared to second transplant with a related donor. However, long-term survivors were identified even after two unrelated SCT. The intensity of second-transplant conditioning did not appear to influence OS, although NRM was lower after reduced-intensity conditioning.
Employment of a different donor for second AlloSCT was generally not associated with better OS, either in the related or unrelated setting. However, following relapse after unrelated-donor AlloSCT, the German study found a trend for improved OS after change to a different unrelated donor in patients without a history of acute or chronic GVHD after first AlloSCT. This suggests that there may be distinct subgroups of patients for whom increased GVT effects with a different donor may offset the risk of NRM. Prospective studies are needed to determine optimal patient selection, donor selection and conditioning regimens for second AlloSCT treatment of relapse.
III. STRATEGIES TO ENHANCE T CELL FUNCTION
Novel Cellular Therapies
Improving anti-tumor potency and specificity of donor cellular therapy for relapse after AlloSCT would optimize GVT and GVHD reactivity and likely improve efficacy. mHAg are important targets for T cell mediated GVHD and GVT reactivity (45). It may be possible to isolate and expand T cells recognizing mHAg selectively expressed on hematopoietic cells to induce GVT without GVHD (46). Alternatively, tumor-associated, over-expressed self antigens like WT-1, proteinase-3, or PRAME are promising targets for T cell directed GVT responses.
While feasible, the generation of tumor-specific CTL has proven time-consuming and often difficult. An alternative strategy to enhance GVT activity is donor T-cell activation and expansion with CD3/CD28 costimulation ex vivo. In preliminary studies, ex-vivo activated DLI (aDLI) yielded several responses notably even in patients with typically DLI-refractory tumors, such as AML, ALL and non-Hodgkin lymphomas, suggesting that aDLI may offer greater GVT potency (47). This also provides a possible strategy to obtain cells for adoptive immunotherapy for relapse prevention or treatment after umbilical cord blood transplant when it is not possible to recontact the donor. Based on a hypothesis that tumor-infiltrating lymphocytes found after AlloSCT relapse are donor cells and an enriched source of tumor-specific lymphocytes, a phase 1 trial demonstrated donor origin, feasibility and safety of administering ex-vivo CD3/CD28 costimulated and expanded tumor-derived donor lymphocytes. It also provided evidence that tumor-antigen reactive donor T cells were expanded, plausibly yielding cell products enriched for tumor-specific CTLs (48). While responses to tumor-derived donor lymphocyte infusion (TDL) were of short duration in the DLI-refractory patients treated, this approach could provide DLI therapy for patients without another source of donor cells.
It is possible to activate and expand donor cells with other biological activities. For instance, cytokine-induced killer (CIK) cells can be generated in vitro and expanded for clinical use. These unique cells are derived from cytotoxic T cells that express CD3 and CD56 and recognize targets through the NKG2D activating receptor. Importantly, cell killing is HLA-unrestricted and TCR-independent, can kill leukemia cells in vitro, and induce minimal GVHD in animal models. Expanded donor CIK cells have been given to a small number of patients with relapsed malignancy after AlloSCT (49). While there was minimal GVHD; only 1/18 patients had a sustained remission; however a number of prolonged remissions, several in patients who did not respond to conventional DLI, suggests that CIK cells may contribute to GVT activity without GVHD. For maximum effect, CIK cells will likely have to be used prior to overt hematologic relapse in high-risk patients, i.e., in a preventive or preemptive approach, or in combination with other relapse therapies.
In addition to the critical role of T cells, NK cells are increasingly implicated as important mediators of GVT activity, particularly in myeloid diseases and in the setting of haploidentical transplant (50); NK cell biology and implications for graft selection and ways to exploit their therapeutic potential were discussed in detail during the Workshop (63; 62). Plausibly, it might be possible to augment NK-mediated GVL through increasing availability of endogenous interleukin-15 (IL-15), a key cytokine for NK cell development, expansion and function, in vivo. This theory can now be tested in the clinic, with recombinant human IL-15 now in clinical trials; alternatively, the novel IL-15 superagonist, ALT-803, which has shown promise in early biological testing (51) is slated to begin clinical testing later this year.
Targeted Therapies
Targeted therapies hold the promise of anti-tumor activity without inducing non-specific GVHD. This is particularly true for targeted antibodies (reviewed in 2009 Workshop Proceedings (1)). A key limitation is that tumor-specific targets are not well defined for most hematologic malignancies. Potential targets have been exploited in previous studies, e.g., with the monoclonal antibodies CD52 (alemtuzumab) and CD20 (rituximab), and with antibody-drug conjugates, e.g., CD33 (gemtuzumab ozogamicin), CD25 (denileukin diftitox) and CD22 (inotuzumab ozogamicin); all small studies demonstrating limited clinical activity in AlloSCT relapse (1). The limited activity might be related in part due to variable or weak target expression or variable or incomplete activity of the targeted agent. In addition, non-specific toxicities develop when the target is broadly expressed on other cell types and tissues.
CD19 is an ideal tumor target, with expression largely restricted to normal and malignant B cells. The antibody blinatumomab is a bi-specific T-cell engaging antibody specific for CD19 and CD3. It serves to direct cytotoxic T cells to CD19-expressing target cells (52) and has activity in both non-Hodgkin’s lymphoma and acute lymphoblastic leukemia (ALL). Impressively, in ALL relapse after conventional chemotherapy blinatumomab induced CR in 68% of patients (53). Few patients have been treated with blinatumomab for ALL relapse after AlloSCT. A small case series reported hematologic CR in 3/3 pediatric patients, though disease rapidly recurred in two of those treated (54). Given its specificity, clinical activity and toxicity profile, it is reasonable to test blinatumomab in a larger group of patients with post-AlloSCT ALL relapse, alone or in combination with other agents.
Targeted cellular therapy may be even more promising since T cells can both expand, amplifying their effect, and persist in vivo, providing long-term vaccine-like anti-tumor activity. Efficient gene transfer techniques now permit genetic modification of T cells to confer novel antigen specificity by stably expressing novel T cell receptors, i.e., chimeric antigen receptors (CARs) on their surface. CAR-modified T cells become activated and kill in an antigen-dependent but HLA-independent manner, making this an attractive approach for any tumor with a defined target. Recently it was shown that T cells expressing a CAR targeting CD19 linked to a potent signaling domain (CD137/4-1BB) demonstrated massive in-vivo expansion, tumor-specific trafficking and long-term persistence in-vivo. CD19-CARs have induced rapid and sustained anti-tumor activity in chemotherapy-refractory CLL (55) and B cell lymphomas (56). These cells have also induced remission for refractory ALL and for relapse of ALL after umbilical cord blood transplant (57). It will be of great interest to continue to test this approach in relapse after AlloSCT, particularly for patients with ALL, CLL and NHL; ongoing trials evaluatingCD19-CAR transduced donor T cells appear promising (57, 58).
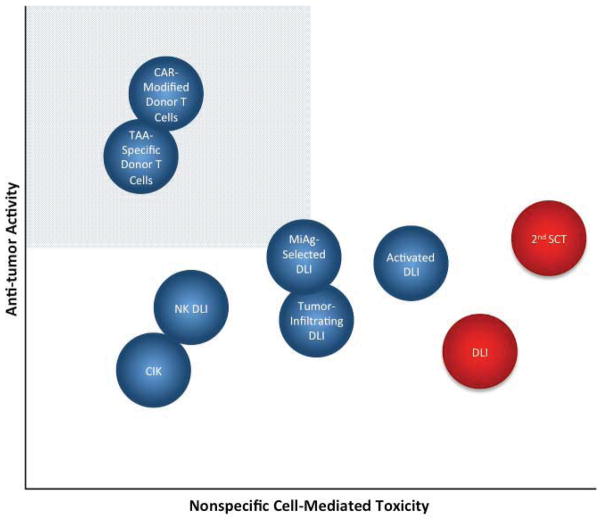
Any new cellular therapy ultimately has to be judged in relationship to conventional DLI or second allogeneic SCT. For most indications other than CML, conventional DLI has some, albeit disappointing, activity and is associated with significant GVHD-related toxicity. Second AlloSCT may be curative for a subset of patients though with extensive morbidity and mortality. Figure 2 depicts a theoretical assessment of the potential for some investigational cellular therapies to treat relapse, with the ideal cell therapy providing maximal antitumor tumor activity with minimal non-specific cell-mediated toxicity. It is hypothesized that non-specific ex-vivo activation of donor T cells, or generation of miAg directed T cells would enhance activity of DLI though limited studies suggest toxicity is similar to DLI. We believe that engineered tumor antigen-specific and CAR-modified donor T cells (such as CD19-directed CARs) have the potential to provide potent anti-tumor activity with limited if any GVHD, a hypothesis supported by early clinical observations (57–59). Numerous other cell therapy approaches hold promise, but as yet have shown limited clinical activity. We recognize that there are limited clinical data for any novel cell therapy other than conventional DLI, and so where any cellular therapy might fit into this idealized perspective could be subjected to vigorous debate. It will certainly require constant modification as well, as we believe the next few years will bring development of new therapies or modifications of existing approaches leading to enhanced activity and limited toxicity to treat relapse after AlloSCT.
Figure 2.
Theoretical relative therapeutic potential of cellular therapies for relapse. The shaded quadrant represents the zone of optimal specificity with respect to tumor vs. off-target cytotoxic tissue damage, which maximizes antitumor potency and minimizes cell-mediated morbidity. Conventional DLI and second AlloSCT (depicted in red) are the currently available cell-based treatments for relapse, against which novel therapies (blue) will be judged.
IV. FUTURE DIRECTIONS AND RESEARCH PRIORITIES
The Scientific Session on Prevention and Treatment of Relapse highlighted ongoing clinical development of new management approaches and, importantly, identified recent field advances that are ripe for clinical development. We remain optimistic about the future potential to treat relapsed disease. While there have been no major breakthroughs in the treatment results, there have been major advances in developing potential novel strategies. Several trial concepts were discussed in the Workshop’s Protocol Planning Committee meetings. The use of novel agents, immune stimulation and modulation, and enhanced cellular therapies all constitute critical areas for future study. Studying novel immune modulators and combinations of immune modulation with cellular therapy are particularly relevant and appropriate for initiation of multi-center prospective trials to treat relapse. A major achievement of the 2nd Workshop was participant commitment to the establishment of an international transplant relapse consortium, and development of a platform for conducting such multi-institutional trials to speed clinical investigation of relapse prevention and treatment strategies – of both novel approaches as well as those more commonly used, albeit understudied.
Acknowledgments
This work was supported in part by the Intramural Research Programs of the National Institute of Health: the National Cancer Institute/Center for Cancer Research and the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Porter DL, Alyea EP, Antin JH, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alyea EP, DeAngelo DJ, Moldrem J, et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2010;16:1037–1069. doi: 10.1016/j.bbmt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroger N, Miyamura K, Bishop MR. Minimal residual disease following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:S94–100. doi: 10.1016/j.bbmt.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9:579–590. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 5.Kroger N, Bacher U, Bader P, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on disease-specific methods and strategies for monitoring relapse following allogeneic stem cell transplantation. part II: chronic leukemias, myeloproliferative neoplasms, and lymphoid malignancies. Biol Blood Marrow Transplant. 2010;16:1325–1346. doi: 10.1016/j.bbmt.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Kroger N, Bacher U, Bader P, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: Methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2010;16:1187–1211. doi: 10.1016/j.bbmt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacher U, Haferlach T, Fehse B, Schnittger S, Kroger N. Minimal residual disease diagnostics and chimerism in the post-transplant period in acute myeloid leukemia. ScientificWorldJournal. 2011;11:310–319. doi: 10.1100/tsw.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oran B, de Lima M. Prevention and treatment of acute myeloid leukemia relapse after allogeneic stem cell transplantation. Curr Opin Hematol. 2011;18:388–394. doi: 10.1097/MOH.0b013e32834b6158. [DOI] [PubMed] [Google Scholar]
- 9.Zhang FH, Ling YW, Zhai X, et al. The effect of imatinib therapy on the outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology. 2013 doi: 10.1179/1607845412Y.0000000052. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Palmer JM, Thomas SH, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119:6379–6381. doi: 10.1182/blood-2012-03-418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharfan-Dabaja MA, Nishihori T, Otrock ZK, Haidar N, Mohty M, Hamadani M. Monoclonal antibodies in conditioning regimens for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lima M, Bonamino M, Vasconcelos Z, et al. Prophylactic donor lymphocyte infusions after moderately ablative chemotherapy and stem cell transplantation for hematological malignancies: high remission rate among poor prognosis patients at the expense of graft-versus-host disease. Bone Marrow Transplant. 2001;27:73–78. doi: 10.1038/sj.bmt.1702726. [DOI] [PubMed] [Google Scholar]
- 14.Elmaagacli AH, Beelen DW, Trenn G, Schmidt O, Nahler M, Schaefer UW. Induction of a graft-versus-leukemia reaction by cyclosporin A withdrawal as immunotherapy for leukemia relapsing after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23:771–777. doi: 10.1038/sj.bmt.1701672. [DOI] [PubMed] [Google Scholar]
- 15.Peggs KS, Kayani I, Edwards N, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2011;29:971–978. doi: 10.1200/JCO.2010.32.1711. [DOI] [PubMed] [Google Scholar]
- 16.Rettinger E, Willasch AM, Kreyenberg H, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118:5681–5688. doi: 10.1182/blood-2011-04-348805. [DOI] [PubMed] [Google Scholar]
- 17.Kumar AJ, Hexner EO, Frey NV, et al. A Pilot Study of Prophylactic Ex Vivo Co-Stimulated Donor Leukocyte Infusions After Reduced Intensity Conditioned Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Askenasy N, Mizrahi K, Ash S, Askenasy EM, Yaniv I, Stein J. Depletion of naive lymphocytes with fas ligand ex vivo prevents graft-versus-host disease without impairing T cell support of engraftment or graft-versus-tumor activity. Biol Blood Marrow Transplant. 2013;19:185–195. doi: 10.1016/j.bbmt.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 22.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood. 2012;119:3361–3369. doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 23.Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–389. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010;36:528–538. doi: 10.1016/j.ctrv.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosi A, Laszlo D, Labopin M, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 26.Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000;96:2712–2716. [PubMed] [Google Scholar]
- 27.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 28.Levine JE, Braun T, Penza SL, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 29.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 30.Guillaume T, Gaugler B, Chevallier P, et al. Escalated lymphodepletion followed by donor lymphocyte infusion can induce a graft-versus-host response without overwhelming toxicity. Bone Marrow Transplant. 2011;47:1112–1117. doi: 10.1038/bmt.2011.231. [DOI] [PubMed] [Google Scholar]
- 31.Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116:1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]
- 32.Lioznov M, El-Cheikh J, Jr, Hoffmann F, et al. Lenalidomide as salvage therapy after allo-SCT for multiple myeloma is effective and leads to an increase of activated NK (NKp44(+)) and T (HLA-DR(+)) cells. Bone Marrow Transplant. 2010;45:349–353. doi: 10.1038/bmt.2009.155. [DOI] [PubMed] [Google Scholar]
- 33.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib-Based Graft-Versus-Host Disease Prophylaxis in HLA-Mismatched Unrelated Donor Transplantation. J Clin Oncol. 2012;30:3202–3208. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weichsel R, Dix C, Wooldridge L, et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res. 2008;14:2484–2491. doi: 10.1158/1078-0432.CCR-07-4393. [DOI] [PubMed] [Google Scholar]
- 36.Robb RJ, Kreijveld E, Kuns RD, et al. Type I interferons control GVHD and GVL responses after transplantation. Blood. 2011 doi: 10.1182/blood-2010-12-325746. [DOI] [PubMed] [Google Scholar]
- 37.Cooper N, Rao K, Goulden N, Amrolia P, Veys P. Alpha interferon augments the graft-versus-leukaemia effect of second stem cell transplants and donor lymphocyte infusions in high-risk paediatric leukaemias. Br J Haematol. 2012;156:550–552. doi: 10.1111/j.1365-2141.2011.08889.x. [DOI] [PubMed] [Google Scholar]
- 38.Collins R, Shpilberg O, Drobyski W, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 39.Tang XW, Zhou QL, Lin ZM, et al. Novel Therapy with Interferon-alpha in Combination with Donor Lymphocyte Infusion for High Risk Acute Leukemia Patients Who Relapsed After Allogeneic Hematopoietic Stem Cell Transplantation. Blood. 2011;118:300–301. [Google Scholar]
- 40.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012 doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw BE, Mufti GJ, Mackinnon S, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transplant. 2008;42:783–789. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 43.Christopeit M, Feldmann U, Finke J, et al. Shift to a New Donor Does Not Improve the Outcome After Second Allogeneic Stem Cell Transplantation (alloSCT) in Acute Leukemia Relapse After a First Allosct - a Risk Factor Analysis by the German Stem Cell Registry (DRST) Blood. 2009;114:1291–1291. [Google Scholar]
- 44.Dominietto A, Bacigalupo A, Bruno B, et al. Second allogeneic HSCT in patients with acute leukaemia relapsed after a first allogeneic transplantation: a retrospective GITMO study. Bone Marrow Transplant Supplement. 2012:47. [Google Scholar]
- 45.Falkenburg JH, Marijt WA, Heemskerk MH, Willemze R. Minor histocompatibility antigens as targets of graft-versus-leukemia reactions. Curr Opin Hematol. 2002;9:497–502. doi: 10.1097/00062752-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Miller JS, Warren EH, van den Brink MR, et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol Blood Marrow Transplant. 2010;16:565–586. doi: 10.1016/j.bbmt.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 48.Hardy NM, Fellowes V, Rose JJ, et al. Costimulated tumor-infiltrating lymphocytes are a feasible and safe alternative donor cell therapy for relapse after allogeneic stem cell transplantation. Blood. 2012;119:2956–2959. doi: 10.1182/blood-2011-09-378398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1679–1687. doi: 10.1016/j.bbmt.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Marcus WD, Xu W, et al. Novel human interleukin-15 agonists. J Immunol. 2009;183:3598–3607. doi: 10.4049/jimmunol.0901244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bargou R, Leo E, Zugmaier G, et al. Tumor Regression in Cancer Patients by Very Low Doses of a T Cell-Engaging Antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 53.Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 54.Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25:181–184. doi: 10.1038/leu.2010.239. [DOI] [PubMed] [Google Scholar]
- 55.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kochenderfer JN, Dudley ME, Maric I, et al. Dramatic Regression of Chronic Lymphocytic Leukemia in the First Patient Treated With Donor-Derived Genetically-Engineered Anti-CD19-Chimeric-Antigen-Receptor-Expressing T Cells After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2011;17:S158a. [Google Scholar]
- 59.Lee DW, Shah N, Stetler-Stevenson M, et al. Autologous-collected anti-CD19 chimeric antigen receptor T cells (19CARTs) for pediatric acute lymphocytic leukemia (ALL) and non-Hodgkin lymphoma (NHL): Clinical activity and cytokine release without graft versus host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) ASCO Meeting Abstracts. 2013;31:10008. [Google Scholar]
- 60.Kneppers E, van der Holt B, Kersten MJ, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118:2413–2419. doi: 10.1182/blood-2011-04-348292. [DOI] [PubMed] [Google Scholar]
- 61.Braun TM, Thall PF, Nguyen H, de Lima M. Simultaneously optimizing dose and schedule of a new cytotoxic agent. Clin Trials. 2007;4:113–124. doi: 10.1177/1740774507076934. [DOI] [PubMed] [Google Scholar]
- 62.Avigan D, Hari P, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: part II. Autologous Transplantation-novel agents and immunomodulatory strategies. Biol Blood Marrow Transplant. 2013 Dec;19(12):1661–9. doi: 10.1016/j.bbmt.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gress RE, Miller JS, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: Part I. Biology of relapse after transplantation. Biol Blood Marrow Transplant. 2013 Nov;19(11):1537–45. doi: 10.1016/j.bbmt.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]