Abstract
Multidrug resistance (MDR) is an important cause of treatment failure in acute lymphoblastic leukemia (ALL). The ABC family of membrane transporters is proposed, albeit with controversy, to be involved in this process. The present study aims to investigate the mRNA expression profile of several genes of this family, including ABCA2, ABCA3, ABCB1/MDR1, MRP1/ABCC1, MRP3/ABCC3, ABCG2/BCRP, and the intracellular transporter MVP/LRP, in childhood ALL, and to evaluate their association with response to therapy. Some genes in the present research are being studied for the first time in Iran. Using quantitative real-time PCR, we evaluated 27 children with ALL at diagnosis and 15 children with normal bone marrow. The status of response to therapy was assessed one year after the onset of therapy through investigating the IgH/TCRγ gene rearrangements. Our findings indicate a considerable and direct relationship between mRNA expression levels of ABCA2, ABCA3, MDR1, and MRP1 genes and positive minimal residual disease (MRD) measured after one year of treatment. Statistical analysis revealed that expression of these genes higher than the cutoff point will raise the risk of MRD by 15-, 6.25-, 12-, and 9-fold, respectively. No relationship was found between of MVP/LRP, MRP3 and ABCG2 genes expression and ALL prognoses. Considering the direct and significant relationship between the increased expression of ABCA2, ABCA3, MDR1, and MRP1 genes and positive risk of MRD in children with ALL, evaluating the expression profile of these genes on diagnosis may identify high risk individuals and help plan a more efficient treatment strategy.
Keywords: acute lymphoblastic leukemia, minimal residual disease, multidrug resistance, ABC transporter
Introduction
Leukemia is the most common type of cancer in children, with approximately 80% of cases pertaining to acute lymphoblastic leukemia (ALL).1 The approach to ALL differs based on the risk factors present.2 Despite advances in therapy, some 20–30% of the patients still experience drug resistance and relapse, with multidrug resistance (MDR) constituting a major cause of this relapse.3,4 During the recent decades, various mechanisms responsible for drug resistance have been extensively studied both in vitro and in vivo.5 One important cause of MDR is increased expression of ATP-binding cassette transporters, or ABC transporters.6,7 In this family, ABCB1/MDR1, ABCC1/MRP1, ABCC3/MRP3, and ABCG2/BCRP genes have been evaluated in numerous studies, with the results being controversial regarding their role in development of drug resistance in pediatric ALL.8-13 There is much less data available on the other two members of this family, namely ABCA2 and ABCA3 genes, in terms of their role in leukemia and its prognosis.14-17 Although not an ABC transporter, the expression of the lung resistance protein (LRP) has been implicated to increase in certain cases of resistance to chemotherapy in some studies.18-20
Despite extensive research, the results on the role of genes of the ABC transporters superfamily and the LRP in ALL prognosis have been limited and controversial. Therefore, the present study first aims to evaluate the mRNA expression profile of ABCA2, ABCA3, MDR1, MRP1, MRP3, BCRP, and LRP genes in children with ALL, and then investigate their prognostic value.
Results
Comparing mRNA levels of MDR genes among controls, patients at diagnosis, and patients with relapsed ALL
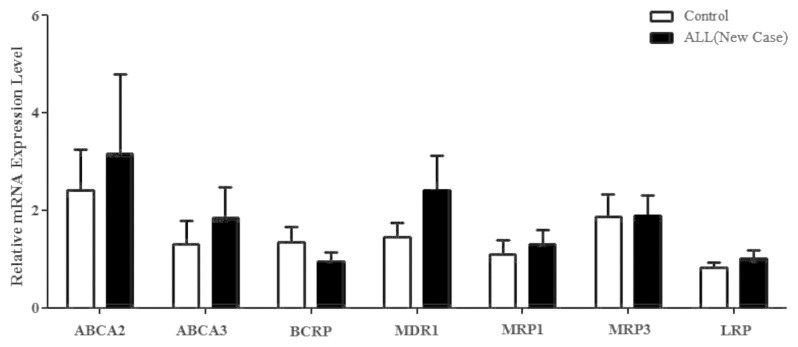
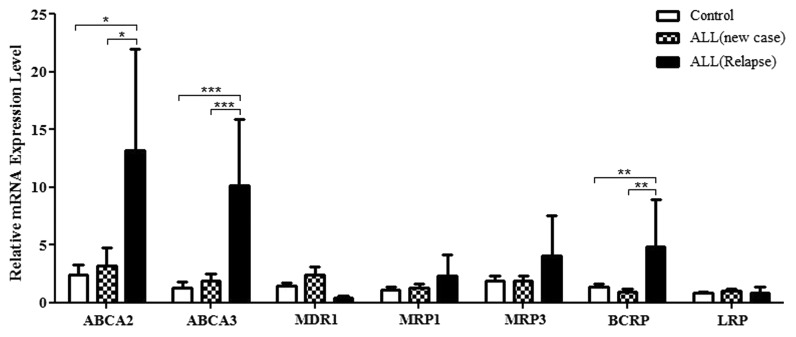
Comparing the relative mRNA expression of studied genes in patients at diagnosis and the control group revealed that although gene expression was generally higher in patients, the difference failed to achieve statistical significance (Fig. 1). Nevertheless, the expression of ABCA2, ABCA3, and ABCG2/BCRP in patients on relapse was significantly different from the control group as well as from the patients at diagnosis: for ABCA2, 13.16 ± 8.82 vs. 3.16 ± 1.62 and 2.41 ± 0.83, respectively (mean ± SEM, P < 0.03); for ABCA3, 10.10 ± 5.75 vs. 1.85 ± 0.61 and 1.31 ± 0.47, respectively (mean ± SEM, P < 0.001); and for ABCG2/BCRP, 4.83 ± 4.12 vs. 0.94 ± 0.2 and 1.35 ± 0.30, respectively (mean ± SEM, P < 0.01) (Fig. 2). No significant relationship was found between phenotype of ALL (B cell and T cell) and gene profile.
Figure 1. The relative mRNA expression levels of the drug-resistant genes ABCA2, ABCA3, BCRP, MDR1, MRP1, and LRP in children with ALL at diagnosis compared with the control group.
Figure 2. The relative mRNA expression levels of the drug-resistant genes in new case ALL children compared with the control group and patients with ALL relapse. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
Prognostic value of gene expression profile in pediatric ALL
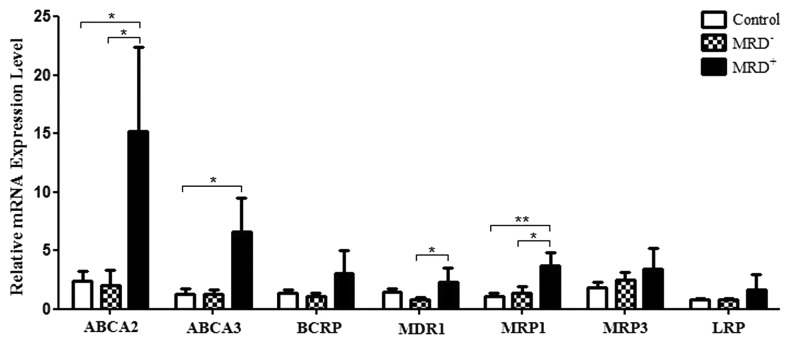
Comparing the mRNA expression level of studied genes in three groups of controls, patients with positive MRD (persisting monoclonal band of IgH or TCγR gene rearrangements after one year of therapy) and patients with negative MRD (elimination of the monoclonal band) indicates a significantly increased expression of 4 out of 7 genes (ABCA2, MRP1, ABCA3, and MDR1) in the MRD+ group vs. the control group (Fig. 3): For ABCA2, 15.18 ± 7.19 vs. 2.41 ± 0.83 (mean ± SEM, P = 0.012); For MRP1, 3.7 ± 1.12 vs. 1.10 ± 0.27 (mean ± SEM, P = 0.002); For ABCA3, 6.64 ± 2.87 vs. 1.31 ± 0.47 (mean ± SEM, P = 0.02); For MDR1, 2.29 ± 1.22 vs. 0.79 ± 0.21, (mean ± SEM, P = 0.049).
Figure 3. Comparing the relative mRNA expression levels of MDR genes in three groups of controls, patients with MRD+, and patients with MRD− .*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
As for the comparison of gene expression at mRNA levels between MRD+ and MRD− patients, assignment of appropriate cut-off points to each gene and analysis with the Pearson coefficient and the Fischer exact test revealed that overexpression of ABCA2, ABCA3, MDR1, and MRP1 above the cutoff points will raise the risk of multidrug resistance (MRD+) in lymphoid leukemia by 15, 6.25, 12, and 9 times, respectively. In the case of LRP, a 1.5-fold increase in gene expression accompanied a 15-fold increase in risk of drug resistance only in the T cell group (P < 0.04) (data not shown). Moreover, we observed that simultaneous mRNA expression of ABCA2 and ABCA3 above the threshold significantly raises the risk of positive MRD by 11.67 times (P = 0.025).
Relationship between gene expression and known prognostic factors
An investigation of the relationship between mRNA expression of the 7 studied genes and the prognostic factors of ALL demonstrated a direct, significant relationship between reduced platelet counts and mRNA expression profile of ABCA3 and MRP3 (P = 0.05 and P = 0.02, respectively). The increased mRNA levels of ABCA3 and MRP1 genes were correlated with the increased levels of CD34 (P = 0.03 and P = 0.04, respectively). The amplified expression of ABCA2 at mRNA levels was related to the high expression of CD10 as a poor prognostic factor of ALL (P = 0.03). Moreover, we observed an inverse, significant relationship between mRNA expression of LRP and serum LDH levels of patients (P = 0.01). The expression levels of other MDR genes revealed no significant relationship with the known prognostic factors (data not shown).
Discussion
The present study on 27 patient, assessed the mRNA expression profile of MDR genes on the onset of the disease (at diagnosis), and revealed no significant difference between the control group compared with patients in any of the 7 genes studied. However, a comparison of the gene expression profile of the control group to the relapsing cases revealed strongly significant differences for ABCA2, ABCA3, and ABCG2 genes. These findings indicate that the mentioned genes may be involved in the development of drug resistance during treatment, not in the progression of leukemia. Based on this assumption, it appears that although these genes, in leukemic cells, are expressed the same as they are in a normal population (at least in the general population of cells), the treatment may change the gene profile and activate these genes. It may alternatively eliminate the drug-susceptible cells (without affecting gene expression) and leave only a clone of resistant cells, thus changing the overall gene expression in bone marrow. On the other hand, the persistence of resistant cells may occur through disorders of the apoptotic pathways. The above hypothesis is quite compatible with initial response to therapy (remission), followed by relapse in the subsequent months or years, and poor response to the same drugs after relapse. The research literature contains numerous and contradictory results. Some studies have reported a different gene expression profile at diagnosis,13 while others, like us, have found similar gene expression.21,22 While it is not clear to what extent these discrepancies reflect the differences in ethnicity, as well as local and temporal factors, it is expectable to find no gene expression difference at the onset of disease, with differences found on relapse.
In our study, we found a relationship between increased expression of some genes with certain known risk factors. However, the significance of these findings is not clear. Response to therapy is a multifactorial issue, influenced by an ensemble of factors, each of which cannot be used solely to make an assumption on response to therapy. Certain relationships, for instance, between CD10 and MRD1,23 low WBC count and MRD1,24 in addition to CD34 and MRP1,25 have been noted in scientific literature; nonetheless, these reports are controversial and without consistency, and in many reports the expression of these genes was found to be unrelated to the mentioned risk factors.12,13,22,26-28
One major objective of this study was to determine whether or not the expression of genes studied may be used as an independent risk factor for drug resistance at the onset of therapy. As previously mentioned, the gene profile of newly diagnosed patients is not significantly different from that of healthy individuals, while the expression is different within patients with ALL. Patients were followed up for a period of one year. MRD was measured using TCγR or IgH gene rearrangement assays in order to identify the population of “drug-resistant patients”, and then compare them to those who were susceptible to therapy (MRD−) and healthy control group (in terms of gene expression). We demonstrated that in cases with higher expression of A2, A3, MRD1, and MRP1 genes (compared with the control group), the risk of drug resistance was notably elevated. The elevated risk of MDR for the abovementioned genes was 15-, 6-, 9-, and 12-fold, respectively.
Considering the controversial reports regarding MDR genes and their prognostic value in multidrug resistance, the studied genes in this project are discussed as follows. Neither G2 nor MRP3 are identified as risk factors for drug resistance in our study. Many other published articles have demonstrated same results regarding these two aforementioned genes.7,13,29 Overall it may at least be stated that the expression of these two genes is not related to the onset or prognosis of pediatric lymphoid leukemia. Regarding A2 and A3 genes, not only do our findings suggest a clear and strong relationship between the overexpression and prognostic value of these genes in lymphoid leukemia, but the few available studies dealing with these genes have also indicated similar results, especially in T-cell leukemia.29,30 Our results showed this association in B-cell lineages. We may conclude that the mRNA expression profile of, at least, ABCA2 and ABCA3 genes may be of prognostic value and help to select an optimal treatment plan, especially in cases where both genes are overexpressed simultaneously.
For MRP1, LRP, and MRD1, controversial results are reported. Some studies suggest a lack of any relationship between mRNA expression profile and prognosis,7,27,31 while others indicate that some of these genes may influence prognosis.1,23,24,32-35 In our study, the relationship was more obvious in the case of overexpression of MRD1 and MRP1 at mRNA levels. The discrepancy in results may be caused by temporal and local factors (the environmental risk factors of cancer), as well as genetic and hereditary characteristics. It should be noted that drug resistance in cancers is a multifactorial issue. In some cases, drug resistance may occur in the absence of any clearly defined risk factor, while in other cases sufficient response to therapy is observed in spite of the poor prognosis of one or more risk factors. Therefore, in patients with overexpression of these MDR genes, it is necessary to repeat the evaluation of response to therapy in different regions and even at different time intervals within a region in order to reach a clear conclusion regarding the prognostic value of these gene expression profiles. Further assessments of gene expression on protein levels and comparing the results to the transcription (mRNA) levels may help resolve some of these controversial reports.
Conclusion
Multidrug-resistant genes include certain genes that interfere with response to therapy. The present study addressing pediatric ALL, demonstrated that only A3, A2, MRD1, and MRP1 genes are significantly related to drug resistance and increased risk of relapse. We propose that the increased mRNA expression levels of the abovementioned genes, particularly A2 and A3, may be considered as risk factors of MDR in childhood ALL. Therefore, it is strongly suggested that the mRNA expression profile of these genes be evaluated at the onset of diagnosis, in order to select new cancer therapeutic strategies. The mRNA expression profile of ABCA2, ABCA3, ABCC3, ABCG2, and LRP was studied for the first time in Iranian children in the present study. Further studies are required to delineate the mechanisms and possible roles of these genes in pathophysiology of multidrug resistance in childhood acute lymphoblastic leukemia.
Materials and Methods
Patients and sampling
All children referred to Sayed-ol-Shohada Hospital, Isfahan, Iran in 2010–12 indicated for bone marrow evaluation for diagnosis were selected for the study. The type of leukemia was diagnosed through phenotype evaluation with flow cytometry (kits provided by Daco and the flow cytometer purchased from Partec) alongside routine tests of CBC count and morphology assessment. Twenty-seven new patients (10 females, 17 males; mean range, 4.8 ± 3.5 y; range, 0.8–14 y) with diagnosis of acute lymphocytic leukemia (6 T-cell and 21 B-cell lineage) entered the study, and those with other type of leukemia or extramedullary involvement at diagnosis were excluded. Fifteen cases with normal bone marrow were used as age- and sex-matched controls. Three patient with bone marrow relapse also were included the study. Sampling was achieved with full consent and in compliance with the ethical protocol and standards of Sayed-ol-Shohada Hospital, Isfahan. One to five milliliters of bone marrow sample or peripheral blood in heparin was obtained and sent on ice to the cellular-molecular biology laboratory of University of Isfahan for cell isolation and RNA extraction. Extraction of mononuclear cells in bone marrow/blood samples from patients and controls was accomplished using LymphoprepTM (Axis-Shield) according to the protocol provided by the manufacturer and based on the buoyant density gradient of blood constituents.
RNA extraction and cDNA synthesis
Extraction of total RNA from the mononuclear cells was performed using RNA plus Mini kit (Qiagen), and the amount and quality of the extracted RNA were assessed using biophotometer (Eppendorf) and gel electrophoresis. Two micrograms of the extracted RNA was transformed to cDNA according to the protocol provided by the cDNA synthesis kit (Fermentas) using a random hexamer primer, RevertAidTM M-MuLV reverse transcriptase and thermocycler (TaKaRa) in a specific temperature plan (sequentially: 5 min at 25 °C, 60 min at 42 °C, and 5 min at 70 °C). The resulting cDNAs were preserved at −20 °C for future steps.
Quantitative real time polymerase chain reaction (qRT-PCR)
Gene expression was assessed at mRNA levels based on SYBR Premix Ex TaqII (Takara) and melting curve analysis using Chromo 4 (Bio-Rad). Four microliters of 0.1 μg/mL synthesized cDNA, 0.8 μL of 100 nM forward and reverse primers, 10 μL of Sybr green, and 6.4 μL of ddH2O were added to the tubes. Quantitative RT-PCR was performed in 35–50 cycles using the following protocol: 8–10 min pre-incubation at 95 °C, 15–30 s denaturation at 95 °C, 30 s annealing at 58–60 °C and 1 min product expansion at 72 °C.The housekeeping gene used in this study for the internal control was glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers used included GADPH, ABCG2, ABCA2, and ABCA3 extracted from previous studies.36 Primer sequences of the endogenous control and drug resistance genes are summarized in Table 1.
Table 1. Primer sequences of the endogenous control and drug resistant genes.
| Gene | Primer sequence (5′ to 3′) | Primer length (bp) | Amplicon length (bp) |
|---|---|---|---|
| ABCA2/ABC2 | F: CCGCACCATC CTTCTGTCCA CCCACC | 26 | 263 |
| R: TGCGGATGAA CTGGGACACC TGGAGA | 26 | ||
| ABCA3/ABC3 | F: GGCCATCATC ATCACCTCCC ACAGCA | 26 | 177 |
| R: AGCGCCTCCT GTTGCCCTTC ACTCTG | 26 | ||
| ABCB1/MDR1 | F: GAGGCCGCTG TTCGTTTCCT TTAGGTC | 26 | 102 |
| R: AGATTCATTC CGACCTCGCG CTCCT | 25 | ||
| ABCC1/MRP1 | F: CGGATGTCAT CTGAAATGGG A | 21 | 103 |
| R: GAGCTGTCTC CTGGATTTGC | 20 | ||
| ABCC3/MRP3 | F: CGCACACCGG CTTAACACTA TCATGG | 26 | 184 |
| R: AAACCAGGAA AGGCCAGGAG GAAATC | 26 | ||
| ABCG2/BCRP | F: CGTGGCCTTG GCTTGTATGA TTGTTA | 26 | 178 |
| R: GGCAAGGGAA CAGAAAACAA CAAAAA | 26 | ||
| LRP/MVP | F: GCTGGCTGAG GTGGAGGTGA AGAAG | 25 | 177 |
| R: GGCTGTGTTG AAGAGGTTGA TGGCAG | 26 | ||
| GAPDH | F: GCCCCAGCAA GAGCACAAGA GGAAGA | 26 | 106 |
| R: CATGGCAACT GTGAGGAGGG GAGAT | 26 |
Assessing response to therapy
All patients were treated using modified ANZCCSG ALL study 8 pilot protocol consisting of induction (VCR, ADR, l-asparaginase, prednisolone), consolidation (6MP, CPM, cytarabine), protocol M (4 injection of High dose MTX), reinduction, reconsolidation, maintenance (6MP, weekly MTX, monthly CPM and VCR), and intrathecal injections. In order to assess response to therapy, patients underwent regular examination of bone marrow or cell count. In addition, PCR-SSCP (single-strand conformation polymorphism) analysis for immunoglobulin heavy chain (IgH) and T-cell receptor gamma (TcRγ) gene rearrangements were performed after one-year treatment and cases with monoclonal IgH or TcRγ gene rearrangements were considered MRD+.37 On the other hand, patients relapsed during the 1-y follow-up were considered as multidrug-resistant ALL cases. One of the 27 new leukemia patients expired because of infection within the first few weeks and one had a CNS relapse within the first year.
The relationship between mRNA expression profile of the abovementioned multidrug resistant genes and the known risk factors of ALL, including reduced platelet count and increased levels of CD34, CD10, and serum LDH, was evaluated using cell blood count tests, flow cytometry, and serologic assays, respectively (Table 2).
Table 2. Primary data of the patients included in the study.
| Condition at the onset of the study | Sex | Age | WBc >20 000 | Subtype | MDR (followed for 1 y) |
Results for RT-PCR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCB1 | ABCC1 | LRP | ABCG2 | ABCC3 | ABCA3 | ABCA2 | |||||||
| Case 1 | New case | M | 14 | - | T cell | + | 4.677135 | 4.262094 | 4.232654 | 0.964598 | 0.748461 | 0.761544 | 6.355475 |
| Case 2 | New case | M | 13 | + | T cell | + | 0.041252 | 0.185767 | 2.229256 | 0.872363 | 0.777546 | 0.159541 | 0.028028 |
| Case 3 | New case | M | 4 | + | T cell | - | 0.110769 | 0.419448 | 0.838895 | 0.618995 | 3.622536 | 2.832351 | 0.036474 |
| Case 4 | New case | M | 1 | - | T cell | - | 0.142164 | 0.100939 | 0.22634 | 0.55787 | 0.772175 | 0.208338 | 0.081221 |
| Case 5 | New case | M | 3 | - | T cell | - | 0.093469 | 0.1991 | 0.468643 | 0.604159 | 3.00424 | 0.523768 | 0.019011 |
| Case 6 | New case | M | 2 | + | T cell | - | 3.107221 | 0.542074 | 0.180687 | 0.0506954 | 0.012931 | 0.042159 | 0.102451 |
| Case 7 | Relapse | M | 14 | - | T cell | N/A | 2.352794 | 0.659468 | 12.54791 | 1.191683 | 0.970634 | 21.43654 | 0.472374 |
| Case 8 | New case | F | 4 | - | B cell | - | 1.141258 | 1.008047 | 0.99762 | 2.44189 | 1.047536 | 1.051173 | 0.954621 |
| Case 9 | New case | F | 3 | - | B l cell | - | 0.886151 | 1.648967 | 1.576322 | 1.99032 | 15.91152 | 1.507335 | 3.278418 |
| Case 10 | New case | F | 4 | + | B cell | - | 1.22317 | 0.323438 | 0.532762 | 0.751581 | 2.389982 | 0.542239 | 0.033101 |
| Case 11 | New case | M | 7 | - | B cell | - | 1.542879 | 3.85455 | 0.580981 | 0.872363 | 2.357079 | 1.555092 | 9.467608 |
| Case 12 | New case | M | 9 | + | Burkitt | + | 1.597289 | 1.527913 | 0.37934 | 2.02511 | 1.166349 | 3.197623 | 1.594385 |
| Case 13 | New case | M | 11 | + | B cell | + | 0.601078 | 5.302095 | 0.440301 | 9.33726 | 9.075644 | 11.89266 | 80.33741 |
| Case 14 | New case | F | 7 | - | B cell | - | 0.483177 | 2.525489 | 0.780009 | Not available | 5.000249 | 14.49014 | 0.416966 |
| Case 15 | New case | M | 8 | - | B cell | + | Not available | Not available | Not available | 0.689203 | 1.004864 | 0.376834 | 43.65273 |
| Case 16 | New case | F | 3 | - | B cell | - | 0.154494 | 0.21562 | 2.087191 | 0.623301 | 2.440201 | 0.388773 | 0.052483 |
| Case 17 | New case | M | 0.8 | + | B cell | - | 0.192193 | 0.220151 | 1.410848 | 1.00905 | 6.439729 | 0.725476 | 0.025524 |
| Case 18 | New case | M | 5 | - | B cell | - | 0.284327 | 0.094835 | 0.272922 | 0.36299 | 1.761739 | 0.1704 | 0.047465 |
| Case 19 | New case | F | 6 | + | B cell | - | 0.128125 | 0.204697 | 0.083421 | 0.0172531 | 1.339784 | 0.19171 | 0.019682 |
| Case 20 | New case | F | 4 | - | B cell | - | 10.37921 | 1.779612 | 1.372268 | 2.63536 | 0.09227 | 0.816203 | 1.03742 |
| Case 21 | New case | M | 3 | + | B cell | + | 0.338124 | 0.134582 | 0.0325 | 0.163572 | 0.072143 | 0.097193 | 0.131944 |
| Case 22 | New case | F | 2 | - | B cell | - | 2.454833 | 1.643262 | 2.44793 | 0.24367 | 0.079771 | 0.144286 | 0.196554 |
| Case 23 | New case | M | 7 | + | B cell | - | 0.446158 | 0.2855 | 0.48517 | 0.575545 | 0.138408 | 0.252963 | 0.312732 |
| Case 24 | New case | F | 3 | + | B cell | + | 0.717288 | 0.287486 | 0.75868 | 0.554016 | 0.527411 | 2.037782 | 2.262629 |
| Case 25 | New case | M | 3 | - | B cell | - | 14.98685 | 1.626265 | 2.044237 | 0.670356 | 1.64376 | 10.0005 | 3.027234 |
| Case 26 | New case | F | 2 | - | B cell | - | 8.880402 | 1.835995 | 1.491292 | 0.0159312 | 0.02480887 | 0.065471 | 0.140437 |
| Case 27 | New case | M | 0.9 | + | B l cell | + | 2.44634 | 0.87452 | 0.417996 | Not available | Not available | Not available | Not available |
| Case 28 | Relapse | M | 0.6 | - | B cell | N/A (expired) |
0.209588 | 0.307054 | 0.074149 | 0.711039 | 0.309283 | 0.804966 | 1.518872 |
| Case 29 | Relapse | F | 4 | - | B cell | N/A | 0.524631 | 0.744516 | 1.443304 | 0.232935 | 0.970634 | 2.679567 | 8.895033 |
| Case 30 | Relapse | M | 7 | - | B cell | N/A | 0.427612 | 8.192905 | 1.758536 | 369.1338 | 11.05786 | 6.198847 | 30.12731 |
Statistical analysis
Analyses were conducted on SPSS 20.0 and GraphPad Prism 5 softwares. The presence and intensity of significant differences were assessed through independent t test and one-way ANOVA. The correlation (direct or indirect) between parameters of interest was studied through calculating the Spearman coefficient. All data are reported as mean ± SEM, with significance levels of P ≤ 0.001, P ≤ 0.01, and P ≤ 0.05.
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
We thank Hadi Moafi (University of British Colombia, Canada) for editing the paper. This work was supported by a research grant from The University of Isfahan/committee of Biotechnology (89/72763 to S Rahgozar).
Authorship Contributions
S Rahgozar and A Moafi contributed to the study design; M Abedi, M Entezar-e-ghaem, and F Montazeri collected data; S Rahgozar, A Moafi, J Moshtaghian, K Ghaedi, and A Esmaeili contributed to analysis and interpretation of data; M Entezar-e-ghaem and M Abedi performed statistical analysis; and A Moafi, S Rahgozar, M Entezar-e-ghaem, and M Abedi wrote the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26603
References
- 1.Styczynski J, Wysocki M, Debski R, Czyzewski K, Kolodziej B, Rafinska B, Kubicka M, Koltan S, Koltan A, Pogorzala M, et al. Predictive value of multidrug resistance proteins and cellular drug resistance in childhood relapsed acute lymphoblastic leukemia. J Cancer Res Clin Oncol. 2007;133:875–93. doi: 10.1007/s00432-007-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–43. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 3.Bakos E, Homolya L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1) Pflugers Arch. 2007;453:621–41. doi: 10.1007/s00424-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 4.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–24. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 5.Filipits M. Mechanisms of cancer: multidrug resistance. Drug Discov Today Dis Mech. 2004;1:229–34. doi: 10.1016/j.ddmec.2004.10.001. [DOI] [Google Scholar]
- 6.Biondi A, Baruchel A, Hunger S, Masera G, Schmiegelow K, Schrappe M, Pui CH. The Eleventh International Childhood Acute Lymphoblastic Leukemia Workshop Report: Ponte di Legno, Italy, 6-7 May 2009. Leukemia. 2009;23:2318–24. doi: 10.1038/leu.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez MA, Scrideli CA, Yunes JA, Valera ET, Toledo SR, Pavoni-Ferreira PC, Lee ML, Petrilli AS, Brandalise SR, Tone LG. mRNA expression profile of multidrug resistance genes in childhood acute lymphoblastic leukemia. Low expression levels associated with a higher risk of toxic death. Pediatr Blood Cancer. 2009;53:996–1004. doi: 10.1002/pbc.22220. [DOI] [PubMed] [Google Scholar]
- 8.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien FE, Dinan TG, Griffin BT, Cryan JF. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. Br J Pharmacol. 2012;165:289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candeil L, Gourdier I, Peyron D, Vezzio N, Copois V, Bibeau F, Orsetti B, Scheffer GL, Ychou M, Khan QA, et al. ABCG2 overexpression in colon cancer cells resistant to SN38 and in irinotecan-treated metastases. Int J Cancer. 2004;109:848–54. doi: 10.1002/ijc.20032. [DOI] [PubMed] [Google Scholar]
- 11.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113:2011–21. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 12.Benderra Z, Faussat AM, Sayada L, Perrot JY, Tang R, Chaoui D, Morjani H, Marzac C, Marie JP, Legrand O. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–72. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 13.Kourti M, Vavatsi N, Gombakis N, Sidi V, Tzimagiorgis G, Papageorgiou T, Koliouskas D, Athanassiadou F. Expression of multidrug resistance 1 (MDR1), multidrug resistance-related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. Int J Hematol. 2007;86:166–73. doi: 10.1532/IJH97.E0624. [DOI] [PubMed] [Google Scholar]
- 14.Mack JT, Brown CB, Tew KD. ABCA2 as a therapeutic target in cancer and nervous system disorders. Expert Opin Ther Targets. 2008;12:491–504. doi: 10.1517/14728222.12.4.491. [DOI] [PubMed] [Google Scholar]
- 15.Marzac C, Garrido E, Tang R, Fava F, Hirsch P, De Benedictis C, Corre E, Lapusan S, Lallemand JY, Marie JP, et al. ATP Binding Cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica. 2011;96:1293–301. doi: 10.3324/haematol.2010.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulevic B, Chen Z, Boyd JT, Davis W, Jr., Walsh ES, Belinsky MG, Tew KD. Cloning and characterization of human adenosine 5′-triphosphate-binding cassette, sub-family A, transporter 2 (ABCA2) Cancer Res. 2001;61:3339–47. [PubMed] [Google Scholar]
- 17.Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, et al. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–10. doi: 10.1158/0008-5472.CAN-3263-2. [DOI] [PubMed] [Google Scholar]
- 18.List AF, Spier CS, Grogan TM, Johnson C, Roe DJ, Greer JP, Wolff SN, Broxterman HJ, Scheffer GL, Scheper RJ, et al. Overexpression of the major vault transporter protein lung-resistance protein predicts treatment outcome in acute myeloid leukemia. Blood. 1996;87:2464–9. [PubMed] [Google Scholar]
- 19.Ryu SJ, An HJ, Oh YS, Choi HR, Ha MK, Park SC. On the role of major vault protein in the resistance of senescent human diploid fibroblasts to apoptosis. Cell Death Differ. 2008;15:1673–80. doi: 10.1038/cdd.2008.96. [DOI] [PubMed] [Google Scholar]
- 20.Berger W, Steiner E, Grusch M, Elbling L, Micksche M. Vaults and the major vault protein: novel roles in signal pathway regulation and immunity. Cell Mol Life Sci. 2009;66:43–61. doi: 10.1007/s00018-008-8364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahjoubi F, Golalipour M, Ghavamzadeh A, Alimoghaddam K. Expression of MRP1 gene in acute leukemia. Sao Paulo Med J. 2008;126:172–9. doi: 10.1590/S1516-31802008000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauerbrey A, Sell W, Steinbach D, Voigt A, Zintl F. Expression of the BCRP gene (ABCG2/MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br J Haematol. 2002;118:147–50. doi: 10.1046/j.1365-2141.2002.03550.x. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan PS, Bhushan B, Singh LC, Mishra AK, Saluja S, Mittal V, Gupta DK, Kapur S. Expression of genes related to multiple drug resistance and apoptosis in acute leukemia: response to induction chemotherapy. Exp Mol Pathol. 2012;92:44–9. doi: 10.1016/j.yexmp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Plasschaert SL, Vellenga E, de Bont ES, van der Kolk DM, Veerman AJ, Sluiter WJ, Daenen SM, de Vries EG, Kamps WA. High functional P-glycoprotein activity is more often present in T-cell acute lymphoblastic leukaemic cells in adults than in children. Leuk Lymphoma. 2003;44:85–95. doi: 10.1080/1042819021000040288. [DOI] [PubMed] [Google Scholar]
- 25.van Grotel M, van den Heuvel-Eibrink MM, van Wering ER, van Noesel MM, Kamps WA, Veerman AJ, Pieters R, Meijerink JP. CD34 expression is associated with poor survival in pediatric T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:737–40. doi: 10.1002/pbc.21707. [DOI] [PubMed] [Google Scholar]
- 26.Mahjoubi F, Akbari S. Multidrug resistance-associated protein 1 predicts relapse in Iranian childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev. 2012;13:2285–9. doi: 10.7314/APJCP.2012.13.5.2285. [DOI] [PubMed] [Google Scholar]
- 27.Valera ET, Scrideli CA, Queiroz RG, Mori BM, Tone LG. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004;122:166–71. doi: 10.1590/S1516-31802004000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakihara T, Tanaka A, Watanabe A, Yamamoto K, Kanto K, Kataoka S, Ogawa A, Asami K, Uchiyama M. Expression of multidrug resistance-related genes does not contribute to risk factors in newly diagnosed childhood acute lymphoblastic leukemia. Pediatr Int. 1999;41:641–7. [PubMed] [Google Scholar]
- 29.Steinbach D, Wittig S, Cario G, Viehmann S, Mueller A, Gruhn B, Haefer R, Zintl F, Sauerbrey A. The multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotype. Blood. 2003;102:4493–8. doi: 10.1182/blood-2002-11-3461. [DOI] [PubMed] [Google Scholar]
- 30.Efferth T, Gillet JP, Sauerbrey A, Zintl F, Bertholet V, de Longueville F, Remacle J, Steinbach D. Expression profiling of ATP-binding cassette transporters in childhood T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2006;5:1986–94. doi: 10.1158/1535-7163.MCT-06-0086. [DOI] [PubMed] [Google Scholar]
- 31.Olson DP, Taylor BJ, La M, Sather H, Reaman GH, Ivy SP. The prognostic significance of P-glycoprotein, multidrug resistance-related protein 1 and lung resistance protein in pediatric acute lymphoblastic leukemia: a retrospective study of 295 newly diagnosed patients by the Children’s Oncology Group. Leuk Lymphoma. 2005;46:681–91. doi: 10.1080/10428190500032612. [DOI] [PubMed] [Google Scholar]
- 32.Gurbuxani S, Sazawal S, Arya LS, Raina V, Marie JP, Bhargava M. MDR1 mRNA expression in young patients with acute lymphoblastic leukaemia. Br J Haematol. 2000;109:897–9. doi: 10.1046/j.1365-2141.2000.109004897.x. [DOI] [PubMed] [Google Scholar]
- 33.Tafuri A, Gregorj C, Petrucci MT, Ricciardi MR, Mancini M, Cimino G, Mecucci C, Tedeschi A, Fioritoni G, Ferrara F, et al. GIMEMA Group MDR1 protein expression is an independent predictor of complete remission in newly diagnosed adult acute lymphoblastic leukemia. Blood. 2002;100:974–81. doi: 10.1182/blood-2001-12-0371. [DOI] [PubMed] [Google Scholar]
- 34.Huh HJ, Park CJ, Jang S, Seo EJ, Chi HS, Lee JH, Lee KH, Seo JJ, Moon HN, Ghim T. Prognostic significance of multidrug resistance gene 1 (MDR1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) mRNA expression in acute leukemia. J Korean Med Sci. 2006;21:253–8. doi: 10.3346/jkms.2006.21.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Sharnouby JA, Abou El-Enein AM, El Ghannam DM, El-Shanshory MR, Hagag AA, Yahia S, Elashry R. Expression of lung resistance protein and multidrug resistance-related protein (MRP1) in pediatric acute lymphoblastic leukemia. J Oncol Pharm Pract. 2010;16:179–88. doi: 10.1177/1078155209351329. [DOI] [PubMed] [Google Scholar]
- 36.Warren MS, Zerangue N, Woodford K, Roberts LM, Tate EH, Feng B, Li C, Feuerstein TJ, Gibbs J, Smith B, et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59:404–13. doi: 10.1016/j.phrs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Poopak B, Pourfatholla AA, Najmabadi H, Vosoogh P, Mortazavi Y, Izadyar M, Hahyavi H, Shahgholi E, Bahoosh G, Haghnejad F. Evaluation of IgH gene rearrengement by polymerase chain reaction in order to clonality determination and MRD evaluation in chilhood ALL. Daneshvar 2005:21-6. [Google Scholar]