Abstract
Objective
HIV+ patients are at increased risk of cardiovascular disease (CVD). This study assessed long-term changes in carotid intima-media thickness (IMT) as a surrogate marker for CVD risk in HIV-infected children and young adults.
Methods
This was a longitudinal, observational study comparing carotid IMT in HIV-infected subjects 2–21 years old to matched controls over 144 weeks.
Results
34 HIV-infected and 29 controls were included in the analysis. Among the HIV-infected group, median age was 10 years, 74% black, and 65% female. 91% were perinatally-infected with 82% on antiretroviral therapy and a median CD4 count of 681 cells/mm3. At baseline, HIV-infected had increased internal carotid artery (ICA) and common carotid artery (CCA) IMT (mm) [ICA- HIV+: 0.90, controls: 0.73 (P<0.01); CCA- HIV+: 1.00, controls: 0.90 (P=0.02)]. Relatively large changes in ICA and CCA IMT were seen from year to year in both groups. However, by week 144, there were no net changes in ICA or CCA IMT within the HIV-infected group. In the controls, CCA increased 0.1 mm and ICA increased 0.17 mm from baseline to week 144. ICA and CCA IMT were similar between groups by 144 weeks.
Conclusion
Despite variations from year to year in carotid IMT in HIV-infected children and healthy controls, likely due to arterial growth and/or luminal diameter change, little or no net change occurred in carotid IMT over the entire 144-week study period. This suggests that only small net changes occur over time in HIV-infected children despite an increased long-term risk of CVD.
Keywords: HIV, atherosclerosis, cardiovascular disease, intima-media thickness, pediatrics, adolescents
INTRODUCTION
HIV-infected individuals are at an increased risk of cardiovascular disease (CVD) [1, 2]. Non-invasive techniques are widely used to assess CVD risk in both adults and children. Carotid intima-media thickness (IMT) measured by ultrasound is one of the most well-accepted and robust methods to estimate subclinical atherosclerotic formation and progression, as it predicts cardiovascular events in the general adult population [3–5]. Although no studies to date have shown the predictive value of IMT in the HIV-infected population, several studies found increased IMT in HIV-infected individuals compared to healthy controls [6–9], and IMT progresses more rapidly over time in HIV-infected patients [7, 8, 10].
A number of cross-sectional studies also found increased carotid IMT in HIV-infected children and young adults compared to healthy controls [6, 11–14], but the changes overtime have not been reported in this population. We were the first to present longitudinal data on carotid IMT in over a 48-week period in HIV-infected children and young adults and healthy, matched controls [15]. Unexpectedly, in both the HIV-infected and control groups, IMT decreased (i.e. improved) over this time period, with more pronounced changes among the HIV-infected group for both internal carotid artery (ICA) and common carotid artery (CCA) IMT. Higher CD4+ T-cell count and longer duration of antiretroviral therapy (ART) may have contributed to these improvements seen at week 48 [15], although we could not rule out a confounding effect of better diet and/or exercise habits as the result of enrollment into this study and subsequent awareness of CVD risk.
Thus, the purpose of this current study was to investigate the longer term changes in IMT, beyond the initial 48 weeks, in HIV infected children and matched healthy controls. Specifically, our objectives were to 1) describe carotid IMT changes over a 144-week period in HIV-infected children and young adults; 2) compare these changes in HIV-infected children and young adults to an age-matched reference group of healthy controls; 3) investigate potential variables, both HIV-related and traditional CVD risk factors, that might be associated with carotid IMT changes in HIV-infected children and young adults.
METHODS
Study design/population
This was a single-site, longitudinal, 144-week observational study of HIV-infected children and young adults and healthy uninfected controls. The primary outcomes were longitudinal measurements of ICA IMT and CCA IMT. Preliminary baseline and 48-week data were reported previously [6, 15]. Data from only subjects who completed 144 weeks are presented here, with a focus on baseline to 96 weeks and from 96 to 144 weeks, given that the most meaningful changes occurred over these time periods.
As previously described [6, 15], HIV-infected children and young adults attending the pediatric HIV clinic at Rainbow Babies and Children’s Hospital/University Hospitals Case Medical Center, Cleveland, Ohio were eligible for enrollment. Inclusion criteria were HIV infection and age 2–21 years. Exclusion criteria included active opportunistic infections, renal failure, diabetes, hypertension, family history of premature CVD, and smoking. Healthy controls were chosen from a larger convenience sample including children of hospital staff, siblings of HIV-infected patients, and patients seen at the general pediatric clinic. The goal was to achieve a group with similar age (±2 years), body mass index (BMI) (±1 kg/m2), and proportions of sex and race. Matching used for selection was not retained in the analysis. Additional exclusion criteria for uninfected controls included current or recent infectious or inflammatory illness, known chronic disease, and receiving any prescription medication. Inclusion and exclusion criteria for both groups were only utilized at enrollment.
The study was reviewed and approved by the Institutional Review Board of the University Hospitals Case Medical Center. All parents or legal guardians and children, when appropriate, gave written informed consent to participate in the study.
Clinical assessments
Blood pressure measurements and standardized anthropometry were performed by the same experienced study coordinator (NS) based on procedure recommendations from the Metabolic Study Group of the AIDS Clinical Trials Group, at baseline, and yearly thereafter. An extensive chart review of HIV+ subjects included demographics, all past and current medical diagnoses, concomitant medications, and detailed ART history. Controls (or guardians) completed questionnaires in order to obtain their relevant demographic and medical information.
Laboratory tests
After ≥8 hours fasting, blood was collected from all subjects for real-time measurements of insulin, glucose, and lipoprotein profile. Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) ((fasting glucose (mg/dL) × fasting insulin (μU/mL))/405).[16] Absolute and percent CD4+ T-cell count and plasma HIV-1 RNA level were concomitantly measured as markers of HIV disease.
Carotid IMT measurements
All carotid ultrasounds were performed by an experienced sonographer and read by an experienced radiologist, both blinded to HIV status. Carotid IMT methods were used as previously described [6]. Briefly, images of the bilateral CCA and ICA were obtained in longitudinal views separately. Images of the near (proximal) and far (distal) wall free of plaques were acquired with a 7–14 MHz AT 1204 linear array transducer (Toshiba American Medical Systems, Tustin, California) operating at 14 MHz with differential harmonics. Three measurements of IMT were obtained at near and far wall of each CCA and ICA. The mean of three measurements at each site (right and left side) was used as final measurement of IMT for that site (for both CCA and ICA, the far and near wall had three IMT measurements each, resulting in a total of 12 measurements per subject). Right and left sides were then averaged, and reported as a single ICA and CCA measurement [17].
Statistical methods
Demographics, clinical characteristics and fasting metabolic parameters are described by study group, and HIV-related characteristics are described for HIV-infected children. Continuous measures are described by medians and ranges, and nominal variables are described with frequencies and percents. Nominal variables were compared using χ2 analysis or Fisher’s exact test. Continuous measures were tested for normality. For between-group comparisons (at each time-point and changes from baseline to 96 weeks and from 96 to 144 weeks), normally-distributed variables were compared using t-tests, and non-normally-distributed variables were compared using Wilcoxon rank sum tests. For within-group changes from baseline to 96 weeks and from 96 to 144 weeks, normally-distributed variables were compared with a paired t-test, and non-normally-distributed variables were compared with Wilcoxon signed rank.
As previously described [15], step-wise regression models were constructed to investigate relationships between outcome measures of carotid IMT and variables of interest within the HIV-infected group. The variables of interest in our study fall naturally into qualitative categories: (1) baseline clinical characteristics, (2) baseline laboratory values, (3) baseline HIV-related factors, (4) change in clinical characteristics during study period, (5) change in laboratory values during study period, and (6) HIV-related factors during study period. In the first stage for each IMT outcome, separate models were created for each of the six aforementioned categories for ICA and CCA separately, and for baseline to 96 weeks and for 96 to 144 weeks for a total of 24 individual models. Variables for inclusion in models were chosen based on clinical significance. Models were maximized based on R2 by interchanging those variables where choices were relevant, e.g. measures of body habitus.
Any variable from each of the first stage models which achieved a P value of ≤0.1, or lacking that, the variable with the lowest P value in the model, was included in the second stage analysis. Any variable from the second stage that remained P<0.1 was then included in the final models. The final models included 4 total: CCA IMT baseline to 96 weeks, ICA IMT baseline to 96 weeks, CCA IMT 96 to 144 weeks, and ICA IMT 96 to 144 weeks. Results are presented as regression coefficients, standard errors of the coefficients, P values, and adjusted R2. This approach was chosen over a composite model with HIV as a variable, because the interest was not simply to determine the effect of HIV on the outcome, but to examine the possibility that a different mechanism is in play in the presence of HIV. Variable selection was also complicated by a large number of candidate variables relative to the overall N, and the correlation of some variables within a given class, e.g. lipids, HIV-related variables.
All analyses were performed using SAS, version 9.2 (SAS Institute, Carey, North Carolina). While variables were screened for admission into multivariable models using P=0.10, the level of significance for final analyses was set at 0.05.
RESULTS
Study population
Baseline characteristics
All 39 HIV-infected patients seen at our clinic were eligible and offered participation in the study; all agreed to participate. Thirty-nine healthy controls were identified and enrolled. By 144 weeks, 5 HIV+ subjects and 10 controls were lost to follow-up or withdrew from the study for transportation or schedule issues. Only those subjects who had data for baseline, 48, 96, and 144 weeks were included in the analysis (HIV+, N = 34; controls, N = 29). The 10 controls who were not included in the analysis matched the 29 subjects who were included in age, sex, race, and BMI.
Baseline demographics, clinical, and laboratory characteristics of the two groups are shown in Table 1. Groups were similar with regard to age, sex, race, BMI, waist circumference (WC), blood pressure, high-density lipoprotein (HDL) cholesterol, and HOMA-IR. HIV-infected group had higher total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides (TG).
Table 1.
Demographics, laboratory, and clinical characteristics of the study population.
| Median (IQR) unless specified otherwise | Baseline | Week 96 | Week 144 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HIV+ (N=34) | Controls (N=29) | HIV+ (N=34) | Controls (N=29) | HIV+ (N=34) | Controls (N=29) | |
|
| ||||||
| Age (years) | 10 (8, 16) | 9 (6,14) | -- | -- | -- | -- |
| Black race, no. % | 25 (74%) | 24 (83%) | -- | -- | -- | -- |
| Female sex, no. % | 22 (65%) | 17 (59%) | -- | -- | -- | -- |
| Body mass index (kg/m2) | 19 (16, 23) | 18 (16, 21) | 20 (17, 23)+ | 20 (17, 24)+ | 22 (17, 24)+ | 20 (17, 23) |
| Waist circumference (in.) | 26 (23, 29) | 24 (20, 28) | 29 (26, 31)+ | 27(23, 31)+ | 30 (27, 33)+ | 28 (23, 31)+ |
| Systolic BP (mm Hg) | 101 (96, 110) | 102 (96, 110) | 100 (98, 112) | 100 (98, 110) | 110 (97, 112)+ | 104 (97, 110) |
| Diastolic BP (mm Hg) | 68 (60, 74) | 70 (60, 70) | 68 (62, 76) | 70 (62, 70) | 70 (64, 72) | 70 (64, 72) |
| Total cholesterol (mg/dL) | 172 (131, 196)* | 148 (131, 165) | 169 (137, 186) | 154 (137, 161) | 160 (135, 183) | 149 (135, 160) |
| HDL cholesterol (mg/dL) | 42 (43, 55) | 50 (43, 62) | 50 (46, 60)*,+ | 53(46, 65) | 51 (48, 58) | 54 (48, 59) |
| LDL cholesterol (mg/dL) | 105 (71, 126)* | 85 (71, 101) | 101 (72, 116) | 88 (72, 96) | 95(71, 113) | 83 (71, 92) |
| Triglycerides (mg/dL) | 85 (36, 116)* | 42 (36, 52) | 84 (34, 109) | 42 (34, 64) | 73 (40, 113) | 46 (40, 70) |
| HOMA-IR | 1.4 (0.6, 2.6) | 1.4 (0.6, 1.7) | 1.8 (0.6, 2.7) | 1.2 (0.6, 2.0) | 2.4 (0.6, 3.3) | 1.4 (0.6, 3.2) |
| Perinatally-infected, no. (%) | 31 (91%) | -- | -- | -- | -- | -- |
| HIV duration (mo.)a | 115 (49, 146) | -- | -- | -- | -- | -- |
| CD4 count (cells/mm3) | 681 (403, 1308) | -- | 781 (528, 954) | -- | 826 (506, 1088) | -- |
| CD4 count % | 32 (29, 38) | -- | 34 (29, 39) | -- | 36 (29, 39) | -- |
| Nadir CD4 count (cells/mm3)b | 279 (131, 448) | -- | 287 (100, 448) | -- | 292 (140, 469) | -- |
| HIV-1 RNA <400 cps/mL, no. (%) | 27 (79%) | -- | 26 (79%) | -- | 23 (72%) | -- |
| Current ART use, no. % | 28 (82%) | -- | 30 (88%) | -- | 30 (88%) | -- |
| Current PI use | 16 (47%) | -- | 17 (50%) | -- | 17 (50%) | -- |
| Current tNRTI use | 17 (50%) | -- | 16 (47%) | -- | 16 (47%) | -- |
| ART duration (mo.) | 72 (23, 105) | -- | -- | -- | -- | -- |
| PI duration (mo.) | 24 (6, 42) | -- | -- | -- | -- | -- |
| tNRTI duration (mo.) | 51 (5, 82) | -- | -- | -- | -- | -- |
N.B.
Significant within-group;
Significant between-groups;
HIV duration was calculated as chronological age for those with vertical transmission, and time from diagnosis for those with behavioral transmission.
CD4 nadir was recorded as the lowest CD4 count at any point prior to and including entry.
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; ART, antiretroviral therapy; PI, protease inhibitor, tNRTI, thymidine-analog nucleoside-reverse transcriptase inhibitors
All but 3 HIV-infected subjects were perinatally-infected. Four subjects were ART-naïve at baseline, but the rest of the subjects were on ART and 79% of all subjects had undetectable HIV-1 RNA levels at baseline. Almost half of all subjects were on a protease-inhibitor (PI)-based regimen, and half were taking thymidine-analog nucleoside-reverse transcriptase inhibitors (tNRTI).
Within-group and between-group changes over study period
From baseline to week 96, BMI and WC increased significantly within both HIV-infected subjects and controls without significant between-group changes (Table 1). High-density lipoprotein cholesterol (HDL-C) increased significantly within the HIV-infected group and compared to the controls.
From week 96 to week 144, BMI, WC, and systolic blood pressure increased significantly within the HIV-infected group without significant differences between HIV+ and control group. Among the controls, only WC increased significantly within the group.
Of the four ART-naïve subjects, all but one started ART during the study period (at enrollment, 2 weeks, 1 month, respectively). Absolute CD4+ T-cell count, CD4 %, and the number of subjects with HIV-1 RNA <400 copies/mL did not change significantly from baseline to 96 weeks or from 96 weeks to 144 weeks.
Carotid IMT
Baseline and Changes over study period
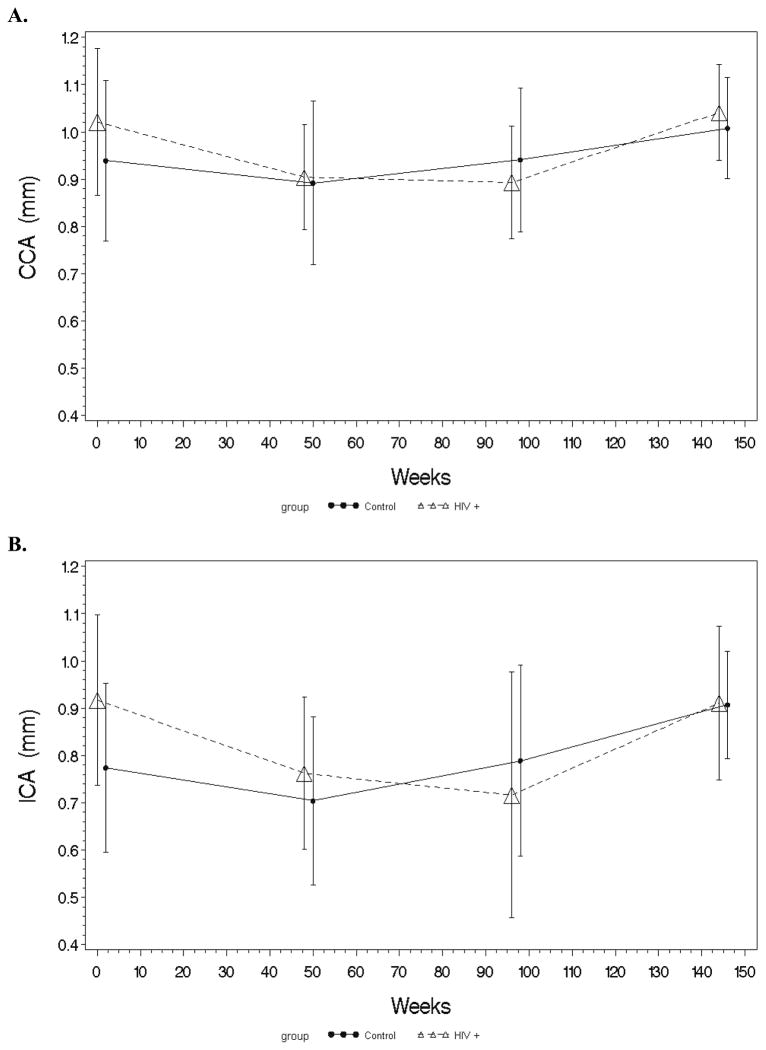
Baseline IMT and changes over the 144-week study period are depicted in Figure 1. At baseline, both CCA and ICA IMT were increased in the HIV+ group compared to controls.
Figure 1. Carotid IMT trends over 144 weeks.
These graphs show the yearly trends for CCA (A) and ICA (B) IMT for HIV-infected subjects and healthy controls over 144 weeks. CCA, common carotid artery; ICA, internal carotid artery; IMT, intima-media thickness.
From baseline to week 96, IMT continued to decrease within the HIV+ group over this entire time period, while IMT decreased initially in the controls from baseline to week 48, but then increased above baseline by week 96. The decrease from baseline to week 96 in the HIV+ group was significant, and was significantly different from the controls. Both ICA and CCA IMT changes followed similar patterns.
From week 96 to week 144, CCA and ICA IMT increased significantly within both HIV+ and control groups. Only the increase in CCA IMT was significant between groups, where the HIV+ group’s IMT increase was greater than the controls.
Regression Analyses
Final regression models for the HIV-infected group for both ICA and CCA from baseline to week 96 and from week 96 to week 144 are shown in Table 2.
Table 2.
Final Regression Models for Changes in Carotid Intima-Media Thickness
| A. Common Carotid Artery
| ||||||
|---|---|---|---|---|---|---|
| Baseline to Week 96 | Week 96 to Week 144 | |||||
|
| ||||||
| Beta | SE (Beta) | P | Beta | SE (Beta) | P | |
| Sex | 0.124 | 0.060 | 0.05 | −0.098 | 0.031 | <0.01 |
| Race | 0.100 | 0.064 | 0.13 | −0.064 | 0.033 | 0.06 |
| WC | 0.003 | 0.003 | 0.35 | |||
| LDL-C | <−0.001 | <0.001 | 0.39 | 0.001 | <0.001 | 0.05 |
| HDL-C TG |
0.001 | <0.001 | 0.16 | |||
| HOMA-IR | −0.032 | 0.015 | 0.04 | 0.011 | 0.009 | 0.23 |
| Change in BMI | 0.028 | 0.016 | 0.10 | |||
| Change in TG | <0.001 | <0.001 | 0.99 | |||
| PI duration at endpoint | −0.002 | <0.001 | 0.03 | |||
|
|
||||||
| R2 = 0.61 | R2 = 0.49 | |||||
| B. Internal Carotid Artery
| ||||||
|---|---|---|---|---|---|---|
| Baseline to Week 96 | Week 96 to Week 144 | |||||
|
| ||||||
| Beta | SE (Beta) | P | Beta | SE (Beta) | P | |
|
| ||||||
| Age | 0.020 | 0.011 | 0.08 | |||
| Race | 0.089 | 0.125 | 0.48 | |||
| WC | 0.001 | 0.010 | 0.91 | |||
| HDL-C | 0.001 | 0.003 | 0.63 | |||
| Change in TG | <−0.001 | <0.001 | 0.46 | |||
| HIV duration at baseline | 0.001 | 0.001 | 0.24 | |||
| ART duration at endpoint | −0.003 | 0.001 | 0.06 | <0.001 | 0.001 | 0.41 |
| PI duration at endpoint | −0.002 | 0.003 | 0.54 | |||
|
|
||||||
| R2 = 0.44 | R2 = 0.27 | |||||
LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; HOMA-IR, homeostasis model assessment of insulin resistance; BMI, body mass index; PI, protease inhibitor; WC, waist circumference; ART, antiretroviral therapy
For changes in CCA IMT from baseline to week 96, only baseline HOMA-IR and PI duration were significant in this model, where the lower the baseline HOMA-IR and the shorter the duration of PI use at 96 weeks were both associated with more negative change (i.e. improvement) in CCA IMT. For changes in CCA IMT from week 96 to week 144, only sex was significant, where male sex was associated with less of an increase in CCA IMT. For changes in ICA IMT from baseline to week 96 and from week 96 to week 144, no variables remained significant in the final models.
DISCUSSION
This study evaluated, for the first time in HIV, changes in subclinical vascular disease, assessed by carotid IMT, in HIV-infected subjects compared to healthy controls. After an initial decrease in IMT in both groups over the first 48 weeks, IMT in the HIV-infected group continued to decrease until 96 weeks and then increased from 96 weeks until week 144, ending at approximately the same thickness as baseline. The healthy controls’ IMT continued to increase from 48 weeks until the end of the study at 144 weeks. Both CCA and ICA IMT followed similar patterns throughout the study period for both groups. By week 144, both groups had similar values compared to one another for both CCA and ICA IMT.
While there have been a number of cross-sectional studies evaluating carotid IMT in HIV-infected children and young adults [6, 11, 12, 18], our cohort study is the first to evaluate longitudinal changes in either HIV-infected or healthy children. Thus, it is generally unknown what the natural course of carotid IMT is in this population. While some cross-sectional studies have suggested that carotid IMT increases with age even during childhood [19, 20], not all studies support this [21, 22]. As Fernhall et al [23] pointed out in a thorough review of the literature, discrepancies among various studies may be due to the fact that IMT changes very little during childhood, and as it changes, so does arterial size and luminal diameter [20, 22]. These complications likely make measuring carotid IMT longitudinally in children much more difficult than in adults. Thus, this may account for the relatively large changes seen per each year in both groups in our study, despite using a single sonographer and radiologist (both blinded to HIV status), and following a specific protocol on the same machine over the entire course of the study. The overall change from baseline to week 144, however, was comparable in the controls to the average yearly change of 0.015 mm/year in healthy adults [24].
In the HIV-infected group, there was no overall net change in either ICA or CCA IMT after 144 weeks of follow-up. This may be because HIV infection, directly or indirectly through heightened inflammation and immune activation, and/or antiretroviral therapy regimens could obscure the normal IMT pattern. In a large longitudinal IMT study of HIV-infected adults, the mean CCA and ICA progression was 0.016 and 0.020 mm per year, respectively [25]. Thus, it is at least reassuring that in HIV-infected children and young adults, there was no net increase over 144 weeks, despite the observed accelerated progression in HIV-infected adults compared to the general population. Furthermore, changes seen from year to year in HIV-infected children are likely insignificant, and only extended longitudinal trials spanning over a decade or more would be clinically meaningful.
In our study, we did investigate variables that may be associated with changes in IMT within the HIV-infected group over study increments that appeared to be the most relevant. From baseline to 96 weeks, lower baseline HOMA-IR and shorter duration of PI use were both associated with more negative (i.e. improvement) changes in CCA IMT; whereas, from week 96 to 144, male sex was associated with less positive change in CCA IMT (i.e. not as bad). While traditional risk factors and PI use have all been associated with increased CVD risk in both adult and pediatric HIV-infected populations [6, 25, 26], these three traditional risk factor variables appeared to be protective against worsening carotid IMT in our study. This, again, may be a reflection of the difficulty in assessing changes in IMT in children over short time periods, or, more likely, this may be an issue of the number of potentially important variables that we examined against a relatively small sample size. This is also likely the reason that no variable remained significant in the final model evaluating changes in ICA IMT.
Despite the aforementioned limitations, longitudinal studies, such as this one, evaluating carotid IMT in HIV-infected children are of paramount importance as the number of long-term survivors of perinatally-infected children and behaviorally-infected adolescents is growing at a significant rate due to combination ART. Furthermore, according to the Centers for Disease Control and Prevention (CDC), there were almost 43,000 new diagnoses of HIV infection in the United States in 2009, with 20% between the ages of 0 and 24 years [27]. Minimizing the risk of complications like CVD during the early HIV infection stage in these children and adolescents would have a considerable impact on not only the length of survival, but on long-term quality of life.
Accordingly, despite the fact the observed changes in IMT during our study period were overall small, we know that atherosclerosis begins during childhood. Postmortem studies have identified fatty streaks in the intima of large arteries in children and adolescents and have correlated with traditional risk factors [28, 29]. Given the additive risk associated with HIV infection, we must continue to longitudinally follow surrogate markers of subclinical atherosclerosis in HIV-infected children and young adults, as well as evaluate interventions which may have a positive impact on their overall CVD risk. This current novel study offers insight into the pathogenesis of CVD in this population, and forms a substrate from which to design additional longitudinal studies evaluating subclinical atherosclerosis in HIV-infected children and young adults.
Acknowledgments
Sources of Support: The study was funded by an independent research grant from GlaxoSmithKline Collaborative Study Group, as well as supported by research grants to Dr. McComsey (R01HD070490) and Dr. Eckard (K23HD069199) from the National Institutes of Health, National Institute of Child Health and Development. The funding agencies had absolutely no role in study design, data collection or analysis.
Footnotes
Disclosure Statement: GAM serves as a consultant and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Merck, and Abbott. GAM currently chairs a DSMB for a Pfizer-funded study. ARE has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline and has served as an advisor to Gilead.. All other authors: no conflicts.
References
- 1.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clinical Infectious Diseases. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. Journal of Clinical Endocrinology & Metabolism. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bots ML, Hoes AW, Hofman A, Witteman JC, Grobbee DE. Cross-sectionally assessed carotid intima-media thickness relates to long-term risk of stroke, coronary heart disease and death as estimated by available risk functions. J Intern Med. 1999;245:269–276. doi: 10.1046/j.1365-2796.1999.0442f.x. [DOI] [PubMed] [Google Scholar]
- 4.Espeland MA, O’Leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3. doi: 10.1186/1468-6708-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogata T, Yasaka M, Yamagishi M, Seguchi O, Nagatsuka K, Minematsu K. Atherosclerosis found on carotid ultrasonography is associated with atherosclerosis on coronary intravascular ultrasonography. J Ultrasound Med. 2005;24:469–474. doi: 10.7863/jum.2005.24.4.469. [DOI] [PubMed] [Google Scholar]
- 6.McComsey GA, O’Riordan M, Hazen SL, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007;21:921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 7.Mercie P, Thiebaut R, Aurillac-Lavignolle V, et al. Carotid intima-media thickness is slightly increased over time in HIV-1-infected patients. HIV Med. 2005;6:380–387. doi: 10.1111/j.1468-1293.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz MW, Stephan C, Harmjanz A, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Thiebaut R, Aurillac-Lavignolle V, Bonnet F, et al. Change in atherosclerosis progression in HIV-infected patients: ANRS Aquitaine Cohort, 1999–2004. AIDS. 2005;19:729–731. doi: 10.1097/01.aids.0000166097.46940.35. [DOI] [PubMed] [Google Scholar]
- 11.de Giuliano IC, de Freitas SF, de Souza M, Caramelli B. Subclinic atherosclerosis and cardiovascular risk factors in HIV-infected children: PERI study. Coronary Artery Disease. 2008;19:167–172. doi: 10.1097/MCA.0b013e3282f6dffb. [DOI] [PubMed] [Google Scholar]
- 12.Charakida M, Donald AE, Green H, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112:103–109. doi: 10.1161/CIRCULATIONAHA.104.517144. [DOI] [PubMed] [Google Scholar]
- 13.de Giuliano IC, de Freitas SF, de Souza M, Caramelli B. Subclinic atherosclerosis and cardiovascular risk factors in HIV-infected children: PERI study. Coron Artery Dis. 2008;19:167–172. doi: 10.1097/MCA.0b013e3282f6dffb. [DOI] [PubMed] [Google Scholar]
- 14.Vigano A, Bedogni G, Cerini C, et al. Both HIV-infection and long-term antiretroviral therapy are associated with increased common carotid intima-media thickness in HIV-infected adolescents and young adults. Curr HIV Res. 2010;8:411–417. doi: 10.2174/157016210791330419. [DOI] [PubMed] [Google Scholar]
- 15.Ross AC, Storer N, O’Riordan MA, Dogra V, McComsey GA. Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J. 2010;29:634–638. doi: 10.1097/inf.0b013e3181d770c4. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18:1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hansen F, Mangell P, Sonesson B, Lanne T. Diameter and compliance in the human common carotid artery--variations with age and sex. Ultrasound Med Biol. 1995;21:1–9. doi: 10.1016/0301-5629(94)00090-5. [DOI] [PubMed] [Google Scholar]
- 20.Jourdan C, Wuhl E, Litwin M, et al. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005;23:1707–1715. doi: 10.1097/01.hjh.0000178834.26353.d5. [DOI] [PubMed] [Google Scholar]
- 21.Lenard Z, Studinger P, Mersich B, Kocsis L, Kollai M. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation. 2004;110:2307–2312. doi: 10.1161/01.CIR.0000145157.07881.A3. [DOI] [PubMed] [Google Scholar]
- 22.Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age, sex, anthropometry and blood pressure. J Hypertens. 1998;16:1593–1602. doi: 10.1097/00004872-199816110-00005. [DOI] [PubMed] [Google Scholar]
- 23.Fernhall B, Agiovlasitis S. Arterial function in youth: window into cardiovascular risk. J Appl Physiol. 2008;105:325–333. doi: 10.1152/japplphysiol.00001.2008. [DOI] [PubMed] [Google Scholar]
- 24.Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34:2985–2994. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 25.Mangili A, Polak JF, Skinner SC, et al. HIV infection and progression of carotid and coronary atherosclerosis: the CARE study. J Acquir Immune Defic Syndr. 2011;58:148–153. doi: 10.1097/QAI.0b013e31822d4993. [DOI] [PubMed] [Google Scholar]
- 26.Sankatsing RR, Wit FW, Vogel M, et al. Increased carotid intima-media thickness in HIV patients treated with protease inhibitors as compared to non-nucleoside reverse transcriptase inhibitors. Atherosclerosis. 2009;202:589–595. doi: 10.1016/j.atherosclerosis.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. HIV/AIDS Statistics and Surveillance. Edited by Editor|. Year|; p.^pp. Pages|. City|: Publisher|. [Google Scholar]
- 28.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 29.Newman WP, 3rd, Wattigney W, Berenson GS. Autopsy studies in United States children and adolescents. Relationship of risk factors to atherosclerotic lesions. Ann N Y Acad Sci. 1991;623:16–25. doi: 10.1111/j.1749-6632.1991.tb43715.x. [DOI] [PubMed] [Google Scholar]