Abstract
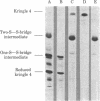
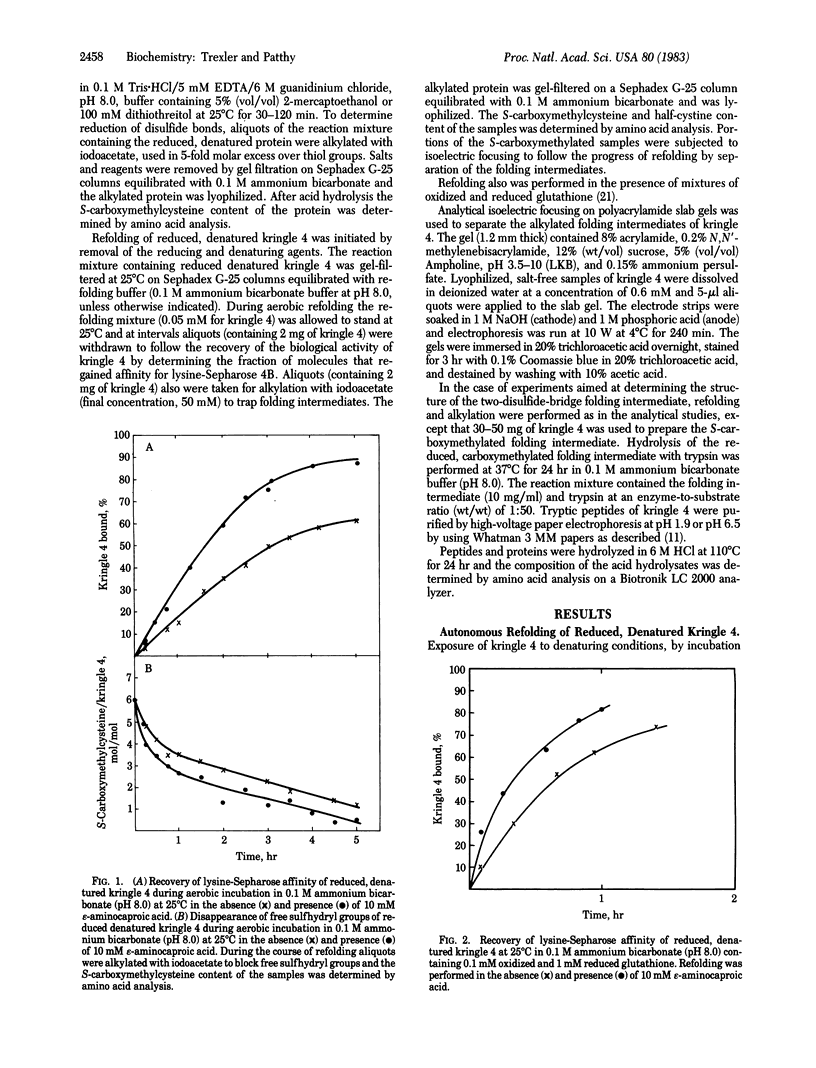
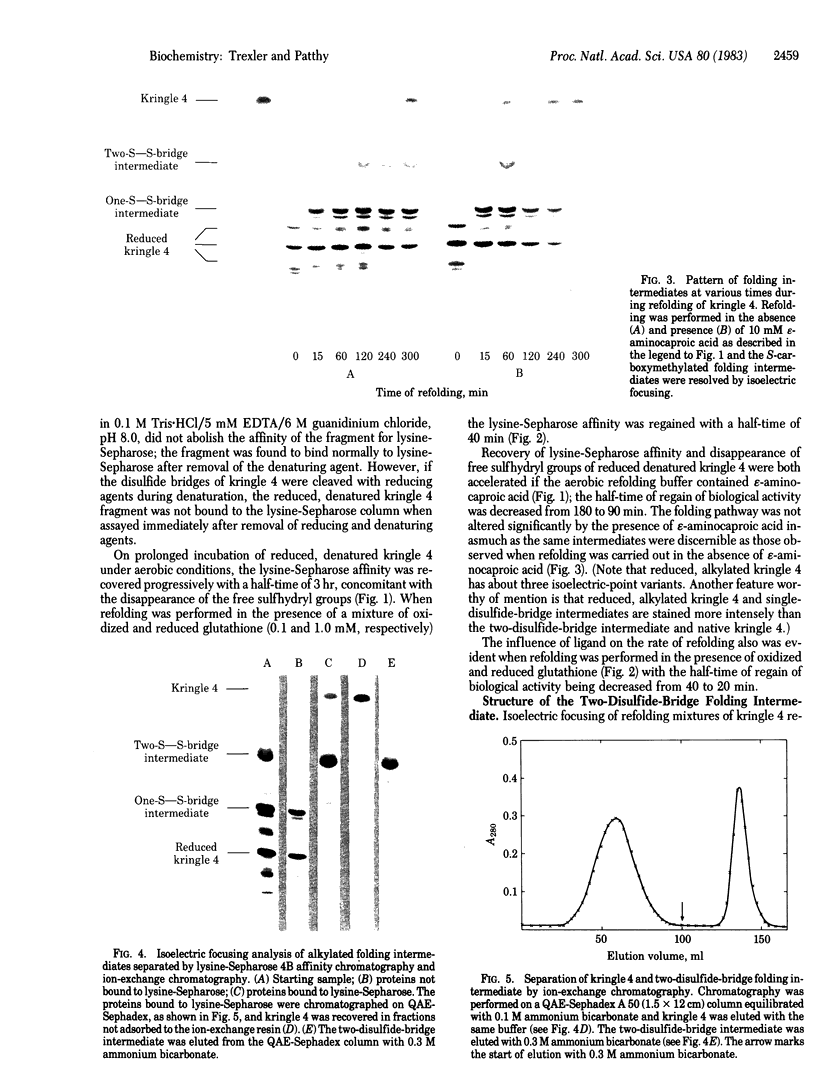
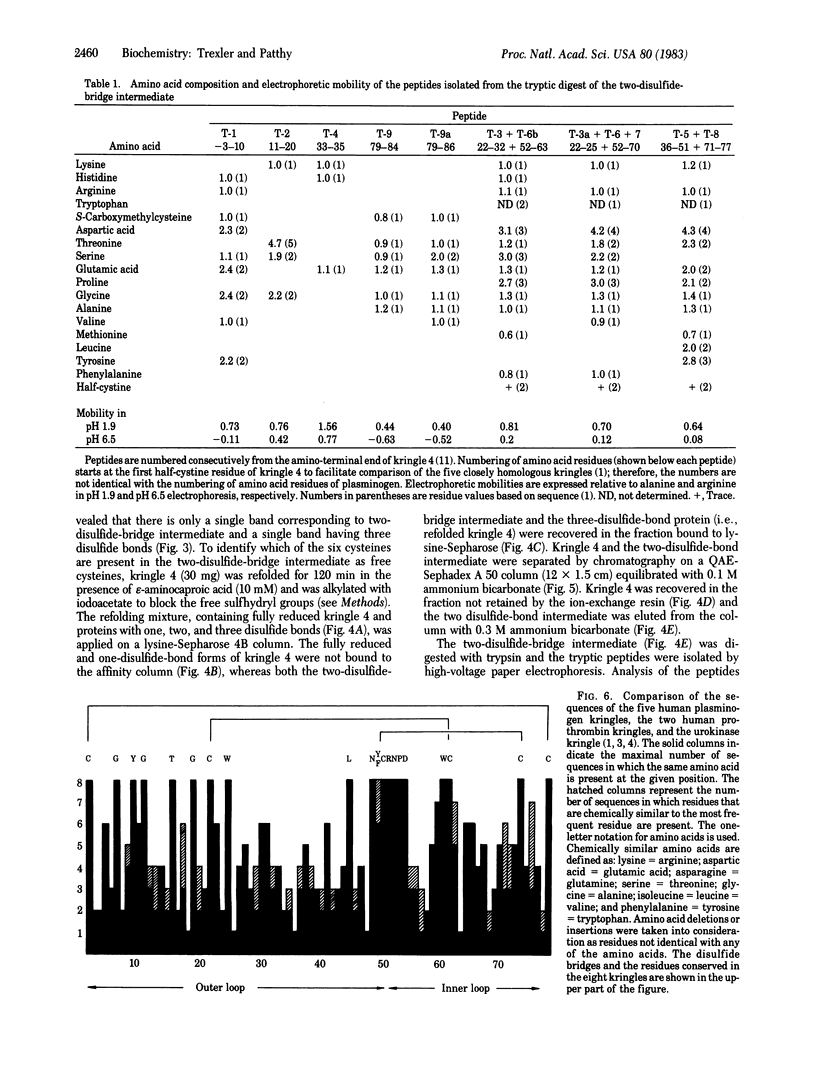
Kringle 4, an 88-residue plasminogen fragment carrying a lysine-binding site, loses its affinity for lysine-Sepharose upon reductive cleavage of its disulfide bridges. Aerobic incubation of the reduced, denatured fragment results in the rapid restoration of the disulfide bonds with concomitant recovery of lysine-Sepharose affinity. The ability of the unfolded fragment to regain its native conformation suggests that the kringle structure is an autonomous folding domain. During refolding of kringle 4 the native disulfide bonds, (formula; see text) and (formula; see text), appears first. The folding intermediate possessing these two disulfide bridges already binds to lysine-Sepharose, indicating that the third native bridge, which in native kringle 4 connects residues Cys1 and Cys79, is not essential for the maintenance of the biologically active conformation of kringle 4. Comparison of the sequences of human prothrombin, urokinase, and plasminogen kringles revealed that the residues surrounding the (formula; see text) and (formula; see text) bridges constitute the most conservative segments of kringles, whereas the residues neighboring the (formula; see text) bridge are not highly conserved. We propose that conservation of various residues in the different kringles reflects their importance for the folding autonomy of kringles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom J. W., Mann K. G. Prothrombin domains: circular dichroic evidence for a lack of cooperativity. Biochemistry. 1979 May 15;18(10):1957–1961. doi: 10.1021/bi00577a017. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Ploplis V. A., Powell J. R., Strickland D. K. The existence of independent domain structures in human Lys77-plasminogen. J Biol Chem. 1981 May 25;256(10):4778–4782. [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Dombrose F. A., Gitel S. N., Zawalich K., Jackson C. M. The association of bovine prothrombin fragment 1 with phospholipid. Quantitative characterization of the Ca2+ ion-mediated binding of prothrombin fragment 1 to phospholipid vesicles and a molecular model for its association with phospholipids. J Biol Chem. 1979 Jun 25;254(12):5027–5040. [PubMed] [Google Scholar]
- Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. IV. The function of the fragment 2 region during activation in the presence of factor V. J Biol Chem. 1974 Dec 25;249(24):7791–7797. [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding. Annu Rev Biochem. 1982;51:459–489. doi: 10.1146/annurev.bi.51.070182.002331. [DOI] [PubMed] [Google Scholar]
- Kurosky A., Barnett D. R., Lee T. H., Touchstone B., Hay R. E., Arnott M. S., Bowman B. H., Fitch W. M. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3388–3392. doi: 10.1073/pnas.77.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch P. G., Rickli E. E., Lergier W., Gillessen D. Localization of individual lysine-binding regions in human plasminogen and investigations on their complex-forming properties. Eur J Biochem. 1980;107(1):7–13. doi: 10.1111/j.1432-1033.1980.tb04617.x. [DOI] [PubMed] [Google Scholar]
- Markus G., Priore R. L., Wissler F. C. The binding of tranexamic acid to native (Glu) and modified (Lys) human plasminogen and its effect on conformation. J Biol Chem. 1979 Feb 25;254(4):1211–1216. [PubMed] [Google Scholar]
- Ploplis V. A., Strickland D. K., Castellino F. J. Calorimetric evaluation of the existence of separate domains in bovine prothrombin. Biochemistry. 1981 Jan 6;20(1):15–21. doi: 10.1021/bi00504a003. [DOI] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry. 1970 Dec 8;9(25):5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- Seegers W. H., Novoa E., Walz D. A., Andary T. J., Hassouna H. I. Effects of prothrombin fragments on thrombin, on thrombin formation, and separation, and separation from Ac-globulin (factor V). Thromb Res. 1976 Jan;8(1):83–97. doi: 10.1016/0049-3848(76)90126-2. [DOI] [PubMed] [Google Scholar]
- Trexler M., Váli Z., Patthy L. Structure of the omega-aminocarboxylic acid-binding sites of human plasminogen. Arginine 70 and aspartic acid 56 are essential for binding of ligand by kringle 4. J Biol Chem. 1982 Jul 10;257(13):7401–7406. [PubMed] [Google Scholar]
- Váli Z., Patthy L. Location of the intermediate and high affinity omega-aminocarboxylic acid-binding sites in human plasminogen. J Biol Chem. 1982 Feb 25;257(4):2104–2110. [PubMed] [Google Scholar]
- Váradi A., Patthy L. Kringle 5 of human plasminogen carries a benzamidine-binding site. Biochem Biophys Res Commun. 1981 Nov 16;103(1):97–102. doi: 10.1016/0006-291x(81)91665-x. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Hewett-Emmett D., Seegers W. H. Amino acid sequence of human prothrombin fragments 1 and 2. Proc Natl Acad Sci U S A. 1977 May;74(5):1969–1972. doi: 10.1073/pnas.74.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetlaufer D. B. Folding of protein fragments. Adv Protein Chem. 1981;34:61–92. doi: 10.1016/s0065-3233(08)60518-5. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. Molecular mechanism of physiological fibrinolysis. Nature. 1978 Apr 6;272(5653):549–550. doi: 10.1038/272549a0. [DOI] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]