Abstract
The precise mechanisms leading to platelet-targeted autoimmunity in immune thrombocytopenia (ITP) are not known. Cellular checkpoints normally regulate immunological self-reactivity during the development of B and T cells through cell deletion, receptor editing, induction of anergy, and extrinsic cellular suppression. When these checkpoints fail, tolerance to self-antigens may be lost. In this review, we summarize the various immune mechanisms contributing to the development of ITP and relate them back to the checkpoint model of autoimmunity. These mechanisms, including increased levels of lymphocyte growth factors, resistance to death signals, and loss of T-regulatory function, result in an environment permissive to the development of platelet-reactive B and T cells. The mechanisms that lead to thrombocytopenia once tolerance for platelet antigens is lost are examined, including complement-dependent and apoptotic pathways. An improved understanding of ITP pathogenesis will ultimately guide the development of better therapies.
Keywords: Immune thrombocytopenia, self-tolerance, platelets, megakaryocytes, autoantibody
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by platelet autoantibodies, low platelet counts, and an increased risk of bleeding. ITP is a clinically and pathologically heterogeneous syndrome, implying that its physical signs and symptoms as well as responses to treatments are variable. Furthermore, the results of immunological tests including platelet autoantibodies are inconsistent across patients.1,2 This diversity suggests that there might be multiple underlying mechanisms and supports the notion that ITP is an overarching designation that includes subtypes of patients with different immune causes of thrombocytopenia and different treatment requirements.3
Loss of self-tolerance for platelet autoantigens is fundamental to the pathogenesis of ITP. Cellular safeguards normally regulate self-reactive receptors during Band T-cell differentiation; however, when these regulatory mechanisms fail, tolerance to self-antigens is lost.4 In ITP the end result is an antibody-mediated attack on platelets and megakaryocytes causing severe thrombocytopenia. Many aspects of immune dysregulation in ITP have been investigated, but the findings often seem disconnected. This review attempts to piece together current knowledge of ITP immune pathogenesis relating it to an established model of autoimmunity. The first part of this review summarizes the immunological failures that may contribute to the loss of tolerance for host platelet antigens. The second part focuses on the mechanisms of thrombocytopenia as they affect both platelets and megakaryocytes.
LOSS OF SELF-TOLERANCE IN ITP
The human immune system is designed to detect and neutralize almost any invading pathogen, yet at the same time recognize and “tolerate” self-antigens. During B-cell differentiation in the bone marrow and T-cell differentiation in the thymus, immune effector cells are exposed to innumerable antigenic targets creating a vast repertoire of B- and T-cell receptors. In the process, some receptors will inadvertently recognize self antigens, including platelet glycoproteins in the case of ITP, and the cells bearing those receptors will become autoreactive. Several regulatory strategies prevent the propagation of autoreactive cells including cell deletion, receptor editing, induction of anergy, and extrinsic cellular suppression.4 Failure at any of these steps may lead to the development of ITP (Table 1).
Table 1.
Normal Immune Checkpoints Preventing the Development of Autoreactivity and the Failure of These Checkpoints in the Development of ITP
| Self-Tolerance Checkpoint | Cytokine or Cellular Dysfunction in ITP | Downstream Effects Contributing to the Development of ITP |
|---|---|---|
| Cell deletion (apoptosis) | ↑ BAFF | Survival and maturation of platelet-reactive B-cells |
| ↑ APRIL | ||
| ↑ Caspase 8 | T-cell resistance to apoptosis in active ITP | |
| ↓ Bax | ||
| ↑ Calpastatin | ||
| ↑ A20 | ||
| Receptor editing (somatic hypermutation V(D)J recombination) | Biased expression and clonal expansion of BCR V(H) repertoire | Expansion of platelet-autoreactive B-cell subsets |
| Biased expression and clonal expansion of TCR Vβ repertoire | Expansion of platelet-autoreactive T-cell subsets | |
| Intrinsic regulation (Induction of inhibitory receptors) | ↓ Antigen-specific anergy (CTLA4 defect?) | Overreactive T cells |
| Extrinsic regulation | ↓ T regs | Activation and proliferation of platelet-reactive T-helper and cytotoxic T cells |
ITP, immune thrombocytopenia; Bax, Bcl-2-associated X protein (see text for specific references; BCR, B-cell receptor; TCR, T-cell receptor; CTLA4, cytotoxic T-lymphocyte antigen 4; BAFF, B-cell activating factor; APRIL, a proliferation-inducing ligand; T regs, T-regulatory cells.
Cell Deletion
In the bone marrow, immunoglobulin (Ig) chain gene rearrangements construct the diversity of B-cell receptors. During differentiation, B cells with immunoglobulin G (IgG) molecules that bind strongly to marrow stromal cells (an indicator of self-reactivity) are destroyed.5 This process of clonal deletion is facilitated by the depletion of growth factors such as B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) and increased expression of Bcl-2 interacting mediator of cell death. BAFF plays a crucial role in B-cell development, survival, and Ig production.6 Excess BAFF protects self-reactive B cells from anergy7 and has been documented in humans with systemic lupus erythematosus, rheumatoid arthritis, and Sjögren syndrome.8–10 A study of 53 patients with chronic ITP found that BAFF levels were elevated in untreated patients with active disease (n = 26) and reduced following immunosuppressive treatment.11 Other studies have supported the association between ITP and high levels of BAFF.12–14 Similarly, levels of APRIL, a ligand that promotes B-cell maturation and survival, were found to be higher in patients with active ITP compared with patients in remission following corticosteroids or splenectomy.15
T cells with self-reactivity are negatively regulated by complex processes. T cells that react with self-peptides coexpressed with major histocompatibility complex I molecules are typically destroyed in the thymus.16 Deletion of self-reactive T cells requires cellular proteins such as the tyrosine kinase ZAP70,17 growth factor receptor-bound protein 2 (GRB2),18 misshapen Nck interacting kinase-related kinase (MINK),19 and proapoptotic signaling pathways. Altered expression of genes involved in apoptosis signaling, including Fas, interferon-gamma (IFN-γ), and interleukin-2 receptor β (IL2RB), Bcl-2-associated X protein (Bax), caspase 8 and A2020,21 have been demonstrated in patients with active ITP, suggesting that autoreactive T cells may be resistant to apoptosis.
The pathogenesis of ITP may also involve dys-regulated expansion of specific T-cell subsets, identified by their cytokine profiles. CD4+ T helper (Th) cells and CD8+ cytotoxic T cells can be categorized as type 1 (producing IFN-γ, interleukin-2 [IL-2], tumor necrosis factor-β [TNF-β]) and type 2 (producing IL-4, IL-5, IL-6, IL-10, IL-13).22 Th1 cytokines tend to promote a proinflammatory response to facilitate macrophage activation, proliferation of cytotoxic T cells and production of opsonizing antibodies.23,24 Th2 responses facilitate B-cell activation and proliferation and promotes antibody production.24 The production of cytokines from both Th1 and Th2 subsets has been termed a Th0 response.25 The balance of Th1 and Th2 subsets regulates the immune response under normal conditions, and this balance is skewed in many auto-immune diseases.26,27 Cytokine profiles in ITP patients tend to show Th0/Th1 polarization,28,29 with increased Th1/Th2 ratios in untreated patients.30 Levels of the Th1 chemokine CXCL10 have been shown to be higher in patients with active ITP compared with patients in remission,31 further suggesting a type 1-mediated response and an association with disease severity. Th17 cells, characterized by the production of the proinflammatory cytokine IL-17, may also be overrepresented in ITP32,33 and may correlate with the levels of Th1 cells.32
Receptor Editing
Self-reactive B cells, which escape destruction in the bone marrow, are induced to continue editing their receptor so that the chances of reactivity with self-antigens is diminished. The normal antibody repertoire shows restriction of V, D, and J gene recombinations34 and somatic mutations in the variable regions of the heavy (VH) and the light (VL) chain lead to diversity in the Ig receptor.35,36 Disruption in the machinery leading to a restricted repertoire has been implicated in ITP. As in other autoimmune diseases, certain VH loci have been shown to be over-represented.37,38 In two studies, patients with ITP had a higher restriction to VH6 gene family usage associated with a high level of somatic mutation in the VH6 genes.39,40 Thus, defects in the selection of the B-cell repertoire may be an important mechanism in the development of ITP.
As with B cells, oligoclonal T-cell expansion is a feature of several autoimmune diseases.41–43 In ITP, biased expression and clonal expansion of the T-cell receptor Vβ repertoire has been demonstrated44–46 and correlated with disease activity.47
Induction of Anergy
Another way of controlling self-reactive lymphocytes is to inhibit their function, that is, render them anergic. Self-reactive B cells may be inhibited by the down-regulation of their receptors, continued expression of death-promoting signaling pathways or prevention of differentiation into long-lived antibody-producing plasma cells via Toll-like receptor-9 (TLR9).48 Normally, T-cell responses can be inhibited by cytotoxic T-lymphocyte antigen 4 (CTLA4) to avoid overreactivity with self-antigens.49 In ITP, the induction of T-cell anergy by CTLA4-Ig, a recombinant fusion protein consisting of the extracellular domain of CTLA4 fused to the constant region of mouse or human Ig, resulted in tolerogenic dendritic cells incapable of stimulating platelet glycoprotein-specific T-cell responses.50,51 These data suggest that the loss of antigen-specific anergy, possibly through defects in CTLA4, may contribute to overreactive T cells in ITP.
Extrinsic Cellular Suppression
T-regulatory cells (T regs), identified by their expression of CD25 and Foxp3, are critical for maintaining immune tolerance by suppressing self-reactive lymphocytes.52 T regs suppress the production of cytokines by CD4+ and CD8+ T cells and limit CD8+ T-cell cytotoxicity.53 Impairment of T regs may contribute to the development of ITP because of decreased number or function.54,55 The number of T regs has been shown to increase in patients with ITP following treatment with coriticosteroids,56 rituximab (monoclonal anti-CD20),57 or both.58 Platelet count responses following rituximab treatment were associated with a normalization of defective T-cell responses,47 suggesting that the depletion of B cells may have important downstream effects on cellular immunity. Chronic ITP patients, treated with a thrombopoietin-receptor agonist (either romiplostim or eltrombopag), have demonstrated improvement in T-reg activity and increased transforming growth factor (TGF)-β1 levels, which were associated with platelet count responses.59
CD4+ Th cells facilitate B-cell activity and auto-antibody production. In chronic ITP, CD4+ Th cells may become stimulated by platelets themselves to secrete IL-2 and facilitate the differentiation of autoreactive B cells.60 CD4+ T cells from patients with ITP recognize and proliferate in response to several distinct epitopes on GPαIIbβ3,61,62 but not the native form of GPαIIbβ3.
PLATELET CROSS-REACTIVITY
Besides cellular mechanisms that may allow self-reactive cells to go unchecked, cross-reactive, cryptic, or altered antigens may deceive the immune system into thinking a self-protein is foreign.63 This process of molecular mimicry is exemplified by secondary ITP due to infection with Helicobacter pylori (H. pylori), human immunodeficiency virus (HIV), or hepatitis C virus (HCV).
In some patients with ITP and concomitant H. pylori infection, eradication of H. pylori can improve platelet counts64 independent of the effect of the antibiotics themselves.65 Antibodies eluted from platelets have specificity for H. Pylori cytotoxin-associated gene A (CagA) protein in some patients, suggesting antigenic mimicry with CagA.66 Platelet count responses to H. pylori eradication are more common in Japan, where infection rates are high and the CagA serotype is prevalent,67 than in North America and Europe, where H. pylori infection rates are low and the CagA serotype is infrequent.64
ITP associated with HIV may be due to cross-reactive antibodies or immune complex formation. Anti-HIV antibodies can cross-react with epitopes on GPβ3, causing platelet lysis.68,69 In addition, anti-HIV-1 gp120 immune complexes can cause accelerated platelet destruction70 and complement-independent platelet lysis.71 Ineffective platelet production may result from HIV infection of megakaryocytes, leading to apoptosis and defective platelet formation.72,73
Molecular mimicry has also been implicated as a mechanism for HCV-induced ITP as antibodies to HCV core envelope 1 have been shown to cross-react with platelet GPβ3.74
MECHANISMS OF THROMBOCYTOPENIA IN ITP
While accelerated platelet clearance is a hallmark of ITP pathogenesis, platelet production does not compensate adequately, suggesting that megakaryocytes are in-jured.75 Impaired platelet production in ITP is supported by evidence from radiolabeled autologous platelet studies showing normal or reduced platelet production in the context of thrombocytopenia,75 and by the remarkable success of thrombopoietin-receptor agonists in improving platelet counts in persons with ITP.76,77 The binding of pathogenic autoantibodies to platelet and megakaryocytes may cause thrombocytopenia by opsonization, the direct activation of complement or apoptotic pathways. Alternatively, cytotoxic T cells may have a direct lytic effect on platelets and/or mega-karyocytes (Fig. 1).
Figure 1.
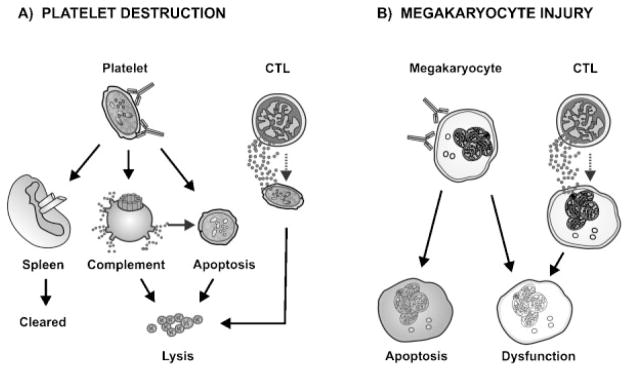
Mechanisms leading to thrombocytopenia in immune thrombocytopenia (ITP). (A) Autoantibody-mediated platelet destruction: Autoantibodies bind to platelets causing Fc-dependent clearance in the spleen, lysis by complement or apoptosis via caspase activation. Cytotoxic T cells (CTL) can also directly mediate platelet lysis. (B) Megakaryocyte injury: Autoantibodies can bind directly to megakaryocytes leading to agglutination, apoptosis, or dysfunction which can result in impaired platelet production. CTL can also impact megakaryocyte function by inhibiting platelet formation.
Pathogenic Autoantibodies
Not all autoantibodies are pathological.78,79 Naturally occurring autoantibodies can be of the IgM, IgG, or IgA class, and tend to be low affinity, polyreactive and germ-line encoded; whereas pathogenic autoantibodies are mainly of the IgG class, exhibit high affinity binding to self-antigens and are genetically mutated.80 In ITP, autoantibodies are most often directed against platelet GPIIbIIIa and GPIbIX, and mapping studies suggest that they target specific and distinct epitopes.81 Using direct glycoprotein-specific assays, antiplatelet autoanti-bodies are detectable in 60 to 70% of ITP patients.1
The autoantibody theory of ITP was conceived from experiments demonstrating that a transferable plasma factor caused thrombocytopenia in healthy volunteers.82 The plasma factor was first identified as platelet-associated IgG and later as platelet glycoprotein-specific IgG.2 Current evidence suggests that platelet autoantibodies may contribute to peripheral platelet destruction by Fc-mediated platelet clearance in the spleen,83 complement activation,84 and/or apoptosis.85,86 In addition, autoantibodies may target mega-karyocytes and interfere with their growth and function.87–89
OPSONIZATION OF PLATELETS
Platelet destruction and clearance by the reticuloendothelial system (RES) is the primary pathogenic mechanism for the development of ITP.90–92 This mechanism at least partially explains the rapid and robust platelet-count responses observed following treatment with intravenous immunoglobulin (IVIg) and Rh-immunoglobulin (for Rh+ individuals).93 Studies using IgG-sensitized red cells, as an in vivo measure of RES function, demonstrated reduced clearance of these cells with increased concentration of plasma monomeric IgG.94 Elevation in the concentration of IgG in the plasma progressively impaired RES function, with a dramatically impairment at concentrations seen after administration of high-dose IVIg. The spleen is the major site of clearance of antibody-coated platelets in ITP and splenectomy leads to a durable remission in up to 66% of patients.95 There is renewed interest in indium-labeled autologous platelet sequestration studies, which have shown a correlation between splenic sequestration patterns and response following splenectomy.96
DIRECT EFFECTS OF PLATELET AUTOANTIBODIES
In addition to targeting platelets for opsonization in the spleen, ITP autoantibodies may have direct effects on platelets as a result of complement activation84,97 or apoptosis.85 Decreased complement components in plasma, and deposition of complement on platelets, have been demonstrated in ITP, suggesting complement deposition may contribute to platelet clearance by complement receptors on macrophages in the spleen or direct platelet lysis.84 ITP plasma has been shown to contain higher complement activation capacity compared with plasma from thrombocytopenic and nonthrombocytopenic controls.97 The effects of ITP autoantibodies on megakaryocyte function are not fully known; however, IgG fractions from ITP sera can induce complement-dependent cytotoxicity to bone marrow megakaryocyte progenitor cells87 and antiglycoprotein antibodies may inhibit proplatelet and megakaryocyte colony formation.88 Some effects of anti-GPIb antibodies may be due to agglutination of megakaryocytes and proplatelet formation rather than a direct effect on megakaryocyte maturation.89
APOPTOSIS
Apoptosis is a noninflammatory form of programmed cell death characterized by cell shrinkage, phosphatidylserine exposure, disruption of mitochondrial membrane potential, DNA fragmentation, cell surface blebbing, and the formation of apoptotic bodies.98 Extrinsic apoptosis pathways involve ligand engagement to death receptors, and intrinsic apoptosis pathways involve members of the Bcl-2 family of proteins. Both require the activation of caspases to mediate intracellular apoptotic signaling ultimately cell death.98 Platelet autoantibodies that induce thrombocytopenia in mice trigger caspase activation in platelets, an effect preventable by the injection of a general caspase inhibitor.85 Anti-GPαIIb can trigger caspase-3 activation, phosphatidylserine exposure, and disruption in the mitochondrial transmembrane potential.86 IVIg may inhibit caspase-3 activation and phosphatidylserine exposure on platelets.86
Apoptotic processes may be more important at the level of the megakaryocyte, as platelet release from mature megakaryocytes in culture requires the induction of apoptotic pathways.99 Indeed, mice that lack proa-poptotic proteins or overexpress antiapoptotic proteins display a mild thrombocytopenic phenotype100,101 and proplatelet formation from megakaryocytes can be attenuated by caspase inhibition.102 Furthermore, in a study investigating familial thrombocytopenia, an apoptosis-enhancing mutation in cytochrome c (G41S) was found to cause premature platelet release and early platelet formation.103 Experimental evidence supports both increased and decreased megakaryocyte apoptosis in ITP. In megakaryocytes cultured with plasma from ITP patients, there was suppression of megakaryocyte growth and maturation.104,105 In ultrastructural studies, most bone marrow megakaryocytes in patients with ITP were extensively damaged and demonstrated structural abnormalities indicative of apoptosis.106
Thrombopoietin, an important factor mediating the growth, development, and ploidy of megakaryocytes,107 has been shown to have antiapoptotic properties108 and thus normally rescues megakaryocytes from early cell death. This may explain the remarkable success of thrombopoietin-receptor agonists in improving platelet counts in patients with ITP. On the other hand, inhibition of megakaryocyte apoptosis has also been shown to lead to thrombocytopenia.109 Reducing apoptosis through reduced levels of TNF-related apoptosis-inducing ligand (TRAIL) was shown to cause impaired platelet production even though megakaryocyte mass was increased.110 Thus, controlled apoptosis appears to be important for proper platelet release, yet dysregulated apoptosis may lead to megakaryocyte injury or death.
Direct Cytotoxic Effect of T Cells
In vitro data suggest that cytotoxic T cells from ITP patients may have direct lytic effects on platelets. CD8+ T cells from patients with active ITP but undetectable platelet autoantibodies bound to platelets in vitro and this causes direct platelet lysis, while CD8+ T cells from patients in remission did not have significant platelet reactivity.21 Furthermore, CD3+ cells from ITP patients showed increased expression of genes involved in cell-mediated cytotoxicity relative to controls, such as TNF-α, perforin, granzyme A, and granzyme B.21,111 Similarly, the expression of FasL and TNFα were increased in CD8+ T cells obtained from patients with chronic ITP.111 Additionally, severe ITP could be induced in a mouse model by antibodies and by CD8+ T cells.112 Although cytotoxic T cells may also target megakaryocytes in chronic ITP, activated CD8+ T cells in the bone marrow of chronic ITP patients have not been shown to cause megakaryocyte lysis ex vivo.113 Rather, CD8+ T cells may prevent megakaryocyte apoptosis, leading to impaired platelet production.113 Taken together, these studies suggest that T-cell dysfunction may be an important feature of chronic ITP and that in some patients, platelets and/or megakaryocytes may be targeted and destroyed by direct T-cell mediated mechanisms.
Summary
The pathogenesis of ITP involves the loss of immune regulation at various levels. In vitro and ex vivo studies suggest that the pathogenesis involves increased levels of lymphocyte growth factors, resistance to death signals, and loss of T-regulatory function, due to development and expansion of self-reactive B and T cells. Platelet antigens may be prone to autoimmune attack due to their structural similarities with antigens of infectious pathogens or their ability to form cryptic or altered epitopes. The final common pathway is the development of autoantibodies and/or cytotoxic T cells that targets platelets and megakaryocytes. Thrombocytopenia occurs because of Fc-dependent platelet clearance in the spleen and megakaryocyte apoptosis. New treatments such as the thrombopoietin-receptor agonists and rituximab have shed light on the mechanistic pathways in ITP. By furthering our understanding of disease pathogenesis, treatments will continue to be refined and tailored to individual patients.
Acknowledgments
We thank Dr. Mark Larche for his insightful comments on the manuscript. Aurelio Santos prepared the illustration. D.M. Arnold is funded by a New Investigator Award from the Canadian Institutes of Health Research in partnership with Hoffmann-LaRoche.
This study was funded by the Canadian Institutes of Health Research in partnership with Amgen Canada (grant #102446).
References
- 1.Warner MN, Moore JC, Warkentin TE, Santos AV, Kelton JG. A prospective study of protein-specific assays used to investigate idiopathic thrombocytopenic purpura. Br J Haematol. 1999;104(3):442–447. doi: 10.1046/j.1365-2141.1999.01218.x. [DOI] [PubMed] [Google Scholar]
- 2.McMillan R. Antiplatelet antibodies in chronic adult immune thrombocytopenic purpura: assays and epitopes. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S57–S61. doi: 10.1097/00043426-200312001-00013. [DOI] [PubMed] [Google Scholar]
- 3.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 5.Hartley SB, Cooke MP, Fulcher DA, et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72(3):325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 6.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168(12):5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 7.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44(6):1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166(1):6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 10.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. 2002;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmerich F, Bal G, Barakat A, et al. High-level serum B-cell activating factor and promoter polymorphisms in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2007;136(2):309–314. doi: 10.1111/j.1365-2141.2006.06431.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XJ, Shi Y, Sun JZ, et al. High-dose dexamethasone inhibits BAFF expression in patients with immune thrombocytopenia. J Clin Immunol. 2009;29(5):603–610. doi: 10.1007/s10875-009-9303-y. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XJ, Shi Y, Peng J, et al. The effects of BAFF and BAFF-R-Fc fusion protein in immune thrombocytopenia. Blood. 2009;114(26):5362–5367. doi: 10.1182/blood-2009-05-217513. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Chen Z, Li H, et al. BAFF and BAFF-R of peripheral blood and spleen mononuclear cells in idiopathic thrombocytopenic purpura. Autoimmunity. 2009;42(2):112–119. doi: 10.1080/08916930802397848. [DOI] [PubMed] [Google Scholar]
- 15.Gu D, Ge J, Du W, et al. Raised expression of APRIL in Chinese patients with immune thrombocytopenia and its clinical implications. Autoimmunity. 2009;42(8):692–698. doi: 10.3109/08916930903214025. [DOI] [PubMed] [Google Scholar]
- 16.Palmer E. Negative selection—clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3(5):383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi N, Takahashi T, Hata H, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426(6965):454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 18.Gong Q, Cheng AM, Akk AM, et al. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2(1):29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 19.McCarty N, Paust S, Ikizawa K, Dan I, Li X, Cantor H. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymocytes. Nat Immunol. 2005;6(1):65–72. doi: 10.1038/ni1145. [DOI] [PubMed] [Google Scholar]
- 20.Olsson B, Andersson PO, Jacobsson S, Carlsson L, Wadenvik H. Disturbed apoptosis of T-cells in patients with active idiopathic thrombocytopenic purpura. Thromb Haemost. 2005;93(1):139–144. doi: 10.1160/TH04-06-0385. [DOI] [PubMed] [Google Scholar]
- 21.Olsson B, Andersson PO, Jernås M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9(9):1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- 22.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 23.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80(3 Pt 1):225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 26.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4 + T cells in the pathogenesis of organ-specific auto-immune diseases. Immunol Today. 1995;16(1):34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 27.Druet P, Sheela R, Pelletier L. Th1 and Th2 cells in autoimmunity. Clin Exp Immunol. 1995;101(Suppl 1):9–12. doi: 10.1111/j.1365-2249.1995.tb06153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semple JW, Milev Y, Cosgrave D, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87(10):4245–4254. [PubMed] [Google Scholar]
- 29.Panitsas FP, Theodoropoulou M, Kouraklis A, et al. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103(7):2645–2647. doi: 10.1182/blood-2003-07-2268. [DOI] [PubMed] [Google Scholar]
- 30.Ogawara H, Handa H, Morita K, et al. High Th1/Th2 ratio in patients with chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;71(4):283–288. doi: 10.1034/j.1600-0609.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 31.Gu D, Chen Z, Zhao H, et al. Th1 (CXCL10) and Th2 (CCL2) chemokine expression in patients with immune thrombocytopenia. Hum Immunol. 2010;71(6):586–591. doi: 10.1016/j.humimm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Ma D, Zhu X, Qu X, Ji C, Hou M. Elevated profile of Th17, Th1 and Tc1 cells in patients with immune thrombocytopenic purpura. Haematologica. 2009;94(9):1326–1329. doi: 10.3324/haematol.2009.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Ma D, Zhang J, et al. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J Clin Immunol. 2010;30(2):253–259. doi: 10.1007/s10875-009-9353-1. [DOI] [PubMed] [Google Scholar]
- 34.Huetz F, Carlsson L, Tornberg UC, Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993;12(5):1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yancopoulos GD, Alt FW. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- 36.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 37.Fischer P, Jendreyko N, Hoffmann M, et al. Platelet-reactive IgG antibodies cloned by phage display and panning with IVIG from three patients with autoimmune thrombocytopenia. Br J Haematol. 1999;105(3):626–640. doi: 10.1046/j.1365-2141.1999.01407.x. [DOI] [PubMed] [Google Scholar]
- 38.Roark JH, Bussel JB, Cines DB, Siegel DL. Genetic analysis of autoantibodies in idiopathic thrombocytopenic purpura reveals evidence of clonal expansion and somatic mutation. Blood. 2002;100(4):1388–1398. [PubMed] [Google Scholar]
- 39.van Dijk-Härd I, Feld S, Holmberg D, Lundkvist I. Increased utilization of the VH6 gene family in patients with autoimmune idiopathic thrombocytopenic purpura. J Autoimmun. 1999;12(1):57–63. doi: 10.1006/jaut.1998.0257. [DOI] [PubMed] [Google Scholar]
- 40.Söderström I, van Dijk-Härd I, Feld S, Hillörn V, Holmberg D, Lundkvist I. Altered VH6-D-JH repertoire in human insulin-dependent diabetes mellitus and auto-immune idiopathic thrombocytopenic purpura. Eur J Immunol. 1999;29(9):2853–2862. doi: 10.1002/(SICI)1521-4141(199909)29:09<2853::AID-IMMU2853>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 41.Kato T, Kurokawa M, Sasakawa H, et al. Analysis of accumulated T cell clonotypes in patients with systemic lupus erythematosus. Arthritis Rheum. 2000;43(12):2712–2721. doi: 10.1002/1529-0131(200012)43:12<2712::AID-ANR11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto Y, Yoon WK, Jee Y, et al. Complementarity-determining region 3 spectratyping analysis of the TCR repertoire in multiple sclerosis. J Immunol. 2003;170(9):4846–4853. doi: 10.4049/jimmunol.170.9.4846. [DOI] [PubMed] [Google Scholar]
- 43.Sun W, Nie H, Li N, et al. Skewed T-cell receptor BV14 and BV16 expression and shared CDR3 sequence and common sequence motifs in synovial T cells of rheumatoid arthritis. Genes Immun. 2005;6(3):248–261. doi: 10.1038/sj.gene.6364166. [DOI] [PubMed] [Google Scholar]
- 44.Hedlund-Treutiger I, Elinder G, Wigzell H, Grunewald J, Wahlström J. T cell receptor V gene usage by CD4+ and CD8+ peripheral blood T lymphocytes in immune thrombocytopenic purpura. Acta Paediatr. 2004;93(5):633–637. [PubMed] [Google Scholar]
- 45.Shimomura T, Fujimura K, Takafuta T, et al. Oligoclonal accumulation of T cells in peripheral blood from patients with idiopathic thrombocytopenic purpura. Br J Haematol. 1996;95(4):732–737. doi: 10.1046/j.1365-2141.1996.d01-1967.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XL, Li YQ, Chen SH, et al. The feature of clonal expansion of TCR Vbeta repertoire, thymic recent output function and TCRzeta chain expression in patients with immune thrombocytopenic purpura. Int J Lab Hematol. 2009;31(6):639–648. doi: 10.1111/j.1751-553X.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 47.Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110(8):2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 48.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4(6):594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 49.Carreno BM, Bennett F, Chau TA, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165(3):1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 50.Peng J, Liu C, Liu D, et al. Effects of B7-blocking agent and/or CsA on induction of platelet-specific T-cell anergy in chronic autoimmune thrombocytopenic purpura. Blood. 2003;101(7):2721–2726. doi: 10.1182/blood-2002-06-1666. [DOI] [PubMed] [Google Scholar]
- 51.Zhang XL, Peng J, Sun JZ, et al. Modulation of immune response with cytotoxic T-lymphocyte-associated antigen 4 immunoglobulin-induced anergic T cells in chronic idiopathic thrombocytopenic purpura. J Thromb Haemost. 2008;6(1):158–165. doi: 10.1111/j.1538-7836.2007.02804.x. [DOI] [PubMed] [Google Scholar]
- 52.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 53.Mellanby RJ, Thomas DC, Lamb J. Role of regulatory T-cells in autoimmunity. Clin Sci (Lond) 2009;116(8):639–649. doi: 10.1042/CS20080200. [DOI] [PubMed] [Google Scholar]
- 54.Sakakura M, Wada H, Tawara I, et al. Reduced Cd4+ Cd25+ T cells in patients with idiopathic thrombocytopenic purpura. Thromb Res. 2007;120(2):187–193. doi: 10.1016/j.thromres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Heck S, Patel V, et al. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112(4):1325–1328. doi: 10.1182/blood-2008-01-135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling Y, Cao X, Yu Z, Ruan C. Circulating dendritic cells subsets and CD4 + Foxp3 + regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79(4):310–316. doi: 10.1111/j.1600-0609.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 57.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–1150. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 58.Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93(1):91–98. doi: 10.1007/s12185-010-0753-z. [DOI] [PubMed] [Google Scholar]
- 59.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–4645. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78(10):2619–2625. [PubMed] [Google Scholar]
- 61.Kuwana M, Kaburaki J, Kitasato H, et al. Immunodominant epitopes on glycoprotein IIb-IIIa recognized by autoreactive T cells in patients with immune thrombocytopenic purpura. Blood. 2001;98(1):130–139. doi: 10.1182/blood.v98.1.130. [DOI] [PubMed] [Google Scholar]
- 62.Sukati H, Watson HG, Urbaniak SJ, Barker RN. Mapping helper T-cell epitopes on platelet membrane glycoprotein IIIa in chronic autoimmune thrombocytopenic purpura. Blood. 2007;109(10):4528–4538. doi: 10.1182/blood-2006-09-044388. [DOI] [PubMed] [Google Scholar]
- 63.Aster RH. Molecular mimicry and immune thrombocytopenia. Blood. 2009;113(17):3887–3888. doi: 10.1182/blood-2008-12-193664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113(6):1231–1240. doi: 10.1182/blood-2008-07-167155. [DOI] [PubMed] [Google Scholar]
- 65.Arnold DM, Bernotas A, Nazi I, et al. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica. 2009;94(6):850–856. doi: 10.3324/haematol.2008.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi T, Yujiri T, Shinohara K, et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124(1):91–96. doi: 10.1046/j.1365-2141.2003.04735.x. [DOI] [PubMed] [Google Scholar]
- 67.Maeda S, Ogura K, Yoshida H, et al. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42(3):338–343. doi: 10.1136/gut.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nardi MA, Liu LX, Karpatkin S. GPIIIa-(49–66) is a major pathophysiologically relevant antigenic determinant for anti-platelet GPIIIa of HIV-1-related immunologic thrombocytopenia. Proc Natl Acad Sci U S A. 1997;94(14):7589–7594. doi: 10.1073/pnas.94.14.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia. Blood. 2005;106(2):572–576. doi: 10.1182/blood-2005-01-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karpatkin S, Nardi M. Autoimmune anti-HIV-1gp120 antibody with antiidiotype-like activity in sera and immune complexes of HIV-1-related immunologic thrombocytopenia. J Clin Invest. 1992;89(2):356–364. doi: 10.1172/JCI115593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106(5):551–561. doi: 10.1016/s0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 72.Sakaguchi M, Sato T, Groopman JE. Human immunodeficiency virus infection of megakaryocytic cells. Blood. 1991;77(3):481–485. [PubMed] [Google Scholar]
- 73.Sundell IB, Koka PS. Thrombocytopenia in HIV infection: impairment of platelet formation and loss correlates with increased c-Mpl and ligand thrombopoietin expression. Curr HIV Res. 2006;4(1):107–116. doi: 10.2174/157016206775197646. [DOI] [PubMed] [Google Scholar]
- 74.Zhang W, Nardi MA, Borkowsky W, Li Z, Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood. 2009;113(17):4086–4093. doi: 10.1182/blood-2008-09-181073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballem PJ, Segal GM, Stratton JR, Gernsheimer T, Adamson JW, Slichter SJ. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest. 1987;80(1):33–40. doi: 10.1172/JCI113060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–1899. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 77.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 78.Dighiero G, Lymberi P, Guilbert B, Ternynck T, Avrameas S. Natural autoantibodies constitute a substantial part of normal circulating immunoglobulins. Ann N Y Acad Sci. 1986;475:135–145. doi: 10.1111/j.1749-6632.1986.tb20863.x. [DOI] [PubMed] [Google Scholar]
- 79.Filion MC, Proulx C, Bradley AJ, et al. Presence in peripheral blood of healthy individuals of autoreactive T cells to a membrane antigen present on bone marrow-derived cells. Blood. 1996;88(6):2144–2150. [PubMed] [Google Scholar]
- 80.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 81.Tsubakio T, Tani P, Woods VL, Jr, McMillan R. Autoanti-bodies against platelet GPIIb/IIIa in chronic ITP react with different epitopes. Br J Haematol. 1987;67(3):345–348. doi: 10.1111/j.1365-2141.1987.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 82.Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38(1):1–10. [PubMed] [Google Scholar]
- 83.Luk SC, Musclow E, Simon GT. Platelet phagocytosis in the spleen of patients with idiopathic thrombocytopenic purpura (ITP) Histopathology. 1980;4(2):127–136. doi: 10.1111/j.1365-2559.1980.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 84.Panzer S, Szamait S, Bödeker RH, Haas OA, Haubenstock A, Mueller-Eckhardt C. Platelet-associated immunoglobulins IgG, IgM, IgA and complement C3 in immune and nonimmune thrombocytopenic disorders. Am J Hematol. 1986;23(2):89–99. doi: 10.1002/ajh.2830230203. [DOI] [PubMed] [Google Scholar]
- 85.Piguet PF, Vesin C. Modulation of platelet caspases and life-span by anti-platelet antibodies in mice. Eur J Haematol. 2002;68(5):253–261. doi: 10.1034/j.1600-0609.2002.01444.x. [DOI] [PubMed] [Google Scholar]
- 86.Leytin V, Mykhaylov S, Starkey AF, et al. Intravenous immunoglobulin inhibits anti-glycoprotein IIb-induced platelet apoptosis in a murine model of immune thrombocytopenia. Br J Haematol. 2006;133(1):78–82. doi: 10.1111/j.1365-2141.2006.05981.x. [DOI] [PubMed] [Google Scholar]
- 87.Usuki Y, Kohsaki M, Nagai K, Ohe Y, Hara H. Complement-dependent cytotoxic factor to megakaryocyte progenitors in sera from patients with idiopathic thrombocytopenic purpura. Int J Cell Cloning. 1986;4(6):447–463. doi: 10.1002/stem.5530040606. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi R, Sekine N, Nakatake T. Influence of mono-clonal antiplatelet glycoprotein antibodies on in vitro human megakaryocyte colony formation and proplatelet formation. Blood. 1999;93(6):1951–1958. [PubMed] [Google Scholar]
- 89.Alimardani G, Guichard J, Fichelson S, Cramer EM. Pathogenic effects of anti-glycoprotein Ib antibodies on megakaryocytes and platelets. Thromb Haemost. 2002;88(6):1039–1046. [PubMed] [Google Scholar]
- 90.Karpatkin S. Autoimmune thrombocytopenic purpura. Blood. 1980;56(3):329–343. [PubMed] [Google Scholar]
- 91.Kelton JG, Gibbons S. Autoimmune platelet destruction: idiopathic thrombocytopenic purpura. Semin Thromb Hemost. 1982;8(2):83–104. doi: 10.1055/s-2007-1005045. [DOI] [PubMed] [Google Scholar]
- 92.Isaka Y, Kambayashi J, Kimura K, et al. Platelet production, clearance and distribution in patients with idiopathic thrombocytopenic purpura. Thromb Res. 1990;60(2):121–131. doi: 10.1016/0049-3848(90)90291-j. [DOI] [PubMed] [Google Scholar]
- 93.Cooper N. Intravenous immunoglobulin and anti-RhD therapy in the management of immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1317–1327. doi: 10.1016/j.hoc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Kelton JG, Singer J, Rodger C, Gauldie J, Horsewood P, Dent P. The concentration of IgG in the serum is a major determinant of Fc-dependent reticuloendothelial function. Blood. 1985;66(3):490–495. [PubMed] [Google Scholar]
- 95.Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104(9):2623–2634. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]
- 96.Sarpatwari A, Provan D, Erqou S, Sobnack R, David Tai FW, Newland AC. Autologous 111 In-labelled platelet sequestration studies in patients with primary immune thrombocytopenia (ITP) prior to splenectomy: a report from the United Kingdom ITP Registry. Br J Haematol. 2010;151(5):477–487. doi: 10.1111/j.1365-2141.2010.08377.x. [DOI] [PubMed] [Google Scholar]
- 97.Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148(4):638–645. doi: 10.1111/j.1365-2141.2009.07995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 99.Falcieri E, Bassini A, Pierpaoli S, et al. Ultrastructural characterization of maturation, platelet release, and senescence of human cultured megakaryocytes. Anat Rec. 2000;258(1):90–99. doi: 10.1002/(SICI)1097-0185(20000101)258:1<90::AID-AR10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 100.Kaluzhny Y, Yu G, Sun S, et al. BclxL overexpression in megakaryocytes leads to impaired platelet fragmentation. Blood. 2002;100(5):1670–1678. doi: 10.1182/blood-2001-12-0263. [DOI] [PubMed] [Google Scholar]
- 101.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 102.De Botton S, Sabri S, Daugas E, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100(4):1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 103.Morison IM, Cramer Bordé EM, Cheesman EJ, et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet. 2008;40(4):387–389. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- 104.Chang M, Nakagawa PA, Williams SA, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102(3):887–895. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 105.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103(4):1364–1369. doi: 10.1182/blood-2003-08-2672. [DOI] [PubMed] [Google Scholar]
- 106.Houwerzijl EJ, Blom NR, van der Want JJ, et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood. 2004;103(2):500–506. doi: 10.1182/blood-2003-01-0275. [DOI] [PubMed] [Google Scholar]
- 107.Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339(11):746–754. doi: 10.1056/NEJM199809103391107. [DOI] [PubMed] [Google Scholar]
- 108.Borge OJ, Ramsfjell V, Veiby OP, Murphy MJ, Jr, Lok S, Jacobsen SE. Thrombopoietin, but not erythropoietin promotes viability and inhibits apoptosis of multipotent murine hematopoietic progenitor cells in vitro. Blood. 1996;88(8):2859–2870. [PubMed] [Google Scholar]
- 109.Houwerzijl EJ, Blom NR, van der Want JJ, Vellenga E, de Wolf JT. Megakaryocytic dysfunction in myelodysplastic syndromes and idiopathic thrombocytopenic purpura is in part due to different forms of cell death. Leukemia. 2006;20(11):1937–1942. doi: 10.1038/sj.leu.2404385. [DOI] [PubMed] [Google Scholar]
- 110.Yang L, Wang L, Zhao CH, et al. Contributions of TRAIL-mediated megakaryocyte apoptosis to impaired megakaryocyte and platelet production in immune thrombocytopenia. Blood. 2010;116(20):4307–4316. doi: 10.1182/blood-2010-02-267435. [DOI] [PubMed] [Google Scholar]
- 111.Zhang F, Chu X, Wang L, et al. Cell-mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;76(5):427–431. doi: 10.1111/j.1600-0609.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 112.Chow L, Aslam R, Speck ER, et al. A murine model of severe immune thrombocytopenia is induced by antibody-and CD8 + T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115(6):1247–1253. doi: 10.1182/blood-2009-09-244772. [DOI] [PubMed] [Google Scholar]
- 113.Li S, Wang L, Zhao C, Li L, Peng J, Hou M. CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br J Haematol. 2007;139(4):605–611. doi: 10.1111/j.1365-2141.2007.06737.x. [DOI] [PubMed] [Google Scholar]


