Abstract
BACKGROUND
Thrombopoietin receptor agonists (TRAs) are effective treatments for immune thrombocytopenia (ITP). However, continuous therapy is generally required to maintain platelet (PLT) count responses.
STUDY DESIGN AND METHODS
In this case series, we describe ITP patients from our practice who achieved durable responses to the TRAs romiplostim and eltrombopag. Patients were classified as having a definite TRA-induced remission if PLT counts increased above 100 × 109/L after TRA treatment and remained above 100 × 109/L even after the medication was discontinued; or a possible TRA-induced remission if PLT counts increased above 100 × 109/L, remained elevated for at least 3 months after the medication was discontinued, but a subsequent relapse occurred or the effect of other disease-modifying therapies could not be excluded.
RESULTS
Of 31 patients with chronic ITP treated with TRAs in our practice, nine patients achieved a PLT count response with either romiplostim (n = 6) or eltrombopag (n = 3) that was maintained even after the medications were discontinued. Three patients met criteria for a definite TRA-induced remission, each after exposure to romiplostim. Patients had ITP for a median of 7.8 years and had failed a median of four prior therapies including eight patients who had a splenectomy. We documented a progressive decline in anti-glycoprotein IIbIIIa PLT autoantibodies in one patient while on treatment.
CONCLUSION
Some patients with ITP can achieve sustained PLT count responses after the use of TRAs. This observation raises the possibility that these agents may restore immune tolerance to PLT antigens in some patients and supports the practice of down titrating the dose.
Immune thrombocytopenia (ITP) is an autoimmune disorder that is characterized by low platelet (PLT) counts and results in an increased risk of bleeding.1 Thrombocytopenia develops because of the loss of tolerance to “self” proteins on PLTs or megakaryocytes, leading to the development of PLT autoantibodies.2 Conventional treatments are aimed at reducing peripheral PLT destruction, whereas a new class of medications called thrombopoietin receptor agonists (TRAs) stimulate mega-karyocyte growth and increase PLT production.3
Romiplostim and eltrombopag are two such thrombopoietic agents that have been approved for the treatment of chronic ITP. In Phase III trials, each has been shown to be remarkably effective compared with placebo or standard of care,4,5 with response rates of 60% to 80% in long-term follow-up studies.6,7 The PLT count response is usually maintained as long as the medication is continued; however, once it is stopped, PLT counts typically drop to pretreatment levels at which point patients may be at increased risk of bleeding.8
We report our observation that some patients treated with either romiplostim or eltrombopag achieved PLT count responses that were sustained even after these medications were discontinued. This observation generates hypotheses about their mechanisms of action and may have implications on prescribing practices.
MATERIALS AND METHODS
Patients were identified from a tertiary referral PLT disorders clinic at an academic hospital. This study was approved by the institutional research ethics board.
We defined a definite TRA-induced remission as 1) the achievement of a PLT count above 100 × 109/L; 2) continuation of PLT count above 100 × 109/L during treatment; and 3) persistence of PLT count above 100 × 109/L even after treatment was discontinued, without the use of concomitant maintenance therapies, rituximab or splenectomy within 2 months before starting the TRA. A possible TRA-induced remission was defined as the achievement of a PLT count above 100 × 109/L that persisted even after the TRA was discontinued, but either a relapse occurred or other disease-modifying treatments had been administered before the TRA. Baseline demographic and laboratory data were summarized descriptively. Sequential PLT counts and all ITP therapies were collected by chart review.
A test for PLT-bound autoantibody was performed where possible using the direct antigen capture assay. Washed PLTs were lysed (100 μL) and added to wells containing monoclonal antibodies to GPIIbIIIa (Raj-1) or GPIbIX (TW-1) as previously described.9 Autoantibodies bound to the glycoprotein were detected using alkaline phosphatase conjugated goat anti-human immunoglobulin G followed by p-nitrophenyl phosphate substrate (optical density [OD], 450 nm).
RESULTS
Patients
This study describes a subset of ITP patients from among the cohort of all patients treated with TRAs from our practice. Between April 2009 and July 2012, we treated a total of 31 ITP patients with TRAs. Of those, eight patients remained on the medication, 23 discontinued therapy, and one patient died. Reasons for discontinuing therapy were lack of response (n = 8), achievement of PLT count response with other therapies (n = 2), inability to pay for the medication (n = 2), development of deep vein thrombosis (n = 1), and sustained PLT count responses off-therapy (n = 9). Those latter patients form the basis of this case series (Table 1).
TABLE 1.
Characteristics of patients (n = 9) with ITP who achieved a durable PLT count response associated with the use of TRAs
| Patient | Age (years) | Sex | ITP duration (years) | Number of prior treatments | Pretreatment PLT count (×109/L) | TRA | Treatment duration (months)* | Remission off TRA (months) | Relapse | Sustained remission† |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Female | 8 | 5 | 19 | Romiplostim | 5 | 20 | No | Definite |
| 2 | 37 | Male | 2 | 3 | 5 | Romiplostim | 0.5 | 28 | No | Definite |
| 3 | 80 | Male | 4 | 3 | 3 | Romiplostim | 11 | 11 | No | Definite |
| 4 | 21 | Female | 2 | 6 | 7 | Eltrombopag | 13 | 26 | No | Possible |
| 5 | 53 | Male | 16 | 4 | 23 | Romiplostim | 14.5 | 3 | Yes | Possible |
| 6 | 57 | Male | 11 | 6 | 6 | Romiplostim | 1 | 31 | No | Possible |
| 7 | 43 | Female | 17 | 3 | 11 | Eltrombopag | 11 | 3.5 | Yes | Possible |
| 8 | 30 | Male | 25 | 9 | 4 | Romiplostim | 0.5 | 8 | Yes | Possible |
| 9 | 21 | Male | 3 | 4 | 13 | Eltrombopag | 5 | 3 | Yes | Possible |
Treatment duration refers to first treatment course for those patients who relapsed.
Definite TRA-induced remission was defined as the achievement of a PLT count above 100 × 109/L after TRA treatment that was maintained during treatment and that persisted even after treatment was discontinued, without the use of concomitant maintenance therapies, rituximab or splenectomy within 2 months before starting the TRA. A possible TRA-induced remission was defined as the achievement of a PLT count above 100 × 109/L that persisted even after the TRA was discontinued, but either a relapse occurred or other disease-modifying treatments had been administered before the TRA.
Of the nine patients with sustained remissions, six had received romiplostim and three had received eltrombopag. Median age of patients was 43 years (interquartile range [IQR], 30–57 years), three (33%) were female, and median duration of ITP before starting treatment was 7.8 years (IQR, 2.9–16.0 years). Before TRA therapy, these nine patients had received a median of four ITP therapies (IQR 3–6), and eight patients had undergone splenectomy. The median weekly stable dose of romiplostim was 1.0 μg/kg subcutaneously, and the median daily stable dose of eltrombopag was 12.5 mg orally. For most patients, medication doses were slowly tapered after a PLT count response was achieved, until the medication was successfully discontinued. Mean PLT count during treatment was 218 × 109/L and mean PLT count after stopping treatment was 270 × 109/L.
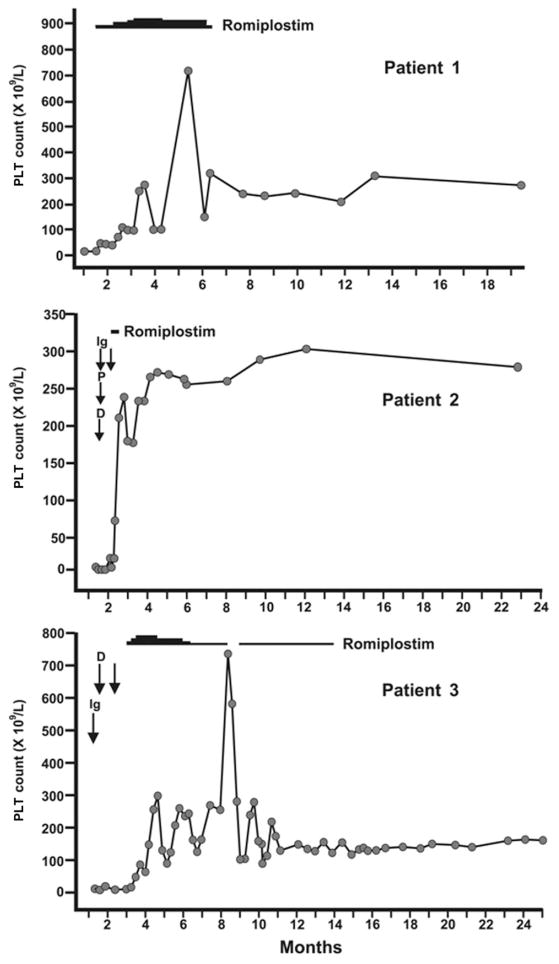
Of the nine patients, three (33%) had a definite TRA-induced remission (Fig. 1). Patient 1 was a 59-year-old female who received romiplostim at a starting dose of 1.0 μg/kg. She had ITP for 7.8 years and had received five prior ITP treatments, including splenectomy 5 years earlier. Before starting romiplostim, the PLT count was 20 × 109/L. After 1 month, the PLT count was 111 × 109/L. The drug was stopped after 5 months and PLT counts remained above 150 × 109/L until the end of follow-up, 20 months later. Patient 2 was a 37-year-old male who received two weekly doses of romiplostim (1.0 μg/kg). He had ITP for 2 years and had received three prior treatments. Before romiplostim was started, the patient had received a 4-week course of prednisone with no effect and the PLT count was 6 × 109/L. After two doses of romiplostim, the PLT count increased to 208 × 109/L and romiplostim was held. No further doses were required, and the patient remained in remission until the end of follow-up 28 months later. Patient 3 was an 80-year-old male who received romiplostim 1.0 μg/kg. He had ITP for 4 years and had received three prior treatments including splenectomy 2 years earlier. Before romiplostim, the PLT count was 10 × 109/L. After 6 weeks of treatment, the PLT count increased to 252 × 109/L. After 14 weeks, the dose was tapered to 0.5 μg/kg weekly and then to 0.5 μg/kg monthly. After 11 months, romiplostim was stopped and the patient remained in complete remission until the end of follow-up 11 months later.
Fig. 1.
Serial PLT counts and treatments for patients with ITP who achieved a definite TRA-induced sustained remission. Ig = intravenous immune globulin; D = dexamethasone; P = prednisone.
Six other patients met criteria for a possible TRA-induced remission either because the effect of other therapies could not be excluded or a relapse occurred. Patient 4 was a 21-year-old female who received eltrombopag (50 mg daily). She had ITP for 2 years and had received six prior treatments. Ten weeks before starting eltrombopag, she had an urgent splenectomy for refractory thrombocytopenia and bleeding. Six weeks before starting eltrombopag, she began a 4-week course of rituximab with no response. When eltrombopag was started, the PLT count was 9 × 109/L, and after 2, 4, and 8 weeks of treatment, the PLT count increased to 45 × 109, 62 × 109, and 247 × 109/L. Eltrombopag was slowly tapered and then stopped after 13 months. The PLT count remained above 150 × 109/L at last follow-up, 26 months after stopping eltrombopag. Patient 5 received romiplostim (1.0 μg/kg) for a PLT count of 23 × 109/L. PLT counts were 358 × 109, 146 × 109, and 243 × 109/L, after 1, 2, and 4 weeks of therapy. The drug was continued at a reduced dose for an additional 14.5 months and then gradually stopped with PLT counts remaining above 100 × 109/L. This patient experienced a brief decrease in the PLT count to 30 × 109/L after an upper respiratory tract illness; however, PLT counts recovered spontaneously. Patient 6 had relapsed ITP. Intravenous immune globulin (IVIG) and dexamethasone produced only brief responses. This patient was started on azathioprine and PLT counts remained below 10 × 109/L after 5 weeks of therapy. At that time, with a PLT count of 6 × 109/L, romiplostim (1 μg/kg) was started and after three weekly doses, the PLT count rapidly increased to 56 × 109, 251 × 109, and 546 × 109/L. Romiplostim was discontinued and PLT counts have remained in the normal range 2.5 years later. Azathioprine was continued, however, and the patient remained on low-dose azathioprine at the time of the last follow-up. Three other patients had durable remissions after the TRA was stopped but relapsed after a median of 3.5 months (IQR, 3.3–5.8 months). Relapses were generally brief and occurred in association with a febrile illness. One patient was successfully re-treated with romiplostim.
PLT autoantibody testing
Of the nine patients with sustained PLT count responses, six had PLT autoantibody testing done, of whom two had detectable PLT autoantibodies. One patient had a strong antiglycoprotein IIbIIIa antibody (OD, 2.5) before starting therapy. The autoantibody titer decreased progressively (OD, 0.22) as the PLT count increased with ongoing treatment.
DISCUSSION
We describe nine patients who unexpectedly achieved durable PLT count responses after stopping TRA treatment. Of those, three patients met our criteria for a definite TRA-induced remission with PLT counts above 100 × 109/L (mostly above 150 × 109/L) that were sustained. All patients had severe ITP and had failed multiple previous therapies. For most patients, the dose of romiplostim or eltrombopag that was sufficient to achieve PLT count responses was low, indeed substantially lower than the mean dose administered in the efficacy trials.4,5,7 Moreover, many patients achieved a high PLT count (mean, 270 × 109/L) even after treatment was stopped. These observations suggest that this subset of patients was highly sensitive to the effect of these medications and may have a more pronounced defect in PLT production rather than PLT destruction.
First-line treatment of ITP in adults includes corti-costeroids and IVIG; however, many patients relapse and require additional treatments.1 Splenectomy remains the most effective second-line treatment;10 however, it is associated with a lifelong risk of bacterial infection and many patients are reluctant to accept it.11 Rituximab is another disease-modifying therapy that is commonly used to treat ITP.12 Across most studies, PLT count responses lasted 10.5 to 11.3 months13,14 and long-term remission rates are low.15 Combination immunosuppressant therapy has been associated with a PLT count response in up to 70% of patients with refractory ITP; however, relapses are common.16
The TRAs are a new class of medications that have been shown to be effective in increasing PLT counts,4,5 decreasing bleeding,17 and improving quality of life18 in patients with ITP. Currently, these medications are mainly used as maintenance therapy for patients with chronic, severe disease. Prolonged follow-up extending to 5 years has shown that the PLT-increasing effect is maintained even with long-term exposure;6,7 however, once these medications are stopped, the PLT count typically falls back to pretreatment levels or even lower, placing the patient at an increased risk of bleeding. Rare side effects of TRAs include thrombosis and marrow reticulin formation,19 although, the true risk of these complications remains uncertain. Potential long-term toxicities and high cost limit their use.20 For that reason, it is our practice to gradually reduce the dose if an excellent PLT count response is achieved.
Strengths of this report are that it is the largest published series to describe sustained remissions with this therapy. A single-patient case report21 and two patients from a large cohort6 have been described after the use of romiplostim. Our report describes our experience with both romiplostim and eltrombopag in real world practice. We were careful to exclude patients who had received rituximab or splenectomy in the preceding 2 months, similar to eligibility criteria used in a recent romiplostim trial,21 to avoid confounding by delayed effects of these treatments.10,13 We chose to report our observations after nine patients with TRA-induced sustained remissions were identified (including three with definite TRA-induced remissions) since this phenomenon occurred with a frequency that was higher than anticipated and had not been previously described using rigorous criteria. It is possible that our strategy of dose-tapering allowed us to more readily identify these patients. It is also likely that our prescribing pattern was more selective than in other jurisdictions, since reimbursement for TRAs in Ontario is restricted to patients with the most severe disease.
Our observations generate hypotheses about the mechanism of actions of these medications. For example, by increasing the exposure to PLT antigens, they may restore immune tolerance to PLTs. In support of that hypothesis was our finding of a progressive decline in PLT autoantibody titer with ongoing exposure to the drug. This concept is similar to immune tolerance induction in patients with hemophilia and inhibitory antibodies to Factor (F)VIII, in whom repeated exposure to FVIII, often given in conjunction with other immune suppressive therapies, causes disappearance of the antibody in up to 70% of patients.22 Epitope mapping studies in ITP have identified peptides of glycoprotein IIIa that are the most immunogenic and support a potential role for ITP immunotherapy in the future.23 In addition, the TRAs themselves may have a direct effect on restoring normal T-regulatory cell function.24 Alternatively, the sustained PLT count responses we observed may represent spontaneous remissions consistent with the natural history of ITP in a small proportion of adults. This explanation is unlikely given the severe nature of the disease among patients in this cohort.
In conclusion, we observed that some patients can maintain PLT count responses even after TRAs are stopped. By titrating to the lowest possible dose and temporarily discontinuing therapy once PLT counts improve, some patients may be spared the need for long-term maintenance therapy.
Acknowledgments
This study was funded by Canadian Institutes for Health Research (Operating Grant #102446).
We thank Aurelio Santos for illustrating the figure and Jim Smith for performing the platelet autoantibody testing. BG performed the research, analyzed the data, and wrote the manuscript; DMA designed the research study, interpreted the results, and analyzed the manuscript; IN interpreted the results and critically revised the manuscript; and JGK designed the research, interpreted the results, and critically revised the manuscript.
ABBREVIATIONS
- ITP
immune thrombocytopenia
- TRA(s)
thrombopoietin receptor agonist(s)
Footnotes
CONFLICT OF INTEREST
DMA received grant funding for investigator-sponsored research in ITP from Amgen Canada, GlaxoSmithKline (GSK) and Hoffmann-LaRoche; was a member of advisory boards for Amgen and GSK; and received speaking honoraria from Amgen, GSK and Hoffmann-LaRoche. JGK was an advisory board member for Amgen and GSK. BG and IN have no conflict of interests to declare.
References
- 1.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 2.Toltl LJ, Nazi I, Jafari R, Arnold DM. Piecing together the humoral and cellular mechanisms of immune thrombocytopenia. Semin Thromb Hemost. 2011;37:631–9. doi: 10.1055/s-0031-1291373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold DM, Nazi I, Kelton JG. New treatments for idiopathic thrombocytopenic purpura: rethinking old hypotheses. Expert Opin Investig Drugs. 2009;18:805–19. doi: 10.1517/13543780902905848. [DOI] [PubMed] [Google Scholar]
- 4.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–99. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 6.Khellaf M, Michel M, Quittet P, Viallard JF, Alexis M, Roudot-Thoraval F, Cheze S, Durand JM, Lefrere F, Galicier L, Lambotte O, Panelatti G, Slama B, Damaj G, Sebahoun G, Gyan E, Delbrel X, Dhedin N, Royer B, Schleinitz N, Rossi JF, Mahévas M, Languille L, Bierling P, Godeau B. Romiplostim safety and efficacy for immune thrombocytopenia in clinical practice: 2-year results of 72 adults in a romiplostim compassionate-use program. Blood. 2011;118:4338–45. doi: 10.1182/blood-2011-03-340166. [DOI] [PubMed] [Google Scholar]
- 7.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–71. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 8.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser JS, Wiznitzer I, Kelly R, Chen CF, Nichol JL. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355:1672–81. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 9.Warner MN, Moore JC, Warkentin TE, Santos AV, Kelton JG. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br J Haematol. 1999;104:442–7. doi: 10.1046/j.1365-2141.1999.01218.x. [DOI] [PubMed] [Google Scholar]
- 10.Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–34. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]
- 11.Wang K, Charles C, Heddle NM, Arnold E, Molnar L, Arnold D. Understanding why patients with immune thrombocytopenia are deeply divided on splenectomy. Health Expect. 2012 Aug 7; doi: 10.1111/j.1369-7625.2012.00806.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold DM, Heddle NM, Carruthers J, Cook DJ, Crowther MA, Meyer RM, Liu Y, Cook RJ, McLeod A, MacEachern JA, Mangel J, Anderson D, Vickars L, Tinmouth A, Schuh AC, Kelton JG. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119:1356–62. doi: 10.1182/blood-2011-08-374777. [DOI] [PubMed] [Google Scholar]
- 13.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, Fraser GA, Lim W, Kelton JG. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 14.Auger S, Duny Y, Rossi JF, Quittet P. Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J Haematol. 2012;158:386–98. doi: 10.1111/j.1365-2141.2012.09169.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel VL, Mahevas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, Kanter J, Neufeld E, Taube T, Ramenghi U, Shenoy S, Ward MJ, Mihatov N, Patel VL, Bierling P, Lesser M, Cooper N, Bussel JB. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–95. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold DM, Nazi I, Santos A, Chan H, Heddle NM, Warkentin TE, Kelton JG. Combination immunosuppressant therapy for patients with chronic refractory immune thrombocytopenic purpura. Blood. 2010;115:29–31. doi: 10.1182/blood-2009-06-222448. [DOI] [PubMed] [Google Scholar]
- 17.Stasi R, Murali M, Michel M, Viallard JF, Giagounidis A, Janssens A, Legg J, Deuson R, Danese MD. Evaluation of bleeding-related episodes in patients with immune thrombocytopenia (ITP) receiving romiplostim or medical standard of care. Int J Hematol. 2012;96:26–33. doi: 10.1007/s12185-012-1088-8. [DOI] [PubMed] [Google Scholar]
- 18.Kuter DJ, Mathias SD, Rummel M, Mandanas R, Giagounidis AA, Wang X, Deuson RR. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 2012;87:558–61. doi: 10.1002/ajh.23163. [DOI] [PubMed] [Google Scholar]
- 19.Cuker A. Toxicities of the thrombopoietic growth factors. Semin Hematol. 2010;47:289–98. doi: 10.1053/j.seminhematol.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Y, Duan X, Xu J, Ni X. TPO receptor agonist for chronic idiopathic thrombocytopenic purpura. Cochrane Database Syst Rev. 2011;(7):CD008235. doi: 10.1002/14651858.CD008235.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, Wang YM, Nie K, Jun S. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118:28–36. doi: 10.1182/blood-2010-10-313908. [DOI] [PubMed] [Google Scholar]
- 22.Kruse-Jarres R. Current controversies in the formation and treatment of alloantibodies to factor VIII in congenital hemophilia A. Hematol Am Soc Hematol Educ Program. 2011;2011:407–12. doi: 10.1182/asheducation-2011.1.407. [DOI] [PubMed] [Google Scholar]
- 23.Sukati H, Watson HG, Urbaniak SJ, Barker RN. Mapping helper T cell epitopes on platelet membrane glycoprotein IIIa in chronic autoimmune thrombocytopenic purpura. Blood. 2007;109:4528–38. doi: 10.1182/blood-2006-09-044388. [DOI] [PubMed] [Google Scholar]
- 24.Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, Yazdanbakhsh K. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116:4639–45. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]