Abstract
Food intake is controlled at the central level by the melanocortin pathway in which the agonist α-MSH binds to melanocortin 4 receptor (MC4R), a Gs-coupled G protein-coupled receptor expressed by neurons in the paraventricular nuclei of the hypothalamus, which signals to reduce appetite. Consumption of a high-fat diet induces hypothalamic accumulation of palmitate, endoplasmic reticulum (ER) stress, apoptosis, and unresponsiveness to prolonged treatment with MC4R agonists. Here we have modeled effects of lipid stress on MC4R by using mHypoE-42 immortalized hypothalamic neurons expressing endogenous MC4R and Neuro2A cells expressing a tagged MC4R reporter, HA-MC4R-GFP. In the hypothalamic neurons, exposure to elevated palmitate in the physiological range induced splicing of X-box binding protein 1, but it did not activate C/EBP-homologous protein or induce increased levels of cleaved caspase-3, indicating mild ER stress. Such mild ER stress coexisted with a minimal loss of MC4R mRNA and yet a profound loss of cAMP signaling in response to incubation with the agonist. These findings were mirrored in the Neuro2A cells expressing HA-MC4R-GFP, in which protein abundance of the tagged receptor was decreased, whereas the activity per receptor number was maintained. The loss of cAMP signaling in response to α-MSH by elevated palmitate was corrected by treatment with a chemical chaperone, 4-phenylbutyrate in both mHypoE-42 hypothalamic neurons and in Neuro2A cells in which protein abundance of HA-MC4R-GFP was increased. The data indicate that posttranscriptional decrease of MC4R protein contribute to lower the response to α-MSH in hypothalamic neurons exposed to even a mild level of lipid stress and that a chemical chaperone corrects such a defect.
Obesity is a major risk factor for the development of metabolic syndrome, which is characterized by hypertension, glucose intolerance, insulin resistance, and dislipidemia. Obesity often precedes development of type 2 diabetes (1). A likely component of the increase in obesity in the last 10 years is the availability of food with high caloric content due to elevated amounts of saturated fatty acids (2, 3). Food intake is controlled at the central level by the melanocortin pathway. In this pathway, leptin and insulin released from adipose tissues and pancreatic islets circulate in the bloodstream and cross the blood-brain barrier to reach the arcuate nucleus of the hypothalamus (4–6). At the arcuate nucleus, leptin and insulin reduce food intake by promoting synthesis and release of the anorexigenic hormone α-MSH by proopiomelanocortin neurons and by inhibiting the release of orexigenic hormones by the neuropeptide Y/agouti gene-related peptide neurons. α-MSH released by the proopiomelanocortin neurons binds to melanocortin 4 receptor (MC4R) expressed by neurons in the paraventricular nuclei of the hypothalamus, which signals to reduce appetite. Conversely, agouti gene-related peptide is an antagonist/inverse agonist of MC4R and acts to increase food intake.
Exposure to a hypercaloric, high-fat (HF) diet induces endoplasmic reticulum (ER) stress and inflammation in the regions of the hypothalamus controlling appetite with increased resistance to anorexigenic hormones such as leptin and insulin (7–15). Because MC4R functions downstream of the insulin and leptin receptors and is therefore distal in the central pathway to control food intake, promoting the activity of this receptor by available potent and stable MC4R agonists appears as a promising approach to reverse or prevent obesity. Moreover, some studies found that mice treated with a HF diet have increased MC4R mRNA and are overresponsive to short-term treatment with melanocortin agonists (7, 16). However, other studies instead find that obese rats exposed to HF-diet have reduced MC4R mRNA (17, 18), central resistance to MC4R agonists (19, 20), and reduced hypothalamic binding to radiolabeled MC4R agonists (21). Importantly, trial studies in humans using potent MC4R agonists were ineffective to treat obesity (22). Modeling lipid stress by using cultured neurons may facilitate studying possible adverse effects of the HF diet on the function of MC4R and finding drugs that correct such defects. In this respect, it has been found humans with obesity have an increased level of circulating free fatty acids (23, 24). Similarly, obese rats and mice exposed to a HF diet have elevated concentrations of circulating free fatty acids, which leads to an accumulation of palmitoyl- and stearoyl-CoA in the hypothalamus as well as insulin resistance (12–14, 25, 26). Importantly, exposing immortalized hypothalamic neurons to elevated palmitate appears to reproduce aspects of the injury found in the hypothalamus of rodents exposed to a HF-diet, including ER stress and insulin resistance (27, 28).
Here we have used mHypoE-42 (N42) immortalized hypothalamic neurons expressing endogenous MC4R and neuronal Neuro 2A (N2A) cells expressing both endogenous MC4R and a tagged MC4R reporter, HA-MC4R-GFP, to determine whether elevated palmitate affects MC4R function. Using these systems, we find that when neuronal cells are exposed to elevated palmitate, the response to MC4R agonists is severely compromised, even when ER stress is mild. By monitoring the reporter HA-MC4R-GFP, it is found that the loss of MC4R function in cells treated with elevated palmitate occurs because of posttranscriptional loss of receptor abundance, rather than the loss of receptor activity. We also find that treating cells exposed to elevated palmitate with the chemical chaperone 4-phenylbutyric acid (PBA) restores normal abundance and function of MC4R.
Materials and Methods
Materials
Lipofectamine 2000, Amplex Red cholesterol assay, and a SuperScript first-strand kit were purchased from Invitrogen. Rat monoclonal antihemagglutinin (HA) antibody (3F10 clone), peroxidase (POD)-conjugated anti-HA antibody (3F10 clone), secondary POD-conjugated antimouse IgG, secondary POD-conjugated antirabbit IgG, secondary fluorescein isothiocyanate (FITC)-conjugated antirabbit IgG, and secondary Cy3-conjugated antirat IgG were purchased from Jackson ImmunoResearch. Primary antibody raised against caspase-3, primary antibody raised against calnexin, and primary antibody raised against glucose-regulated protein 78 (GRP78) were purchased from Cell Signaling. Primary antibody raised against the KDEL amino acid sequence (product ab12223) was purchased from Abcam. Primary antibody against 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) (product sc-27578), primary antibody against Na+/K+ ATPase α-1 antibody clone C464.6, and primary antibody raised against C/EBP-homologous protein (CHOP) (product sc-575) were purchased from Santa Cruz Biotechnology. Protease inhibitor mixture (Complete Mini), and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) tablets were purchased from Roche Applied Science. α-MSH, 3-isobutyl-1-methylxanthine (IBMX), PBA, and methyl-β-cyclodextrin were purchased from Sigma. Bicinchoninic assay (BCA) protein assay reagent and enhanced chemiluminescence detection kits were purchased from PerkinElmer Life Sciences. Primary antibody raised against actin clone C4 was purchased from Millipore. Formaldehyde (16%) was from Ted Pella Inc. A direct cAMP enzyme immunoassay kit was from Enzo Life Science Inc (product ADI-900–066). Embryonic mouse hypothalamic cell line, N42, was purchased from Cedarlane Laboratories. An RNeasy Plus minikit was purchased from QIAGEN. SsoAdvanced SYBR Green supermix was purchased from Life Science Research.
Cell culture
N42 cells and N2A cells stably transfected with HA-MC4R-GFP (29) were cultured in DMEM with L-glutamine and sodium pyruvate supplemented with 10% fetal bovine serum and penicillin/streptomycin. The N2A cells stably expressing HA-MC4R-GFP have been previously described.
Gel electrophoresis and immunoblotting
Cell lysates were prepared by scraping cells into sample buffer containing protease inhibitors. Samples were sonicated prior to loading onto SDS-PAGE gel (8% polyacrylamide). The separated proteins were transferred to a nitrocellulose membrane then immunoblotted with the indicated antibodies.
Determination of HA-MC4R-GFP at the cell surface by enzyme-linked immunoassay
This assay was carried out using the N2AHA-MC4R-GFP cell line as described previously (29). Briefly, cells were washed with DMEM and incubated in DMEM for 1 hour at 37°C. Cells were incubated for 1 hour at 4°C with POD-conjugated anti-HA antibodies (25 mU/mL) in DMEM. Cells were washed with ice-cold DMEM and fixed with formaldehyde. Cells were washed with PBS and then incubated with the ABTS substrate. The reaction of ABTS with the POD enzyme results in an oxidized product, which absorbs light at 405 nm wavelength. ODs were measured by a microplate spectrophotometer (Epoch; Bio-Tek Instruments, Inc).
Assay to determine cAMP
Cells were washed three times with DMEM and then incubated for 1 hour with DMEM at 37°C. The cells were treated with DMEM containing 0.5 mM IBMX for 10 minutes at 37°C and then stimulated with 0.5 mM IBMX and 100 nM α-MSH or 1 μM forskolin for 15 minutes at 37°C. Samples were collected in 0.1 M HCl containing 0.5 mM IBMX, spun at top speed in a tabletop centrifuge, and analyzed according to the manufacturer's instructions. The collected supernatants in 0.1 M HCl were also used to determine protein concentration with the BCA protein assay.
Quantification of cell protein content
Total cell protein was measured using the BCA protein assay kit following the manufacturer's instructions. Cell lysates were collect from 24-well plates.
PCR measurement of X-box binding protein 1 (XBP-1) splicing
Sample mRNA was extracted using an RNeasy Plus minikit (QIAGEN). Extracted mRNA was converted to cDNA using a SuperScript kit (Invitrogen). Target sequences were amplified with the primer sequences as follows: murine XBP-1, forward, 5′-GAGTCCGCAGCAGGTG-3′; reverse, 5′-GTGTCAGAGTCCATGGGA-3′. Amplification steps occurred with a melting temperature of 94°C, annealing temperature of 58°C, and extension temperature of 72°C, with 1 minute of extension time for 35 cycles. PCR products were run on a 2.5% agarose gel and DNA bands were quantified using Image J software (National Institutes of Health, Bethesda, Maryland).
Measurement of MC4R, HA-MC4R-GFP, and actin mRNA by quantitative PCR
Sample mRNA was extracted using an RNeasy Plus minikit (QIAGEN). Extracted mRNA was converted to cDNA using a SuperScript first-strand kit (Invitrogen). Quantitative PCR was performed using the SsoAdvanced SYBR Green supermix (Life Science Research) and primers as follows: murine MC4R, forward, 5′-CTCCCAACTTCTACAGGCATAC-3′, reverse, 5′-CTCCTTGAACTGATCCAACCTC-3′; green fluorescent protein, forward, 5′-AAGGGCGAGGAGCTGTTCACC-3′, reverse, 5′-AGGTGAACTTCAAGATCCGCCA-3′; and murine actin, forward, 5′-ACCTTCTACAATGAGCTGCG-3′, reverse, 5′-CTGGATGGCTACGTACATGG-3′. The thermocycler used was Bio-Rad MJ Mini personal thermocycler.
Quantification of CHOP activation by immunofluorescence microscopy
Fixed cells plated on glass coverslips were permeabilized with PBS containing detergent [0.2% (w/vol) Triton X-100] and 100 μg/mL ovalbumin and then probed for 1 hour at room temperature in the same solution containing rabbit primary antibodies against CHOP. Coverslips were washed with PBS/Triton X-100/ovalbumin solution and then stained with FITC- conjugated secondary antibodies, Alexa Fluor 555-conjugated phalloidin to visualize actin, and Hoescht 33342 to visualize nuclei for 1 hour at room temperature. Coverslips were washed and mounted on slides for imaging using the Olympus FV1000 laser-scanning microscope. Images of cells were captured at random. The intensity of the FITC fluorescence (CHOP immunostaining) in a region of interest drawn around the nucleus stained by Hoescht 33342 was measured by using the ImageJ software (National Institutes of Health).
Measurements of cell count and cell viability by trypan blue exclusion
Cells were trypsinized and suspended in complete DMEM culture medium. Cells were treated with trypan blue (0.4%) and loaded to a hemocytometer for counting.
Drug treatment
For treatment with PBA, cells were exposed to vehicle alone (dimethylsulfoxide) or to PBA (at 2 mM for N2AHA-MC4R-GFP cells and at 0.25 mM for N42 cells) in complete media for 16 hours at 37°C until cells were harvested. The concentration of 2 mM was selected to treat N2AHA-MC4R-GFP cells based on our previous experiments (30, 31). However, because 2 mM PBA appeared to reduce the cell number of the N42 neurons, experiments were carried out to expose N42 neurons to different concentrations of the drug (0, 0.1, 0.25, 0.5, 1, and 2 mM). Because PBA at concentration at or below 0.25 mM did not reduce the N42 cell number, treatment with the drug at this concentration was used for all subsequent experiments using the N42 cells. For treatment with tunicamycin, cells were exposed to 1 μg/mL tunicamycin in complete media for 16 hours at 37°C until cells were harvested.
Cell treatment with palmitate
Palmitate stock was prepared as100 mM sodium palmitate in 0.1 M NaOH heated to 72°C. Essentially free fatty acid (FFA)-free BSA was prepared as a 10% solution and then sterile filtered. For 5 mM stock, 50 μL of 72°C 100 mM palmitate stock was combined with 950 μL 10% FFA-free BSA at 37°C. To treat cells, 5 mM palmitate in 10% FFA-free BSA is added directly to complete media. Control cells were treated with equivalent amounts of 10% FFA-free BSA.
Statistical analysis
Data from three or more independent experiments are expressed as mean ± SD. A Student's t test was used to test for significant differences between two conditions. For experiments with three or more conditions, a one-way ANOVA was performed with a Bonferroni posttest to compare all pairs of columns.
Results
N42 immortalized hypothalamic neurons and N2AHA-MC4R-GFP cells exposed to elevated palmitate have profound loss of MC4R signaling and minimal loss of MC4R mRNA
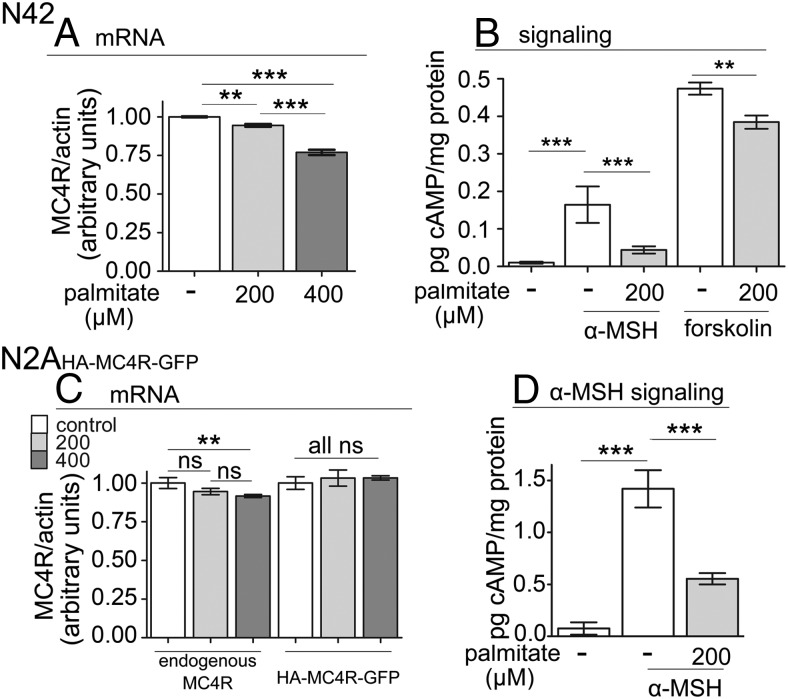
In the hypothalamus both MC4R and melanocortin 3 receptor bind to α-MSH to signal by coupling to Gs (32). Recently, Belsham and collaborators (33, 34) developed and characterized extensively several clones of immortalized hypothalamic neurons. These immortalized neurons have been used to model effects of dislipidemia on ER stress and insulin signaling by exposing them to elevated palmitate and inflammatory cytokines (27, 28). We have chosen for our studies the N42 immortalized hypothalamic neurons (33, 34), which express MC4R, but not melanocortin 3 receptor, so that the extent of cAMP signaling in response to α-MSH can be directly related to MC4R function. In obese humans the concentration of palmitate is increased to approximately 0.2 mM (23, 24, 35). Therefore, we exposed the cultured N42 hypothalamic neurons to elevated palmitate at 0.2 mM and above (0.4 mM) for 24 hours to model the in vitro effects of dislipidemia on MC4R function. In the N42 neurons exposed to 0.2 and 0.4 mM palmitate, there was minimal and mild loss, respectively (∼6% and 23% of control), of MC4R mRNA expression normalized to actin mRNA (Figure 1A). We determined whether exposure to elevated palmitate inhibited signaling by MC4R. In N42 cells not exposed to elevated palmitate, acute stimulation with 100 nM α-MSH increased the amount of cAMP by approximately 15-fold (Figure 1B). Differently, when cells were exposed to 0.2 mM palmitate for 24 hours, the level of cAMP generated in response to 100 nM α-MSH (or to 1 μM α-MSH; not shown) was severely reduced (by ∼70%). In the N42 hypothalamic neurons exposed to forskolin, a drug that activates directly adenylate cyclase, treatment with 0.2 mM palmitate decreased the amount of cAMP being generated in response to 100 nM α-MSH but only by 20%. These experiments indicate that, upon exposure to elevated palmitate, loss of cAMP generation in response to α-MSH is dependent largely upon loss of MC4R function, rather than because of reduced activity of adenylate cyclase.
Figure 1.
Elevated palmitate induces profound loss of cAMP signaling in response to α-MSH in N42 hypothalamic neurons and immortalized N2AHA-MC4R-GFP cells. A, N42 cells were treated without or with palmitate at the indicated concentrations for 24 hours. Cells were collected for mRNA isolation and quantitative PCR performed using primers specific to MC4R and actin. Data are shown as the amount of MC4R transcript normalized to that of actin. B, N42 cells were treated with palmitate as in A and then stimulated with 100 nM α-MSH or 1 μM forskolin. Samples were collected to measure cell cAMP levels and protein concentration. C, N2AHA-MC4R-GFP cells were treated with palmitate and the amount of MC4R and HA-MC4R-GFP transcripts, normalized to that of actin, was determined as in A. D, N2AHA-MC4R-GFP cells were treated with palmitate as in A, and cAMP and protein concentration was determined. Data are expressed as mean ± SD. **, P < .01; ***, P < .001.
It is possible that the profound loss of MC4R function in neurons exposed to elevated palmitate is due to changes in the abundance of the receptor and/or in its intrinsic activity. However, a direct determination of the amount of endogenous MC4R protein expressed in N42 cells by Western blot analysis cannot be carried out because high-affinity antibodies against the protein are unavailable. In addition, monitoring changes in the expression level of an exogenous, tagged MC4R in N42 hypothalamic neurons is difficult because these cells are resistant to transfection. We have previously generated a stably transfected neuroblastoma N2AHA-MC4R-GFP cell line expressing a tagged reporter, HA-MC4R-GFP, in addition to endogenous MC4R (29). We reasoned that the N2AHA-MC4R-GFP cell line would allow the evaluation of possible changes in MC4R protein abundance and/or activity because it is possible to measure the level of expression the tagged receptor, HA-MC4R-GFP. In this respect, the N2AHA-MC4R-GFP cell line appears to be a reliable model to study MC4R function because the exogenous receptor is functional and expressed at relatively low levels (29, 36) and because the modulation of cAMP signaling in response to α-MSH is similar to that of immortalized hypothalamic neurons by being attenuated by prolonged exposure to α-MSH and by changes in the cell membrane cholesterol content (37, 38). However, it is not known whether exposure to elevated palmitate would also have similar effects on the endogenous and exogenous MC4R mRNA expression and on MC4R signaling in response to α-MSH as compared with the hypothalamic neurons. To test that, N2AHA-MC4R-GFP cells were incubated with palmitate at 0.2 and 0.4 mM. Treatment with elevated fatty acid had minimal or no effects on the abundance of the mRNA encoding for endogenous MC4R and for the tagged HA-MC4R-GFP reporter (Figure 1C). Functionally, in N2AHA-MC4R-GFP cells not exposed to elevated fatty acid, stimulation with α-MSH increased the level of cAMP by approximately 18-fold (Figure 1D), similar to our observations in the N42 hypothalamic neurons. When N2A cells were exposed to 0.2 mM palmitate, stimulation with α-MSH increased cAMP levels only by 8-fold, with an approximately 60% decrease in signaling as compared with N2AHA-MC4R-GFP cells incubated without the elevated concentration of the fatty acid. Therefore, in the N2AHA-MC4R-GFP neuronal cells, exposure to elevated palmitate induces either mild or no loss of abundance of MC4R mRNA together with a profound loss of response to acute exposure to α-MSH. In conclusion, N2AHA-MC4R-GFP neuronal cells mirror the minimal loss of MC4R mRNA abundance and the profound loss of MC4R function observed in the N42 cells in response to elevated palmitate.
Exposure to elevated palmitate affects the abundance, but not the activity, of the MC4R reporter HA-MC4R-GFP
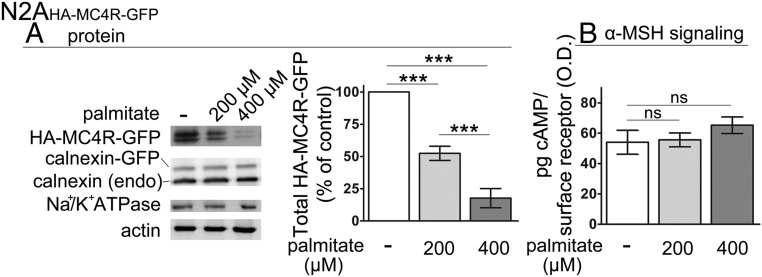
The profound loss of the response to α-MSH in N42 neurons and N2AHA-MC4R-GFP cells exposed to elevated palmitate, in the face of only mild decrease of MC4R transcript, suggests that that either decreased abundance of MC4R protein and/or reduced ability of MC4R to signal underlies resistance to α-MSH. Because N2AHA-MC4R-GFP cells mirror the effects of exposure to elevated palmitate observed in the immortalized hypothalamic neurons, we measured by Western blot the abundance of the reporter HA-MC4R-GFP. Treatment of N2AHA-MC4R-GFP cells with palmitate at 0.2 and 0.4 mM decreased the expression of HA-MC4R-GFP protein by approximately 52% and approximately 73%, respectively (Figure 2A). In contrast, the abundance of endogenous calnexin, an ER resident protein, and transiently expressed calnexin-green fluorescent protein (GFP), expressed by using the same vector as HA-MC4R-GFP, was unchanged, and so was the abundance of the α-subunit of Na+/K+ ATPase, which, like MC4R, traverses the secretory pathway to reach the plasma membrane. Thus, the loss of HA-MC4R-GFP abundance by exposure to elevated palmitate is specific. Because in N2AHA-MC4R-GFP cells, the abundance of HA-MC4R-GFP mRNA is unchanged (Figure 1C), the loss of HA-MC4R-GFP protein is dependent on a posttranscriptional mechanisms.
Figure 2.
Exposure to elevated palmitate within the physiological range induces the loss of HA-MC4R-GFP protein abundance in N2AHA-MC4R-GFP cells. A, Lysates derived from N2AHA-MC4R-GFP cells treated as in Figure 1A were analyzed by Western blot. Abundance of HA-MC4R-GFP is normalized to that of actin. Quantification of data is derived from three independent experiments including that presented in the figure. B, N2AHA-MC4R-GFP cells were treated with palmitate as in Figure 1A. The cAMP generated in response to stimulation with α-MSH and abundance of HA-MC4R-GFP at the cell surface, respectively, were measured as described in Materials and Methods. The graph shows the abundance of intracellular cAMP divided by the abundance of HA-MC4R-GFP at the cell surface (expressed in OD units). Data are expressed as mean ± SD. ***, P < .001.
To test whether, in addition to a loss of abundance of HA-MC4R-GFP, there was also loss of activity of the receptor residing at the cell surface, we measured the ratio of cAMP generated to the amount of receptor at the cell surface in response to an acute treatment with α-MSH (38). By this assay, exposure to palmitate at 0.2 mM did not change the amount of cAMP generated per HA-MC4R-GFP receptor number (Figure 2B), indicating that activity of the receptor is maintained. The data obtained using N2AHA-MC4R-GFP cells together with the functional data in the N42 hypothalamic neurons indicate that the profound and similar loss of cAMP signaling in response to acute stimulation with α-MSH observed in both cell lines exposed to elevated palmitate is due to a loss of abundance of MC4R protein.
In N42 hypothalamic neurons exposure to elevated palmitate within the physiological range induces mild ER stress
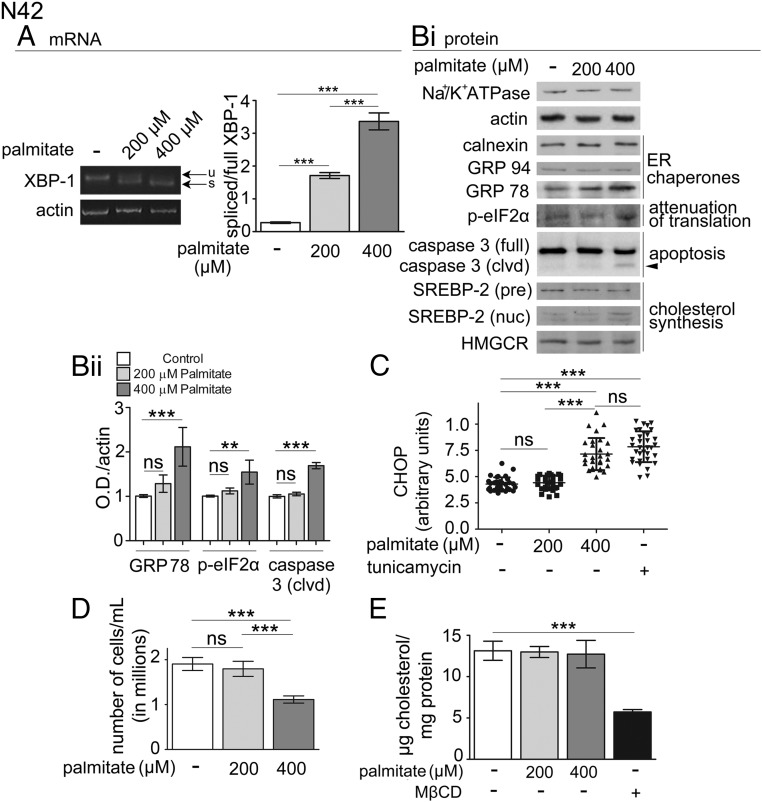
In obese rodents exposed to a HF diet, dislipidemia increases the level of palmitate in the hypothalamus and induces ER stress, inflammation, and resistance to leptin and insulin (7–11). ER stress induces the unfolded protein response (UPR), a complex pathway to reestablish ER homeostasis and, when this fails, to promote cell apoptosis (39). A major branch of the UPR is initiated by inositol-requiring enzyme 1-α, which splices XBP-1 mRNA to generate the transcription factor XBP-1s. XBP-1s regulates the expression of genes to expand the ER folding capacity including that of the ER chaperone GRP78/immunoglobin heavy chain-binding protein (BiP) (40, 41). To determine whether exposure to elevated palmitate causes ER stress in the N42 hypothalamic neurons, we monitored the level of XBP-1 splicing by measuring the ratio XBP-1s to XBP-1. When N42 cells were exposed to palmitate at 0.4 mM, the ratio XBP-1s mRNA to XBP-1 mRNA increased by approximately 12-fold (Figure 3A), and the abundance of GRP78/BiP protein increased by approximately 2-fold (Figure 3B). Conversely, when cells were exposed to palmitate at 0.2 mM, the ratio XBP-1s mRNA to XBP-1 mRNA was increased to a lower level (by ∼6 fold, Figure 3A) and abundance of GRP78/BiP did not change (Figure 3B).
Figure 3.
Exposure to elevated palmitate within the physiological range induces mild ER stress in N42 hypothalamic neurons. A, N42 cells were treated as in Figure 1A, and splicing of XBP-1 was measured by RT-PCR as described in Materials and Methods; u, unspliced XBP-1; s, spliced XBP-1. B, N42 cells were treated as in Figure 1A and samples were collected to determine by Western blot analysis the abundance of the indicated proteins (i). Quantification of the level of expression of the indicated proteins (ii) was derived from three experiments including that shown in Bi. SREBP-2, sterol regulatory element-binding protein-2. C, N42 cells were treated as in Figure 1A or with 1 μg/mL tunicamycin (tun), as indicated. The graph shows the integrated fluorescence density of CHOP localized in the nucleus. D, N42 cells were treated with palmitate as in Figure 1A, and viable cells labeled by trypan blue staining were counted. E, N42 cells were treated with palmitate as in Figure 1A and then analyzed for cholesterol content. Data are expressed as mean ± SD. **, P < .01; ***, P < .001.
Another branch of the UPR is initiated by the autophosphorylation of protein kinase-like endoplasmic reticulum kinase, which in turn phosphorylates the α-subunit of eukaryotic translation initiation factor (eIF2) α, to eIF2αP to attenuate protein translation. In N42 neurons exposed to palmitate at 0.4 mM, but not at 0.2 mM, levels of eIF2αP detected by Western blot analysis were increased (by ∼1.6-fold, Figure 3B). If ER stress is not resolved, apoptosis is induced. In N42 neurons exposed to palmitate at 0.4, but not at 0.2 mM, levels of the transcription factor CHOP in the nucleus was also increased by approximately 2-fold, to the same level as that induced by incubation with tunicamycin, a drug that induces a classical form of ER stress by increasing the load of deglycosylated, unfolded protein in the ER (Figure 3C and Supplementary Figure 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). In addition, in N42 neurons exposed to palmitate at 0.4, but not at 0.2 mM, the cleaved, active form of caspase-3 was also expressed (Figure 3B, arrowhead), and the number of viable cells was decreased (by ∼50%, Figure 3D). Together these results indicate that exposure to 0.4 mM palmitate induces in the N42 hypothalamic neurons severe and unrecoverable ER stress, leading to cell death.
Conversely, exposure of N42 neurons to palmitate at 0.2 mM appears to induce a milder injury because neither the proapoptotic pathway CHOP was activated (Figure 3C) nor apoptosis itself was taking place, monitored by increased cleaved caspase-3 and reduced cell number (Figure 3, B and D). We have previously found that acute depletion of cell cholesterol by methyl-β-cyclodextrin decreases cAMP signaling in N42 hypothalamic neurons exposed to α-MSH (38). The UPR also modulates the cell lipid metabolism (42), including synthesis of cholesterol, by regulating the abundance of a transcription factor, sterol-regulatory element-binding protein-2 (43), which in turn promotes the expression of several genes including that for HMGCR, the rate-limiting enzyme in the synthesis of cholesterol (44). However, in N42 neurons exposed to palmitate at 0.2 and 0.4 mM, the abundance of the nuclear form of sterol-regulatory element-binding protein-2, HMGCR (Figure 3B) and, importantly, cholesterol (Figure 3E) were not changed.
In N2AHA-MC4R-GFP cells, exposure to elevated palmitate within the physiological range induces mild ER stress, thus mirroring effects observed in the N42 hypothalamic neurons
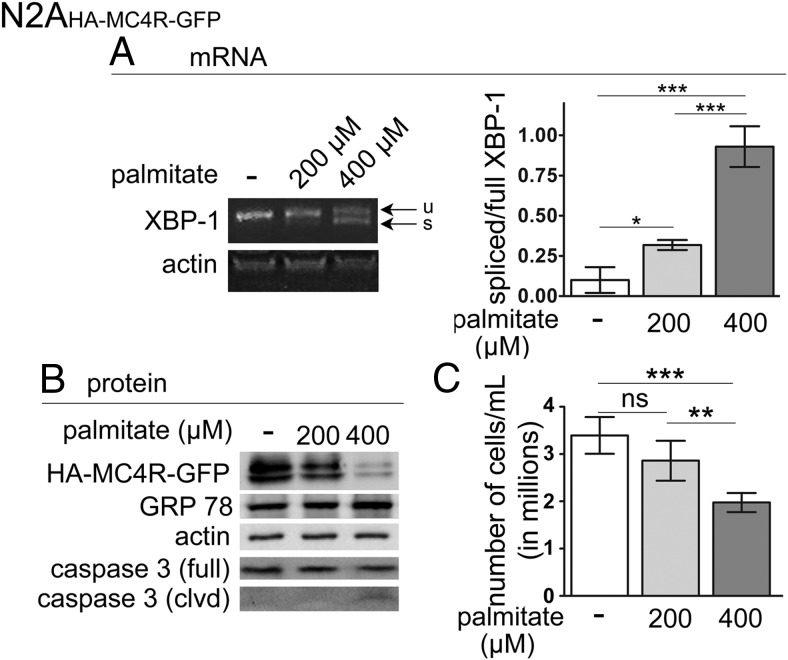
Also in N2AHA-MC4R-GFP cells, exposure to elevated palmitate increased the abundance of XBP-1s mRNA in a dose-dependent manner (Figure 4A). In addition, only exposure to the higher concentration of palmitate induced increased the levels of GRP78/BiP, the appearance of the cleaved form of caspase-3, and decreased the number of viable N2AHA-MC4R-GFP cells, indicating that apoptosis was taking place (Figure 4, B and C). Therefore, in N2AHA-MC4R-GFP cells, exposure to 0.2 and 0.4 mM palmitate induces mild and severe ER stress, respectively, similar to what was observed in the N42 hypothalamic neurons. Together with data in Figures 1–3, these experiments indicate that elevated palmitate causes a profound loss of MC4R abundance and function, even under conditions of mild ER stress in both N42 neurons and N2AHA-MC4R-GFP cells.
Figure 4.
Exposure to elevated palmitate within the physiological range induces mild ER stress in N2AHA-MC4R-GFP cells. A, N2AHA-MC4R-GFP cells were treated with and without elevated palmitate as described in Figure 1A. Splicing of XBP-1 was analyzed as in Figure 3A; u, unspliced XBP-1; s, spliced XBP-1. B, N2AHA-MC4R-GFP cells were treated as in Figure 1A; samples were collected to determine by Western blot analysis the abundance of the indicated proteins. C, N2AHA-MC4R-GFP cells were treated with elevated palmitate as in A, and viable cells labeled by trypan blue staining were counted. Data are expressed as mean ± SD. *, P < .05; ***, P < .001.
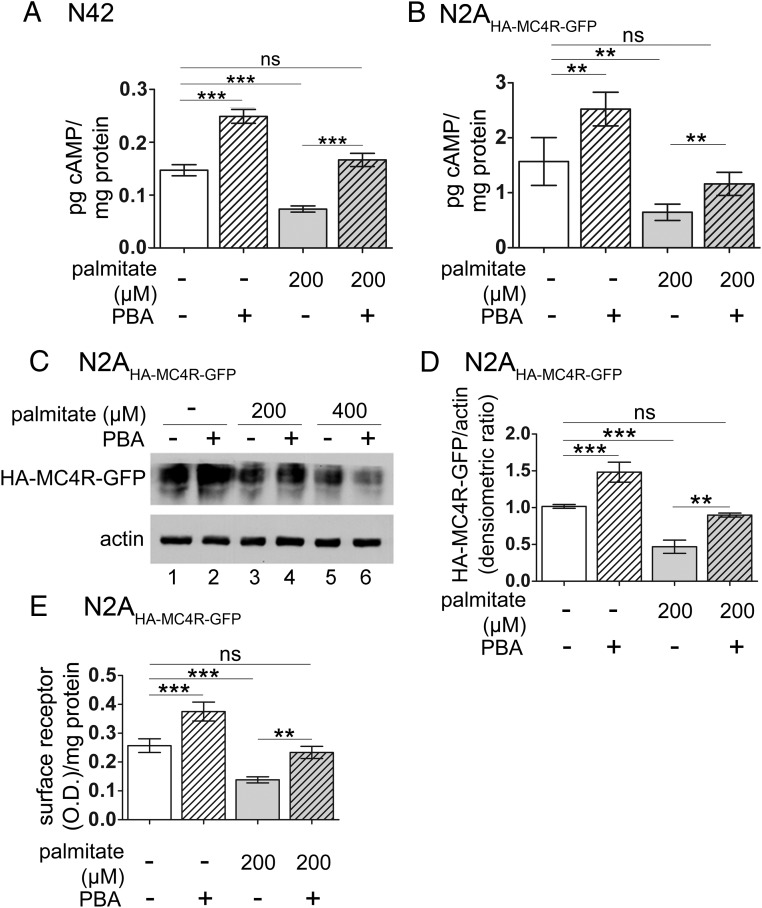
PBA reestablishes optimal response to α-MSH in N42 hypothalamic neurons and N2AHA-MC4R-GFP cells treated with elevated palmitate by restoring abundance of MC4R
We have found previously that PBA, a chemical chaperone that reduces ER stress (10, 45–48), increases the abundance and function of overexpressed wild-type-MC4R and variants of MC4R linked to human obesity, which misfold and are retained in the ER (30, 31, 49–53). Here we asked whether PBA would also rescue function of endogenous MC4R in cells exposed to elevated palmitate. We have previously found that treatment with 2 mM PBA promotes folding of tagged MC4R in the N2A cells (30, 31). However, incubation with PBA at 2 mM, a concentration that did not have adverse effects on N2A cells, instead reduced the cell number of the N42 hypothalamic neurons. We therefore carried out experiments to determine the highest concentration of PBA in the medium, which did not affect N42 cell number and which was found to be 0.25 mM (not shown). When N42 hypothalamic neurons were treated with 0.25 mM PBA for 16 hours, stimulation with α-MSH induced generation of cAMP to a significantly higher level (by ∼70%) than that of cells that were not exposed to the chemical chaperone (Figure 5A, lanes 1 and 2). Also, when N42 cells were exposed to the elevated palmitate at 0.2 mM, the treatment with PBA increased the generation of cAMP in response to acute stimulation with α-MSH (by ∼120%, Figure 5A, lanes 3 and 4) and to the same level as control cells not exposed to the elevated fatty acid (Figure 5A, lanes 1 and 4). These experiments suggest that PBA corrects a propensity of endogenous MC4R to misfold whether or not cells are treated with elevated palmitate.
Figure 5.
PBA restores signaling in response to α-MSH in N42 hypothalamic neurons and N2AHA-MC4R-GFP cells exposed to elevated palmitate by increasing the abundance of the receptor. A and B, N42 cells (A) and N2AHA-MC4R-GFP cells (B) were treated with and without palmitate at the indicated concentrations and with PBA at 0.25 mM (N42 cells) and 2 mM (N2AHA-MC4R-GFP cells). Cells were stimulated without and with α-MSH at 100 nM. Samples were collected to measure cAMP levels and protein concentration as in Figure 1A. In the graphs, the cAMP levels of cells kept in the basal condition are subtracted from those of cells in the stimulated condition. C, Lysates derived from N2AHA-MC4R-GFP cells treated as in Figure 1A were analyzed by Western blot with anti-HA and antiactin antibodies. D, Abundance of HA-MC4R-GFP is normalized to that of actin. The quantification in the graph is derived from three independent experiments including that shown in C. E, N2AHA-MC4R-GFP cells were treated with palmitate as in Figure 1A. The cAMP generated in response to stimulation with α-MSH is measured as in A. Data are expressed as mean ± SD. **, P < .01; ***, P < .001.
In N2AHA-MC4R-GFP cells, treatment with 2 mM PBA increased the level of cAMP generated in response to stimulation with α-MSH (by ∼60%, Figure 5B, lanes 1 and 2), whereas this effect was more attenuated at lower concentrations of the drug (<35% increase of cAMP response to agonist in cells treated with 0.5 mM PBA, not shown). Treatment with 2 mM PBA also increased the level of cAMP generated in response to stimulation with α-MSH in N2AHA-MC4R-GFP cells exposed to elevated palmitate (by ∼60%, Figure 5B, lanes 3 and 4) and to the same level as that of control cells that were not exposed to the elevated fatty acid (Figure 5B, lanes 1 and 4). Together the data indicate that exposure to PBA, albeit at different concentrations, restores function of endogenous and exogenous MC4R in palmitate-treated N42 neurons and N2AHA-MC4R-GFP cells, respectively, to the same level as that in untreated control cells. A likely mechanism by which PBA increases the response to α-MSH in palmitate-treated neurons is by restoring abundance of MC4R protein. In the N2AHA-MC4R-GFP cells treated with 0.2 mM palmitate, incubation with PBA restored total abundance of HA-MC4R-GFP, detected by Western blot analysis (Figure 5, C, lanes 1–4, and D). In the N2AHA-MC4R-GFP cells treated with 0.2 mM palmitate, incubation with PBA also increased the expression of HA-MC4R-GFP at the cell surface (Figure 5E, lanes 3 and 4) to the same level as in control cells that were not treated with the elevated fatty acid (Figure 5E, lanes 1 and 4). Conversely, addition of PBA to cells exposed to 0.4 mM palmitate decreased the total abundance of HA-MC4R-GFP (Figure 5C, lanes 5 and 6). These experiments indicate that, in hypothalamic neurons exposed to mild lipid stress, PBA restores the signaling response to α-MSH by increasing the total abundance of MC4R and expression of the receptor at the cell surface. The experiments also indicate that administration of PBA cannot recover and may actually worsen the loss of MC4R when lipid stress induces severe ER stress (Figure 5C).
Discussion
Profound loss of α-MSH-mediated cAMP signaling precedes severe ER stress in immortalized hypothalamic neurons exposed to elevated palmitate
A key finding of this paper is that exposure of hypothalamic neurons to elevated palmitate induces a profound loss of cAMP signaling in response to exposure to α-MSH. Strikingly, we find that such loss of response to α-MSH appears even before cells develop severe ER stress. It has been previously found that in the hypothalamus of rodents treated for prolonged time with a HF diet, there is accumulation of metabolites derived from palmitate (25) as well as increased phosphorylation of eIF2α and activation of caspase-3, indicating that severe ER stress and apoptosis are taking place (54). Our data indicate that when hypothalamic neurons are exposed to lipid stress, the loss of MC4R function, measured by decreased cAMP signaling in response to α-MSH, is already occurring at a stage at which splicing in XBP-1 is taking place, but phosphorylation of eIF2α and activation of caspase-3 and proapoptotic CHOP are not yet induced. The profound loss of MC4R signaling upon even mild level lipid stress may contribute to the inability of stable and potent melanocortins to reverse obesity in rodents and humans (22, 55, 56).
Lipid stress promotes resistance to α-MSH by inducing a loss of MC4R abundance by posttranscriptional mechanisms
It has been reported that exposure to a HF diet is associated with decreased levels of hypothalamic MC4R mRNA in obese rodents (17, 18). In contrast, other studies indicate increased MC4R mRNA in the hypothalamus of obese rats (7, 16). These observations suggest that a HF diet has variable effects on hypothalamic MC4R mRNA abundance, likely depending on the specific type of diet and the modality by which the diet was delivered. Our data, in which cultured hypothalamic neurons are exposed to mild lipid stress, indicate that profound loss of signaling in response to acute stimulation with α-MSH and loss of MC4R protein can coexist with even minimally reduced levels of an endogenous MC4R transcript. It is found that, also in N2AHA-MC4R-GFP cells, exposure to elevated palmitate had minimal effects on the abundance of the transcripts encoding for endogenous MC4R and exogenous HA-MC4R-GFP, whereas instead affecting profoundly signaling in response to acute stimulation with α-MSH. Thus, the N2AHA-MC4R-GFP cell line reproduces effects of elevated palmitate observed in the cultured hypothalamic neurons with respect to MC4R mRNA abundance and response to α-MSH. A major obstacle to detect effects of lipid stress on MC4R abundance is that the expression of the receptor cannot be directly assessed at the protein level because high-affinity antibodies able to detect endogenous levels of receptor in the hypothalamus or in hypothalamic neurons have not yet been developed. By using N2AHA-MC4R-GFP cells, we find that elevated palmitate in the physiological range reduces protein abundance of tagged MC4R.
Together these observations suggest that lipid stress promotes resistance to α-MSH by inducing a loss of MC4R abundance by posttranscriptional mechanisms. The conclusion that lipid stress impairs MC4R abundance and cAMP signaling in hypothalamic neurons is consistent with in vivo studies, in which obese rats exposed to a HF diet have central resistance to MC4R agonists monitored by measuring food intake (19, 20) and with a study in which it is found that binding to radiolabeled MC4R agonists is reduced in the hypothalamus of rats with obesity induced by a HF diet (21). The study presented here also indicates that HA-MC4R-GFP is a reliable reporter to monitor the effects of lipid stress on endogenous MC4R. In the future, expression of the tagged MC4R in the paraventricular nucleus may facilitate monitoring the adverse effects of a HF diet on endogenous MC4R and possible reversal of such effects by PBA.
Here we also find that exposure to elevated palmitate in the physiological range did not alter the amount of cAMP generated per HA-MC4R-GFP receptor number at the cell surface. Thus, the loss of MC4R signaling in response to acute exposure to α-MSH is due to a loss in protein abundance of the receptor, rather than to loss of receptor activity. This is different from the effects induced by reduced membrane cholesterol, which causes loss of MC4R signaling in response to acute stimulation with α-MSH because a population of receptors at the plasma membrane becomes inactive (38). Interestingly, insulin-deficient diabetic mice have decreased cholesterol synthesis in the hypothalamus, which leads to increased food intake and weight gain (57). However, rodents exposed to HF diet do not have altered cholesterol levels in the hypothalamus (58), a feature reproduced here in the cultured hypothalamic neurons exposed to elevated palmitate. Our data indicate that loss of MC4R function in response to lipid stress can occur by different mechanisms, namely loss of receptor activity and loss of receptor abundance, depending on whether the injury is due to decreased cell cholesterol or exposure to elevated palmitate, respectively.
PBA corrects the loss of MC4R protein induced by cell exposure to elevated palmitate
We find that PBA, a chemical chaperone that reduces ER stress (10, 45–48), restored acute response to α-MSH in hypothalamic neurons and in the N2AHA-MC4R-GFP cells treated with elevated palmitate. PBA also restored abundance of the HA-MC4R-GFP reporter in the N2AHA-MC4R-GFP cells. Thus, our data indicate that PBA can sensitize neurons exposed to elevated palmitate to α-MSH, likely by promoting abundance of MC4R protein. A possible explanation for the loss of MC4R abundance in response to exposure to elevated palmitate may be that the folding capacity of the ER, monitored here by lack of increased abundance of ER chaperones, does not increase in response to lipid stress. The lack of expansion of the folding capacity of the ER observed here in the immortalized hypothalamic neurons appears also to occur in hepatocytes of mice in which lipid stress was induced by a HF diet (59). Lack of an increase of the folding capacity of the ER in the face of stress may affect more severely MC4R, which appears to have an inherent propensity to misfold (30), than other proteins traversing the ER. In this respect, we find here that, in response to lipid stress, although expression of the tagged MC4R is severely compromised, the abundance of another protein traversing the ER to reach the plasma membrane, the α1 subunit of Na+ K+ ATPase (60), is not decreased. Other investigators have found that obesity induced by a HF diet changes the cell lipid composition of the hypothalamus by increasing levels of palmitoyl- and stearoyl-CoA and specific sphingolipids (12, 58). Although mechanisms by which elevated palmitate causes reduced MC4R protein are, at present, only speculative, it will be of future interest to determine whether ER stress, perhaps consequent to signaling by elevated palmitate and/or changes in the ceramide lipid composition of the cell (42, 61, 62), contribute to promote the loss of the MC4R protein.
Within the melanocortin system, it appears that ER stress in obese mice leads also to reduced leptin signaling, despite unchanged mRNA levels of the leptin receptor (7, 10). In addition, it has been found that the signaling of the insulin receptor by hypothalamic neurons is also attenuated by cell exposure to elevated fatty acids by a mechanism that implicates ER stress (27). These data, taken together with the work presented here, indicate that ER stress may act to reduce the activity of multiple receptors involved in the melanocortin pathway, including that of MC4R. Our work provides the first evidence that the loss of signaling of the MC4R is due to the loss of receptor abundance, which takes place, even when there is mild ER stress, and occurs without the loss of receptor activity. Finally, our work suggests that combinatorial treatment with chemical chaperones and MC4R agonists may promote MC4R signaling in lipid-stressed hypothalamic neurons in vivo by rescuing the abundance of the receptor.
Additional material
Supplementary data supplied by authors.
Acknowledgments
This work was supported by the National Institutes of Health Grant R01-DK080424 (to G.B.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ABTS
- 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)
- BCA
- bicinchoninic assay
- BiP
- immunoglobin heavy chain-binding protein
- CHOP
- C/EBP-homologous protein
- eIF2
- eukaryotic initiation factor
- ER
- endoplasmic reticulum
- FFA
- free fatty acid
- FITC
- fluorescein isothiocyanate
- GFP
- green fluorescent protein
- GRP78
- glucose-regulated protein 78
- HA
- hemagglutinin
- HF
- high-fat
- HMGCR
- 3-hydroxy-3-methylglutaryl coenzyme A reductase
- IBMX
- 3-isobutyl-1-methylxanthine
- MC4R
- melanocortin 4 receptor
- N42
- mHypoE-42
- N2A
- Neuro 2A
- P
- phosphorylation
- PBA
- 4-phenylbutyric acid
- POD
- peroxidase
- UPR
- unfolded protein response
- XBP-1
- X-box binding protein 1
- XBP-1s
- splicing of XBP-1 mRNA.
References
- 1. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332–341. [DOI] [PubMed] [Google Scholar]
- 2. Cascio G, Schiera G, Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev. 2012;8(1):2–17. [DOI] [PubMed] [Google Scholar]
- 3. Lottenberg AM, Afonso MdS, Lavrador MSF, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 2012;23(9):1027–1040. [DOI] [PubMed] [Google Scholar]
- 4. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. [DOI] [PubMed] [Google Scholar]
- 5. Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. [DOI] [PubMed] [Google Scholar]
- 6. Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15(10):1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5(3):181–194. [DOI] [PubMed] [Google Scholar]
- 8. De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. [DOI] [PubMed] [Google Scholar]
- 9. Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9(1):35–51. [DOI] [PubMed] [Google Scholar]
- 11. Thaler JP, Yi C-X, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benoit SC, Kemp CJ, Elias CF, et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J Clin Invest. 2009;119(9):2577–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R981–R986. [DOI] [PubMed] [Google Scholar]
- 14. Clegg DJ, Gotoh K, Kemp C, et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145(11):4880–4889. [DOI] [PubMed] [Google Scholar]
- 16. Stofkova A, Skurlova M, Kiss A, Zelezna B, Zorad S, Jurcovicova J. Activation of hypothalamic NPY, AgRP, MC4R, AND IL-6 mRNA levels in young Lewis rats with early-life diet-induced obesity. Endocr Regul. 2009;43(3):99–106. [PubMed] [Google Scholar]
- 17. Gutierrez-Aguilar R, Kim D-H, Woods SC, Seeley RJ. Expression of new loci associated with obesity in diet-induced obese rats: from genetics to physiology. Obesity (Silver Spring, Md). 2012;20(2):306–312. [DOI] [PubMed] [Google Scholar]
- 18. Chen H, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: interaction with postnatal nutritional environment. PloS One. 2009;4(7):e6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woods SC, D'Alessio DA, Tso P, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83(4):573–578. [DOI] [PubMed] [Google Scholar]
- 20. Clegg DJ, Benoit SC, Air EL, et al. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology. 2003;144(7):2941–2946. [DOI] [PubMed] [Google Scholar]
- 21. Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin, and melanocortin binding associated with moderate-fat diet and predisposition to obesity. Endocrinology. 2007;148(1):310–316. [DOI] [PubMed] [Google Scholar]
- 22. Krishna R, Gumbiner B, Stevens C, et al. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: energy intake predicts lack of weight loss efficacy. Clin Pharmacol Ther. 2009;86(6):659–666. [DOI] [PubMed] [Google Scholar]
- 23. Shojaee-Moradie F, Baynes KCR, Pentecost C, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50(2):404–413. [DOI] [PubMed] [Google Scholar]
- 24. Sabin MA, De Hora M, Holly JMP, et al. Fasting nonesterified fatty acid profiles in childhood and their relationship with adiposity, insulin sensitivity, and lipid levels. Pediatrics. 2007;120(6):e1426–e1433. [DOI] [PubMed] [Google Scholar]
- 25. Posey KA, Clegg DJ, Printz RL, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296(5):E1003–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peyot M-L, Pepin E, Lamontagne J, et al. β-Cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59(9):2178–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinology. 2010;151(2):576–585. [DOI] [PubMed] [Google Scholar]
- 28. Choi SJ, Kim F, Schwartz MW, Wisse BE. Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids. Am J Physiol Endocrinol Metab. 2010;298(6):E1122–E1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohammad S, Baldini G, Granell S, Narducci P, Martelli AM, Baldini G. Constitutive traffic of melanocortin-4 receptor in Neuro2A cells and immortalized hypothalamic neurons. J Biol Chem. 2007;282(7):4963–4974. [DOI] [PubMed] [Google Scholar]
- 30. Granell S, Mohammad S, Ramanagoudr-Bhojappa R, Baldini G. Obesity-linked variants of melanocortin-4 receptor are misfolded in the endoplasmic reticulum and can be rescued to the cell surface by a chemical chaperone. Mol Endocrinol. 2010;24(9):1805–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Granell S, Serra-Juhe C, Martos-Moreno GA, et al. A novel melanocortin-4 receptor mutation MC4R-P272L associated with severe obesity has increased propensity to be ubiquitinated in the ER in the face of correct folding. PloS One. 2012;7(12):e50894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tao Y-X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31(4):506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) α to ERβ in clonal hypothalamic neurons. Mol Endocrinol. 2006;20(9):2080–2092. [DOI] [PubMed] [Google Scholar]
- 34. Mayer CM, Fick LJ, Gingerich S, Belsham DD. Hypothalamic cell lines to investigate neuroendocrine control mechanisms. Front Neuroendocrinol. 2009;30(3):405–423. [DOI] [PubMed] [Google Scholar]
- 35. Serlie MJ, Meijer AJ, Groener JE, et al. Short-term manipulation of plasma free fatty acids does not change skeletal muscle concentrations of ceramide and glucosylceramide in lean and overweight subjects. J Clin Endocrinol Metab. 2007;92(4):1524–1529. [DOI] [PubMed] [Google Scholar]
- 36. Shinyama H, Masuzaki H, Fang H, Flier JS. Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology. 2003;144(4):1301–1314. [DOI] [PubMed] [Google Scholar]
- 37. Granell S, Molden BM, Baldini G. Exposure of MC4R to agonist in the endoplasmic reticulum stabilizes an active conformation of the receptor that does not desensitize. Proc Natl Acad Sci USA. 2013;110(49):E4733–E4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDaniel FK, Molden BM, Mohammad S, et al. Constitutive cholesterol-dependent endocytosis of melanocortin-4 receptor (MC4R) is essential to maintain receptor responsiveness to α-melanocyte-stimulating hormone (α-MSH). J Biol Chem. 2012;287(26):21873–21890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197(7):857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. [DOI] [PubMed] [Google Scholar]
- 41. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. [DOI] [PubMed] [Google Scholar]
- 42. Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15(5):623–634. [DOI] [PubMed] [Google Scholar]
- 43. Zeng L, Lu M, Mori K, et al. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004;23(4):950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer's bottle to Scap's MELADL. J Lipid Res. 2009;50(suppl):S15–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and β-cell dysfunction in humans. Diabetes. 2011;60(3):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem. 2007;282(38):27905–27912. [DOI] [PubMed] [Google Scholar]
- 48. Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997;100(10):2457–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095. [DOI] [PubMed] [Google Scholar]
- 50. Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12(5):561–574. [DOI] [PubMed] [Google Scholar]
- 51. Lubrano-Berthelier C, Durand E, Dubern B, et al. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003;12(2):145–153. [DOI] [PubMed] [Google Scholar]
- 52. Nijenhuis WA, Garner KM, van Rozen RJ, Adan RA. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem. 2003;278(25):22939–22945. [DOI] [PubMed] [Google Scholar]
- 53. Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144(10):4544–4551. [DOI] [PubMed] [Google Scholar]
- 54. Moraes JC, Coope A, Morari J, et al. High-fat diet induces apoptosis of hypothalamic neurons. PloS One. 2009;4(4):e5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51(5):1337–1345. [DOI] [PubMed] [Google Scholar]
- 56. Bluher S, Ziotopoulou M, Bullen JW, Jr, et al. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes. 2004;53(1):82–90. [DOI] [PubMed] [Google Scholar]
- 57. Suzuki R, Lee K, Jing E, et al. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 2010;12(6):567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borg ML, Omran SF, Weir J, Meikle PJ, Watt MJ. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol. 2012;590(Pt 17):4377–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tokhtaeva E, Sachs G, Vagin O. Assembly with the Na,K-ATPase α(1) subunit is required for export of β(1) and β(2) subunits from the endoplasmic reticulum. Biochemistry. 2009;48(48):11421–11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15(5):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yue JTY, Lam TKT. Lipid sensing and insulin resistance in the brain. Cell Metab. 2012;15(5):646–655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.