Abstract
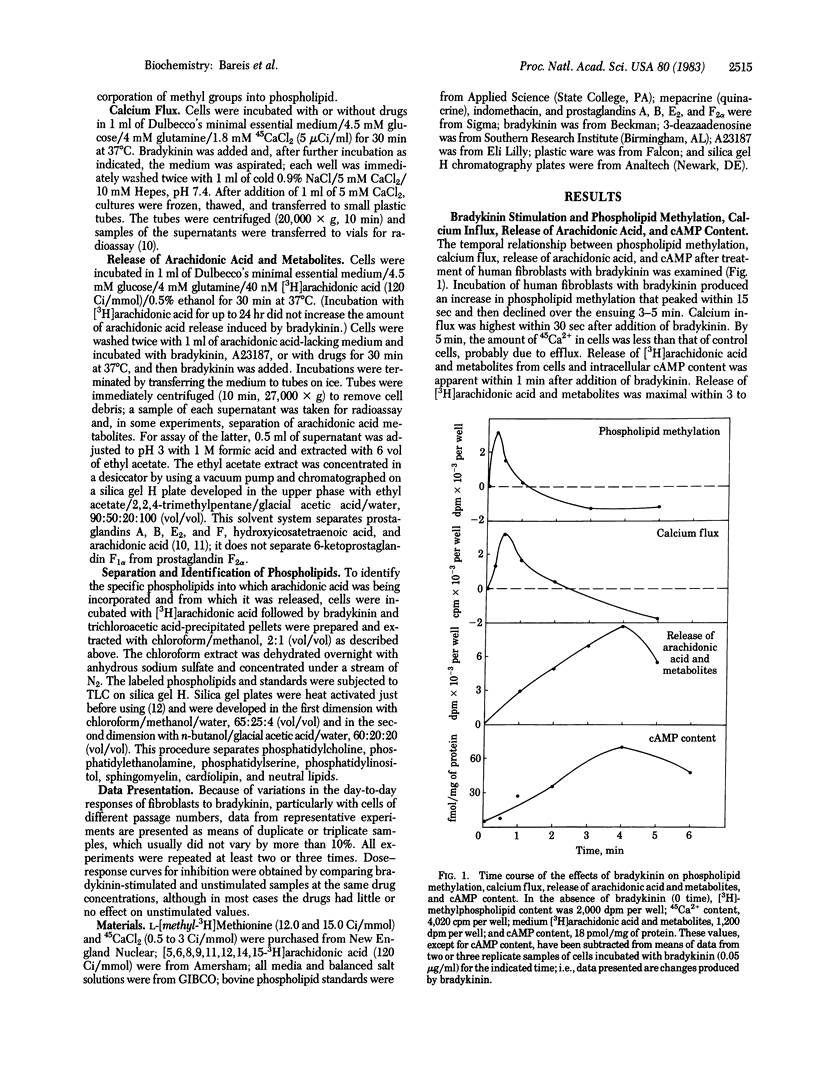
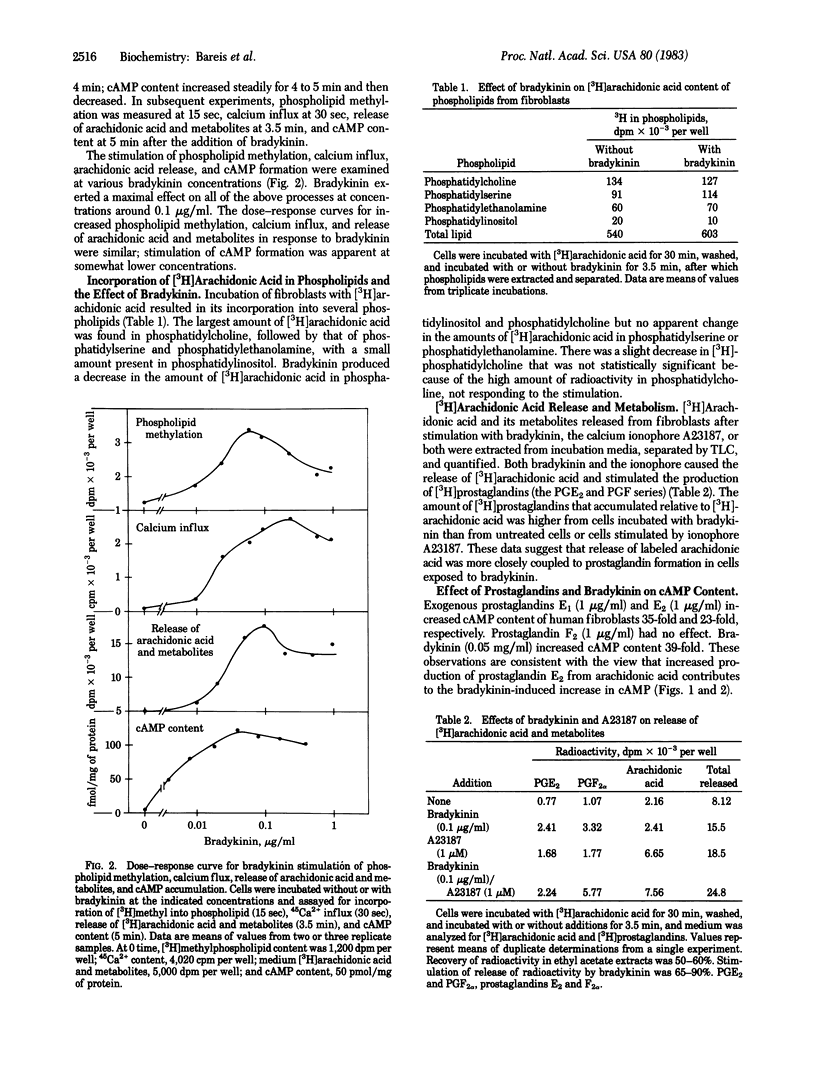
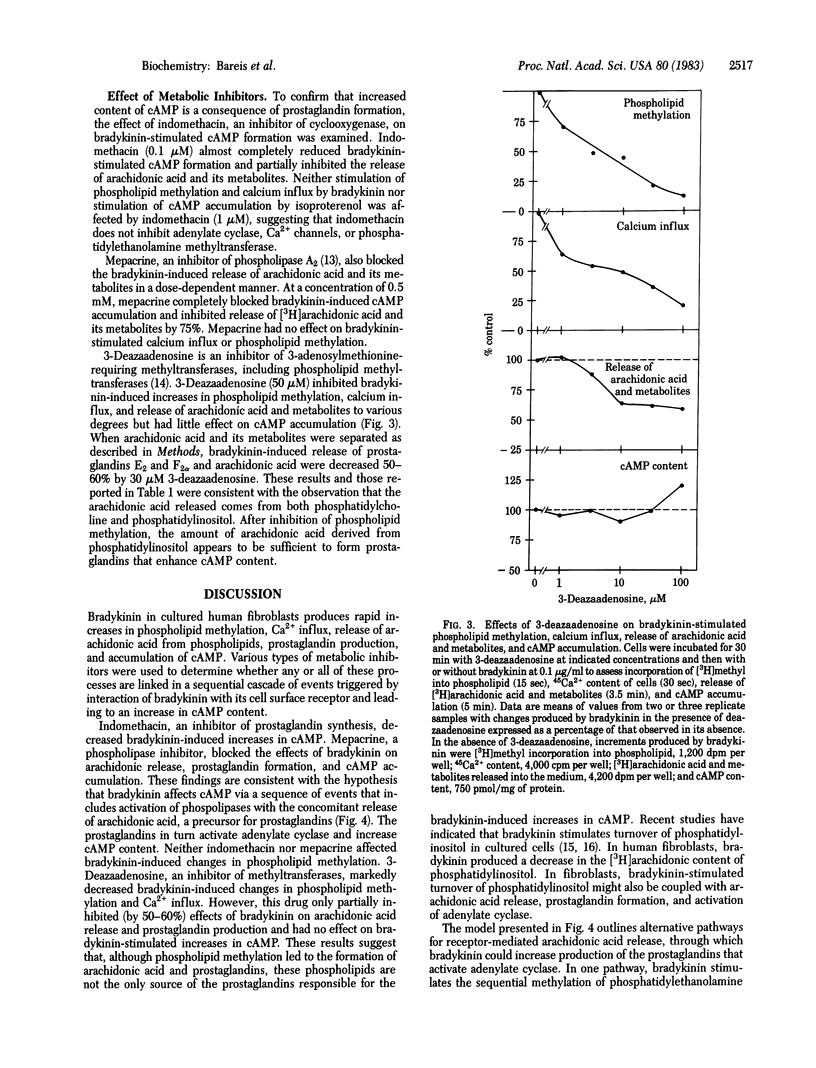
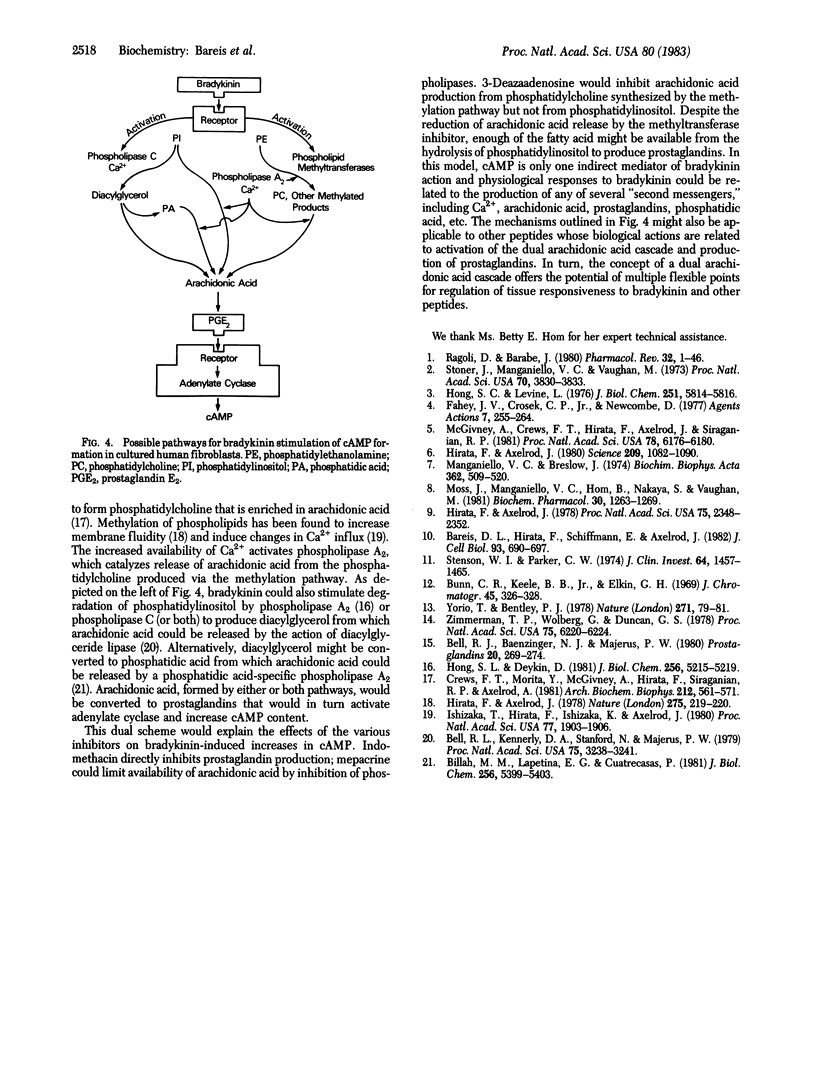
The biochemical events that lead to bradykinin stimulation of cAMP accumulation in human fibroblasts were examined. Treatment of human fibroblasts with bradykinin increases phospholipid methylation, Ca2+ influx, arachidonic acid release, prostaglandin formation, and cAMP content. The dose-response curves of bradykinin for the increase in the above changes were similar. In human fibroblasts, exogenous arachidonic acid was mainly incorporated into phosphatidylcholine, followed by phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol. Bradykinin caused a release of arachidonic acid from methylated phospholipids (phosphatidylcholine) and phosphatidylinositol. 3-Deazaadenosine, a methyltransferase inhibitor, almost completely inhibited bradykinin-stimulated phospholipid methylation and Ca2+ influx and partially reduced arachidonic acid release and prostaglandin formation but had no effect on cAMP formation. Mepacrine, a phospholipase inhibitor, blocked bradykinin-induced arachidonic acid release, prostaglandin release, and cAMP accumulation. Indomethacin, a cyclooxygenase inhibitor, blocked the effect of bradykinin on cAMP accumulation. Prostaglandins E1 and E2, but not F2 alpha, increased accumulation of cAMP. These observations indicate that bradykinin generates cAMP via arachidonic acid release and subsequent formation of prostaglandins. Our findings suggest that arachidonic acid can arise from either phosphatidylcholine synthesized by the methylation pathway or phosphatidylinositol.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bareis D. L., Hirata F., Schiffmann E., Axelrod J. Phospholipid metabolism, calcium flux, and the receptor-mediated induction of chemotaxis in rabbit neutrophils. J Cell Biol. 1982 Jun;93(3):690–697. doi: 10.1083/jcb.93.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. L., Baenziger N. L., Majerus P. W. Bradykinin-stimulated release of arachidonate from phosphatidyl inositol in mouse fibrosarcoma cells. Prostaglandins. 1980 Aug;20(2):269–274. doi: 10.1016/s0090-6980(80)80045-1. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Bunn C. R., Keele B. B., Jr, Elkan G. H. A technique for improved thin-layer chromatography of phospholipids. J Chromatogr. 1969 Dec 2;45(2):326–328. doi: 10.1016/s0021-9673(01)86223-3. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Morita Y., McGivney A., Hirata F., Siraganian R. P., Axelrod J. IgE-mediated histamine release in rat basophilic leukemia cells: receptor activation, phospholipid methylation, Ca2+ flux, and release of arachidonic acid. Arch Biochem Biophys. 1981 Dec;212(2):561–571. doi: 10.1016/0003-9861(81)90399-4. [DOI] [PubMed] [Google Scholar]
- Fahey J. V., Ciosek C. P., Jr, Newcombe D. S. Human synovial fibroblasts: the relationships between cyclic AMP, bradykinin, and prostaglandins. Agents Actions. 1977 Jul;7(2):255–264. doi: 10.1007/BF01969984. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic methylation of phosphatidylethanolamine increases erythrocyte membrane fluidity. Nature. 1978 Sep 21;275(5677):219–220. doi: 10.1038/275219a0. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic synthesis and rapid translocation of phosphatidylcholine by two methyltransferases in erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 May;75(5):2348–2352. doi: 10.1073/pnas.75.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Deykin D. The activation of phosphatidylinositol-hydrolyzing phospholipase A2 during prostaglandin synthesis in transformed mouse BALB/3T3 cells. J Biol Chem. 1981 May 25;256(10):5215–5219. [PubMed] [Google Scholar]
- Hong S. L., Levine L. Stimulation of prostaglandin synthesis by bradykinin and thrombin and their mechanisms of action on MC5-5 fibroblasts. J Biol Chem. 1976 Sep 25;251(18):5814–5816. [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganiello V. C., Breslow J. Effects of prostaglandin E1 and isoproterenol on cyclic AMP content of human fibroblasts modified by time and cell density in subculture. Biochim Biophys Acta. 1974 Oct 8;362(3):509–520. doi: 10.1016/0304-4165(74)90146-9. [DOI] [PubMed] [Google Scholar]
- McGivney A., Crews F. T., Hirata F., Axelrod J., Siraganian R. P. Rat basophilic leukemia cell lines defective in phospholipid methyltransferase enzymes, Ca2+ influx, and histamine release: reconstitution by hybridization. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6176–6180. doi: 10.1073/pnas.78.10.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Hom B. E., Nakaya S., Vaughan M. Effects of d- and l-propranolol on the responsiveness of human fibroblasts to choleragen and prostaglandin E1. Biochem Pharmacol. 1981 Jun 1;30(11):1263–1269. doi: 10.1016/0006-2952(81)90307-5. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner J., Manganiello V. C., Vaughan M. Effects of bradykinin and indomethacin on cyclic GMP and cyclic AMP in lung slices. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3830–3833. doi: 10.1073/pnas.70.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorio T., Bentley P. J. Phospholipase A and the mechanism of action of aldosterone. Nature. 1978 Jan 5;271(5640):79–81. doi: 10.1038/271079a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Wolberg G., Duncan G. S. Inhibition of lymphocyte-mediated cytolysis by 3-deazaadenosine: evidence for a methylation reaction essential to cytolysis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6220–6224. doi: 10.1073/pnas.75.12.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]


