Abstract
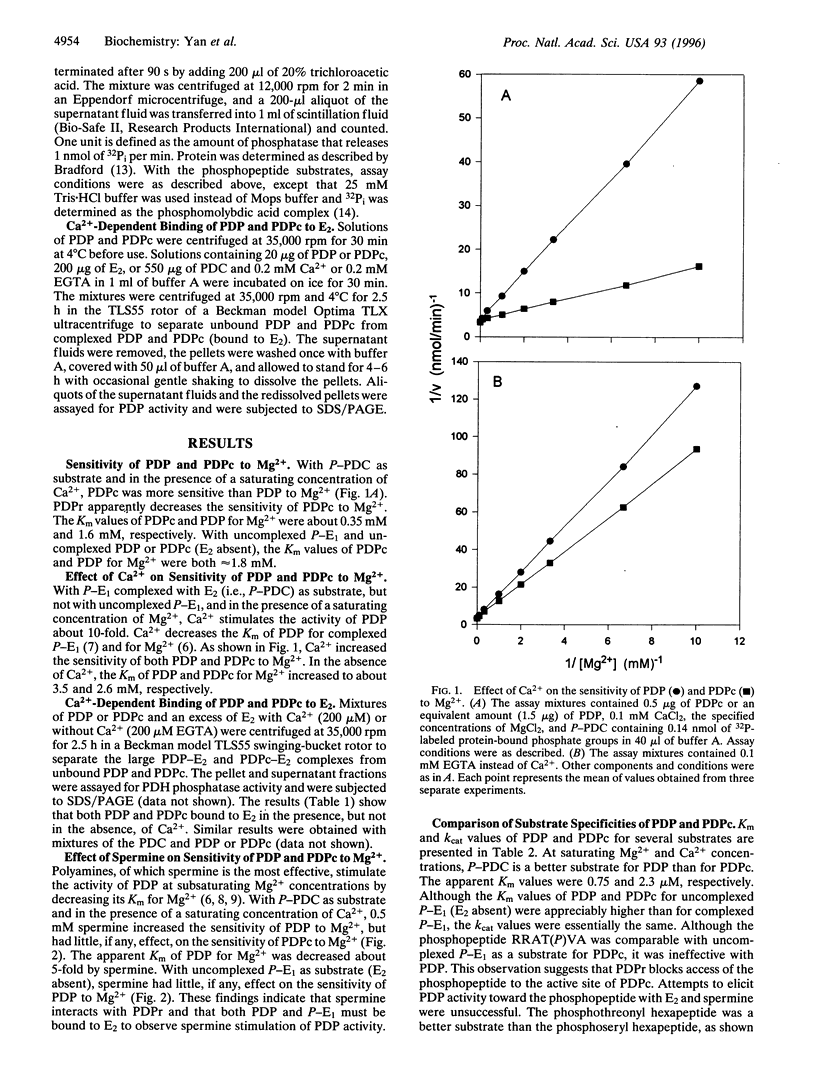
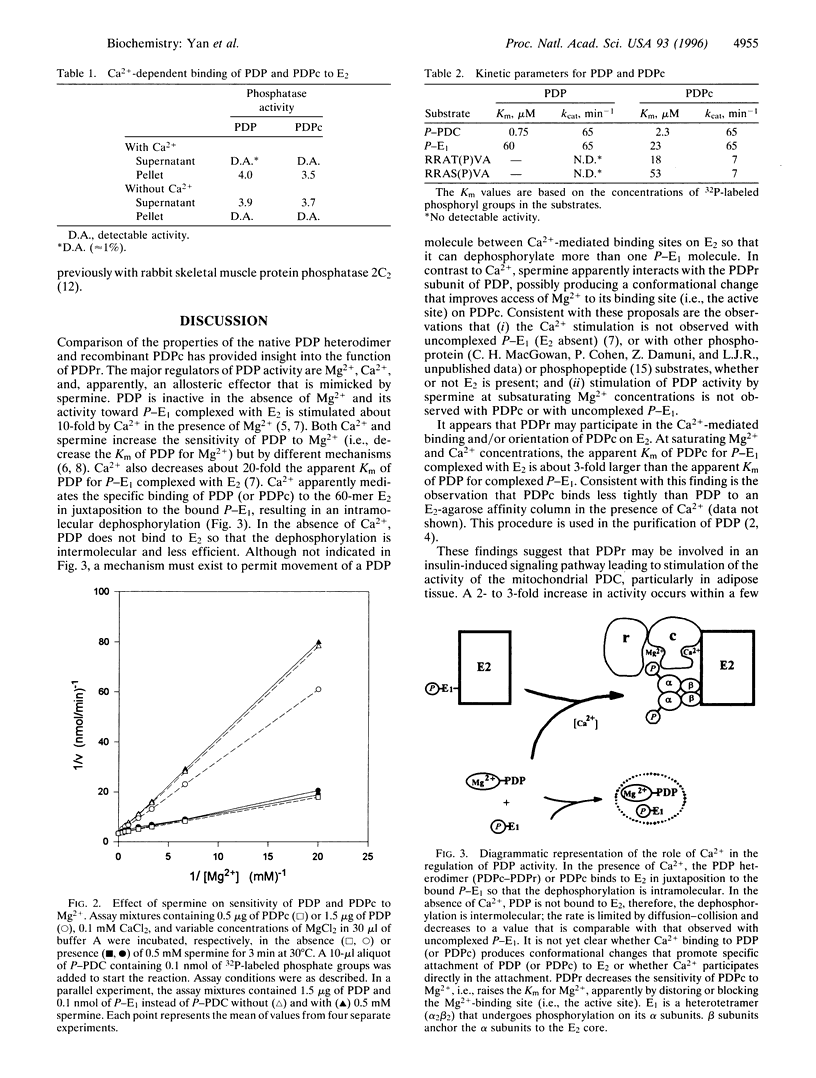
Bovine pyruvate dehydrogenase phosphatase (PDP) is a Mg2+-dependent and Ca2+-stimulated heterodimer that is a member of the protein phosphatase 2C family and is localized to mitochondria. Insight into the function of the regulatory subunit of PDP (PDPr) has been gained. It decreases the sensitivity of the catalytic subunit of PDP (PDPc) to Mg2+. The apparent Km of PDPc for Mg2+ is increased about 5-fold, from about 0.35 mM to 1.6 mM. The polyamine spermine increases the sensitivity of PDP but not PDPc to Mg2+, apparently by interacting with PDPr. PDPc but not PDP can use the phosphopeptide RRAT(P)VA as a substrate. These observations are interpreted to indicate that PDPr blocks or distorts the active site of PDPc and that spermine produces a conformational change in PDPr that reverses its inhibitory effect. These findings suggest that PDPr may be involved in the insulin-induced activation of the mitochondrial PDP in adipose tissue, which is characterized by a decrease in its apparent Km for Mg2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corkey B. E., Duszynski J., Rich T. L., Matschinsky B., Williamson J. R. Regulation of free and bound magnesium in rat hepatocytes and isolated mitochondria. J Biol Chem. 1986 Feb 25;261(6):2567–2574. [PubMed] [Google Scholar]
- Damuni Z., Humphreys J. S., Reed L. J. Stimulation of pyruvate dehydrogenase phosphatase activity by polyamines. Biochem Biophys Res Commun. 1984 Oct 15;124(1):95–99. doi: 10.1016/0006-291x(84)90921-5. [DOI] [PubMed] [Google Scholar]
- Davis P. F., Pettit F. H., Reed L. J. Peptides derived from pyruvate dehydrogenase as substrates for pyruvate dehydrogenase kinase and phosphatase. Biochem Biophys Res Commun. 1977 Apr 11;75(3):541–549. doi: 10.1016/0006-291x(77)91506-6. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Midgley P. J., Rutter G. A., Thomas A. P., McCormack J. G. Studies into the mechanism whereby insulin activates pyruvate dehydrogenase complex in adipose tissue. Ann N Y Acad Sci. 1989;573:285–296. doi: 10.1111/j.1749-6632.1989.tb15005.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donella Deana A., Mac Gowan C. H., Cohen P., Marchiori F., Meyer H. E., Pinna L. A. An investigation of the substrate specificity of protein phosphatase 2C using synthetic peptide substrates; comparison with protein phosphatase 2A. Biochim Biophys Acta. 1990 Feb 19;1051(2):199–202. doi: 10.1016/0167-4889(90)90194-i. [DOI] [PubMed] [Google Scholar]
- Larner J., Huang L. C., Suzuki S., Tang G., Zhang C., Schwartz C. F., Romero G., Luttrell L., Kennington A. S. Insulin mediators and the control of pyruvate dehydrogenase complex. Ann N Y Acad Sci. 1989;573:297–305. doi: 10.1111/j.1749-6632.1989.tb15006.x. [DOI] [PubMed] [Google Scholar]
- Lawson J. E., Niu X. D., Browning K. S., Trong H. L., Yan J., Reed L. J. Molecular cloning and expression of the catalytic subunit of bovine pyruvate dehydrogenase phosphatase and sequence similarity with protein phosphatase 2C. Biochemistry. 1993 Sep 7;32(35):8987–8993. doi: 10.1021/bi00086a002. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pelley J. W., Pettit F. H., Hucho F., Randall D. D., Reed L. J. -Keto acid dehydrogenase complexes. XV. Purification and properties of the component enzymes of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):327–342. doi: 10.1016/0003-9861(72)90151-8. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moule S. K., Edgell N. J., Welsh G. I., Diggle T. A., Foulstone E. J., Heesom K. J., Proud C. G., Denton R. M. Multiple signalling pathways involved in the stimulation of fatty acid and glycogen synthesis by insulin in rat epididymal fat cells. Biochem J. 1995 Oct 15;311(Pt 2):595–601. doi: 10.1042/bj3110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Reed L. J. Pyruvate dehydrogenase complex from bovine kidney and heart. Methods Enzymol. 1982;89(Pt 500):376–386. doi: 10.1016/s0076-6879(82)89067-8. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Pratt M. L., Maher J. F., Roche T. E. Purification of bovine kidney and heart pyruvate dehydrogenase phosphatase on Sepharose derivatized with the pyruvate dehydrogenase complex. Eur J Biochem. 1982 Jul;125(2):349–355. doi: 10.1111/j.1432-1033.1982.tb06690.x. [DOI] [PubMed] [Google Scholar]
- Rahmatullah M., Roche T. E. Component requirements for NADH inhibition and spermine stimulation of pyruvate dehydrogenaseb phosphatase activity. J Biol Chem. 1988 Jun 15;263(17):8106–8110. [PubMed] [Google Scholar]
- Saltiel A. R. Insulin generates an enzyme modulator from hepatic plasma membranes: regulation of adenosine 3',5'-monophosphate phosphodiesterase, pyruvate dehydrogenase, and adenylate cyclase. Endocrinology. 1987 Mar;120(3):967–972. doi: 10.1210/endo-120-3-967. [DOI] [PubMed] [Google Scholar]
- Teague W. M., Pettit F. H., Wu T. L., Silberman S. R., Reed L. J. Purification and properties of pyruvate dehydrogenase phosphatase from bovine heart and kidney. Biochemistry. 1982 Oct 26;21(22):5585–5592. doi: 10.1021/bi00265a031. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Diggle T. A., Denton R. M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J. 1986 Aug 15;238(1):83–91. doi: 10.1042/bj2380083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollny E., Watkins K., Kramer G., Hardesty B. Purification to homogeneity and partial characterization of a 56,000-dalton protein phosphatase from rabbit reticulocytes. J Biol Chem. 1984 Feb 25;259(4):2484–2492. [PubMed] [Google Scholar]


