Abstract
Background
Mibampator, an AMPA receptor potentiator, was evaluated for treatment of agitation and aggression (A/A) in Alzheimer’s disease (AD).
Methods
Outpatients (n=132) with probable AD and A/A randomized to 12 weeks of double-blind treatment with 3 mg po mibampator or placebo were assessed using the 4-domain NPI-4-A/A derived from the Neuropsychiatric Inventory. Secondary measures included the Cohen-Mansfield Agitation Inventory, Cornell Scale for Depression in Dementia, Frontal Systems Behavior inventory (FrSBe), and ADAS-Cog. Efficacy was analyzed using mixed-effects model repeated measures from baseline to endpoint. Adverse events (AEs), labs, vital signs and ECGs were monitored.
Results
Baseline characteristics were comparable between groups. Both groups improved on the NPI-4-A/A, but without group differences. Among secondaries, mibampator was significantly better (p=.007) than placebo only on the FrSBe. AEs were similar between groups. One death occurred in the placebo group.
Conclusion
Possible explanations for no significant group differences include caregiver, drug target engagement, and design issues.
Keywords: AMPA, Alzheimer’s disease, mibampator, agitation, aggression, neuropsychiatric symptoms, glutamate, Neuropsychiatric Inventory
INTRODUCTION
Agitation and aggression (A/A) are frequently observed in patients with Alzheimer’s disease (AD) (Sulzer et al., 2008; Ballard et al., 2009) and encompass a range of verbal and motor disturbances such as restlessness, cursing, aggression, hyperactivity, combativeness, wandering, repetitive calling out, irritability, and disinhibition (Cohen-Mansfield et al., 1995). Agitation and aggression are common behavioral manifestations of AD neuropathology and can co-occur associated with delusions, hallucinations, depression, anxiety, and frontal-lobe symptoms (Hirono et al., 2000; Tekin S, 2001). Aggression and agitation have been linked to the neurodegeneration of frontolimbic regions in AD (Hirono et al., 2000; Tekin S, 2001). Outpatients with AD and high neuropsychiatric symptom (NPS) severity incur significantly higher costs of unpaid care, long-term care, medication, and physician visits (Murman et al., 2002) and their monthly total care costs are more than double the costs for those without NPS (Herrmann et al., 2004). Symptoms of A/A carry a particular burden on caregivers, resulting in reduced quality of life (Shin et al., 2005), and increased likelihood of institutionalization (Gilley et al., 2004).
Indicated pharmacotherapy (cholinesterase inhibitors or memantine) targeting the cognitive and functional symptoms of AD have limited effectiveness for NPS (Trinh et al., 2003), and currently there are no Food and Drug Administration (FDA)-approved drugs specifically for the treatment of NPS in AD. Pharmacologic therapy for the more severe behavioral symptoms of dementia has traditionally included conventional and atypical antipsychotics (Doody et al., 2001). However, antipsychotics are not consistently more effective than placebo nor do they reduce health care costs (Rosenheck et al., 2007). Moreover, the FDA has issued a black-box warning for use of antipsychotics in elderly with dementia due to an increased risk of death, resulting in an unfavorable risk-to-benefit assessment (Jeste et al., 2008). Practitioners have limited options to treat NPS including A/A aside from antipsychotics because antidepressants and anticonvulsants have not demonstrated efficacy and benzodiazepines are associated with falls and impaired cognition (Herrmann and Lanctôt, 2007). There is a need for novel pharmaceutical approaches.
Alzheimer’s disease has widespread effects on neurotransmitter systems, most prominently producing a deficit in the basal forebrain cholinergic projecting system but also affecting noradrenergic, dopaminergic and serotonergic systems (Reinikainen et al., 1990). Dysregulation of norepinephrine, dopamine, serotonin and gamma-aminobutyric acid (GABA) brain systems have been implicated in behavioral changes in AD (Herrmann et al., 2004; Lopez et al., 1996). Dopamine and serotonin involvement have been suggested by relationships to A/A with or without psychosis in postmortem and genomic studies (Sweet et al., 2001; Holmes et al., 2001; Sukonick et al., 2001).
Abnormalities of glutamatergic homeostasis also occur in AD (Madsen et al., 1994; Blanchard et al., 2004). The amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are one of several subtypes of ionotropic glutamate receptors (Gates et al. 2001) and are responsible for the majority of excitatory neurotransmission in the central nervous system (CNS) (Yamada, 2000; Chappell et al., 2007). AMPA receptor potentiators augment glutamatergic synaptic responses that control fast synaptic transmission by binding to allosteric sites on neurons and slowing the desensitization process, thereby enhancing AMPA receptor signaling (Vandergriff et al., 2001). Activation of AMPA receptors strengthens synapses and changes in glutamatergic synaptic transmission contribute to neural plasticity in the CNS (Yamada, 2000). Activation of AMPA receptors is capable of increasing expression of brain-derived neurotrophic factor (BDNF) in vitro (Wu et al., 2004) and in vivo (Mackowiak et al., 2002). AMPA receptor potentiators can promote long-term changes in glutamatergic synaptic signaling and modulate trophic pathways (Lynch, 2004). Excess levels of beta-amyloid oligomers in brains of patients with AD alter neurotransmission of glutamate through effects on AMPA receptors (Zhao et al., 2008). AMPA receptor impairment has been reported to play a role in disrupting long-term potentiation in the hippocampus by compromising glutamatergic-dependent synaptic circuitry (Yamada, 2000). Destruction or alteration of AMPA receptor subunits correlates with severity of neurotoxicity and severity of AD (Walton and Dodd, 2007). Mibampator (LY451395) is a biarylpropylsulfonamide AMPA receptor potentiator (O’Neill, 2004) previously assessed in a Phase 2 trial for its effects on cognition using the ADAS-Cog in 181 patients with mild-to-moderate AD dementia (Chappell et al., 2007). No efficacy for cognition was found, but a significant improvement on the NPS secondary measure was evident. The current study was designed to further assess the efficacy and safety of mibampator in patients with AD and with clinically significant A/A symptoms, as measured by the 4-domain A/A subscale of the Neuropsychiatric Inventory (NPI-4-A/A).
METHODS
Participants and Design
The study was carried out in accordance with the Declaration of Helsinki and its amendments at sites in the United States. Written informed consent was obtained from all patients and/or their caregivers or legal representatives prior to the performance of any procedures. This was a multicenter, randomized, double-blind, placebo-controlled, Phase 2 trial in patients who met DSM-IV-TR and National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s disease and Related Disorders Association (NINCDS/ADRDA) criteria for probable AD. Entered patients had clinically significant, persistent A/A that was disruptive to daily living or put themselves or others in harm’s way for at least 3 days per week for at least 4 weeks prior to study entry. Patients were male or non-fertile female community-dwelling ≥60 years old with a reliable caregiver who was in frequent or daily contact with the patient. Inclusion criteria were: Mini Mental State Examination (MMSE) (Folstein et al., 1975) score from 6 to 26, NPI-10 total score ≥10, and NPI-4-A/A ≥4 on one domain at Visit (V) 1 (screening) and V2 (randomization). A CT or MRI brain scan within 2 years of study entry could not be inconsistent with AD and Modified Hachinski Ischemia Scale scores had to be ≤4. In addition, patients could not meet DSM-IV-TR criteria for delirium and/or have a Delirium Rating Scale-Revised-98 (DRS-R98) (Trzepacz et al., 2001) score ≥18.
Prior to randomization, patients were discontinued from concomitant psychotropic medications and could not have unstable medical problems or other major neurological or psychiatric disorders. Stable doses of four antidepressant medications (sertraline, citalopram, escitalopram, fluoxetine) and acetylcholinesterase inhibitors (AChEIs) and memantine used for AD were allowed because it was determined they had low potential to interfere with efficacy or safety. Use of strong CYP450 3A/4 inhibitors was disallowed.
There were three study periods (SP): 3- to 28-day screening (SP I), 12-week, double-blind treatment (SP II), and 1-week single-blind washout (SP III). Of the 24 sites that screened patients, 23 randomized patients in a 1:1 manner to receive BID doses of mibampator or placebo. The protocol specified a total enrollment of 150 patients, but enrollment was slower than expected and was stopped early with 132 randomized patients.
Procedures
The interactive voice response system (IVRS) was used to assign blisterpacks containing double-blind study drug to each patient. Site personnel confirmed that they had located the correct blisterpacks by entering a confirmation number found on the blisterpacks into the IVRS. Patients were seen for face-to-face assessments at 3-week intervals during SPII. The primary outcome measure, the NPI-4-A/A subscale, was administered during those visits as were other A/A measures. Certain secondary measures were assessed at 6-week intervals (Figures 3 and 4). Telephone visits during the intervening weeks monitored patient safety and assessed Caregiver Global Impressions of the patient’s A/A.
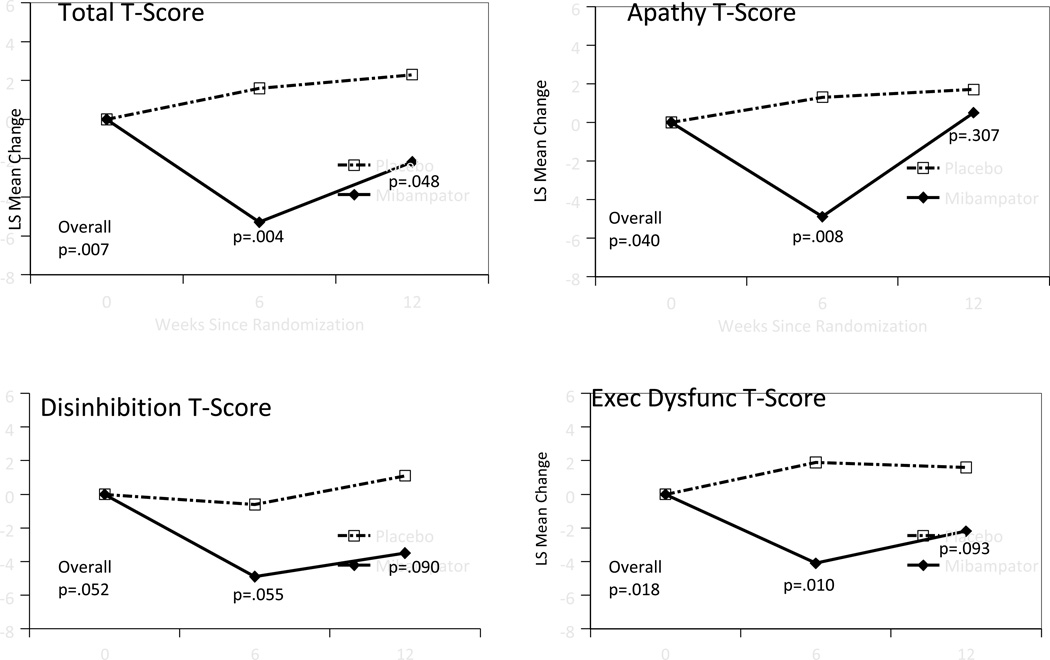
Figure 3.
FrSBe total and subscale least square means change from baseline after treatment (MMRM analysis)
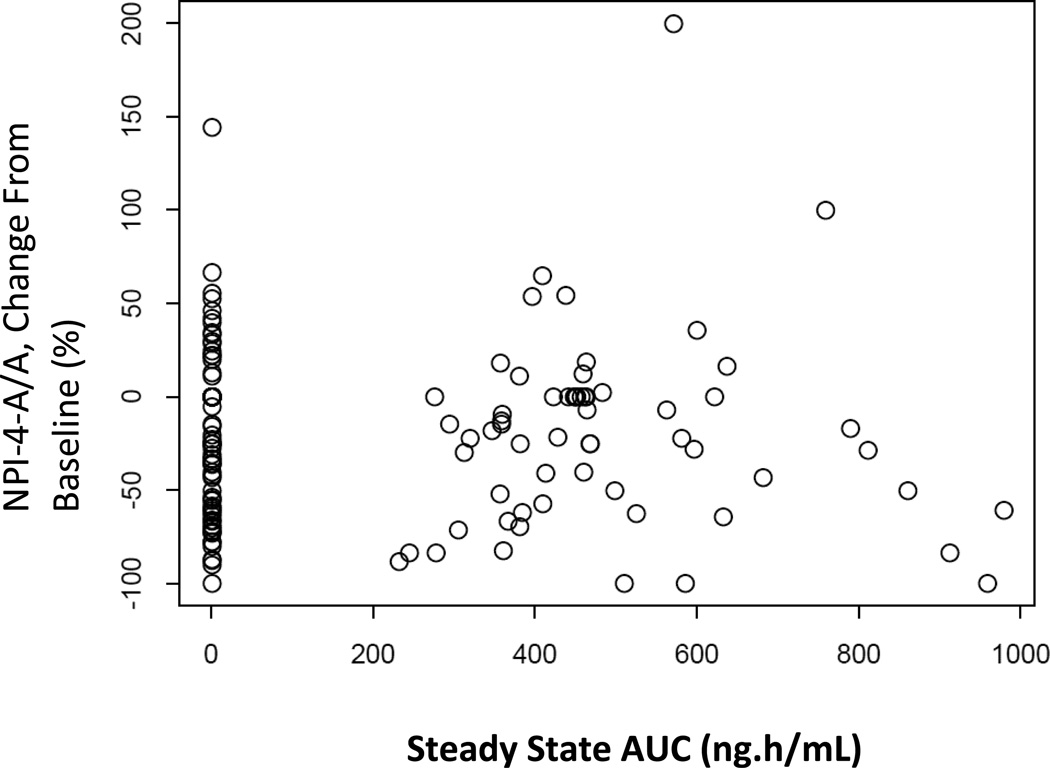
Figure 4.
Pharmacokinetic and pharmacodynamic (PK/PD) relationship comparing least square means change from baseline to endpoint on NPI-4 A/A and blood plasma PK/PD
Measures
The Neuropsychiatric Inventory (NPI-10)
(Cummings et al., 1994) is a widely used, valid and reliable scale measuring noncognitive NPS in dementia, with each of 10 domains rated by the caregiver during a clinician interview, along with severity of caregiver distress. The extent of distress associated with each domain comprises the NPI-Distress (NPI-D) scale. The NPI-4 A/A is a 4-domain subscale chosen as the primary efficacy measure, which combines the following domains: agitation/aggression; aberrant motor behavior; irritability/emotional lability; and disinhibition. Every domain is assessed by a screening question and if the answer is “no,” subquestions are not pursued. If the screening question is answered “yes,” all subquestions are queried and the worst is used to rate that domain for frequency and severity (maximum of 12 points per domain). However, for the four domains included in the NPI-4-A/A, all subquestions were queried irrespective of the response to the stem question. The theoretical rationale for the development of the NPI-4-A/A and partial validation is described in Dennehy et al., 2012.
Cohen-Mansfield Agitation Inventory-Community version (CMAI-C)
(Cohen-Mansfield et al., 1995) is a 36-item item questionnaire assessing A/A in community-dwelling older adults with dementia. Caregivers rate frequency of symptoms from 1=never to 7=several times per hour. The scale has demonstrated reliability and validity in AD patients (Weiner et al., 2000).
Cornell Scale for Depression in Dementia (CSDD)
(Alexopoulos et al., 1988) is a 19-item scale for severity of depressive symptoms in patients with dementia. A clinician interviews both the caregiver and patient and utilizes this information to rate symptoms as absent, mild/intermittent, or severe. This scale is sensitive, valid and reliable with a score range from 0 to 38 where ≥ 8 suggests clinical depression.
Frontal Systems Behaviors Scale (FrSBe)
(Grace and Malloy, 2001) is a 46-item ecological measure of behaviors associated with prefrontal cortical dysfunction. We used the informant form. T-scores for total and subscale (dysexecutive, disinhibition, and apathy) scores are reported.
Clinical Global Impression, Severity-Agitation/Aggression (CGI-S-AA), and Severity-Global Functioning (CGI-S-GF)
(Guy, 1976) are 7-point, single-item Likert scales for overall severity of symptoms of A/A and global functioning based on the investigator’s general clinical experience with a similar patient population, ranging from 0 (normal) to 7 (most severely ill).
Alzheimer’s Disease Assessment Scale-Cognitive 14 item scale (ADAS-Cog14)
(Rosen et al., 1984) measures cognitive impairment across domains for orientation, verbal memory, language, praxis, delayed free recall, digit cancellation, and maze completion. Total scores range from 0 to 90 where higher scores indicate more cognitive deficits.
Alzheimer’s Disease Cooperative Study Activities of Daily Living Inventory (ADCS-ADL)
(Galasko et al., 2004) is a 23-item inventory, administered by interview with the caregiver. If the patient attempted the particular ADL during the past 4 weeks, the caregiver rates the patient’s performance level based on descriptions. Total ADCS-ADL scores range from 0 to 78 where higher scores indicate more independence in daily functioning.
Safety
Summaries of treatment-emergent adverse events (TEAEs), changes in laboratory tests, and changes in vital signs and electrocardiograms (ECGs) were used to assess safety. Standardized tremor and cerebellar examinations were done at every visit. TEAEs were defined as events that first occurred or worsened after baseline.
Study Intervention
After 1 week of twice-daily treatment with either 3-mg mibampator or placebo, a one-time dose reduction to 1 mg twice daily due to intolerability was permitted. Patients who received a dose reduction continued at 1 mg twice-daily for the remainder of the study. The use of benzodiazepines was not permitted except for rescue doses of 0.5 mg lorazepam up to 3 doses in a day and 6 doses in a 3-week period, for patients who experienced severe agitation, defined as sudden, uncontrollable aggressive outbursts with potential for serious harm to themselves or others. Lorazepam was not allowed to be taken in the 24 hours preceding a clinic visit.
Compliance with study medication was assessed at each visit by direct questioning of the caregiver and by capsule counts. Patients who missed >20% of their prescribed study medication, altered the prescribed amount of medication, or violated concomitant medication specifications were discontinued from the study.
A series of Alzheimer’s Association pamphlets and a small paperback on coping with behavioral disturbances in Alzheimer’s (Robinson et al., 2007) were provided to caregivers at prescribed intervals over the course of study participation, including at the point of randomization. The intent of this intervention was to standardize educational influences, but these materials were not emphasized or elaborated upon by study personnel.
Pharmacokinetics and Pharmacodynamics (PK/PD)
Plasma samples were obtained prior to administration of study drug and after 3, 6, 9, and 12 weeks of treatment with study drug. These samples were analyzed for mibampator concentration at Alta Analytical Laboratory located in El Dorado Hills, CA, USA using a validated LC-API/MS/MS method (BPLY451A). The lower limit of quantification for mibampator was 0.050 ng/mL, and the upper limit of quantification was 25.000 ng/mL. Samples above the limit of quantification were diluted to yield results within the calibrated range. Pharmacokinetic data were analyzed with population methodology, utilizing NONMEM VII, Level 1.2 (ICON Development Solutions, Ellicott City, MD). The influence of body weight on volume of distribution was investigated as a covariate during model development. The final model was used to estimate steady-state Cmax and AUC during a dosing interval for all subjects receiving mibampator. Exploratory graphical analyses of the PK/PD relationship between AUC values and NPI-4, NPI-10, and ADAS-cog were conducted. All subjects were used in these analyses, with placebo subjects assigned an AUC of 0 ng·h/mL. Change in PD parameters was defined as the percent difference between baseline and end of treatment. For patients who terminated the study early, the PD value observed at their last visit was used to calculate the change in PD parameters.
STATISTICAL ANALYSIS
The statistical software package nQuery was used to calculate sample size. A sample size of 150 patients (75 per arm) provides 80% power for the study to detect an effect size of 0.41 in mean change from baseline to endpoint of the NPI A/A subscore between mibampator 3 mg BID and placebo. A similar effect size was observed in a previous mibampator study. Assignment to treatment groups was determined by a computer-generated random sequence using an IVRS. Randomization was stratified by investigative site and severity of neuropsychiatric symptoms (NPI-10 total score < 30 at baseline versus NPI-10 total score ≥30 at baseline). Block size was 4. Data were analyzed using SAS® software (version 9.1.3). Demographics and rating scales are described using mean and standard deviation (SD). The null hypothesis for all measures was that the mean change from baseline to last visit of the active treatment phase was equivalent for patients on mibampator versus placebo, after accounting for differences at baseline. In addition, within- and between-group comparisons to baseline were made at each week and over all weeks (main treatment effect). Efficacy analyses were evaluated at 0.05, one-sided alpha level of significance for treatment group comparisons. Safety analyses were conducted using a2-sided, 0.05 level of significance. The primary population was intention-to-treat (ITT). A patient was included in the ITT population if s/he had a baseline and at least one postbaseline efficacy measurement. The analysis was by original assigned groups (ITT); 63 patients were randomized to mibampator and 69 patients were randomized to placebo. The primary efficacy analysis included data from 54 mibampator patients and 62 placebo patients. The remaining 16 patients (9 mibampator, 7 placebo) dropped out of the study before Week 3 or did not complete the NPI-4 A/A subscale at Week 3, the first visit at which NPI-4 A/A was scheduled to be measured. All randomized patients were included in the safety analyses.
The null hypothesis for all continuous measures collected at multiple times postbaseline was tested by a likelihood-based, mixed-effects model repeated measures analysis (MMRM). The model included the fixed categorical effects of treatment, investigator, baseline NPS severity, visit, and treatment-by-visit interaction, as well as the continuous covariates of baseline score and baseline score-by-visit interaction score. Baseline NPS severity was stratified into high NPS (NPI-10 ≥30) and low NPS (NPI-10 < 30) using the NPI-10 score at the randomization visit. Changes in laboratory measures were conducted on rank-transformed data due to the non-normal nature of some measures. The model for baseline treatment comparisons included baseline NPS severity, treatment, and investigator. For the primary efficacy measure, NPI-4 A/A, additional analyses were performed. Subgroups for medical and psychiatric indices were analyzed using a similar MMRM model with additional terms for subgroup, subgroup-by-treatment, and subgroup-by-treatment-by-visit. A secondary analysis of change from baseline to last observation carried forward (LOCF) endpoint was done. The model included treatment, investigator, and baseline NPS severity, with baseline score as a continuous covariate. This LOCF model was also used for measures collected only at baseline and endpoint, such as weight and ECG parameters.
Treatment differences for changes in categorical measures were assessed using Fisher’s exact test. Measures included percentages of TEAEs during the 12-week, double-blind, acute phase (SP II) and the 1-week, double-blind, washout period (SP III), and treatment-emergent high, low, or abnormal lab values during SP II.
RESULTS
Subjects
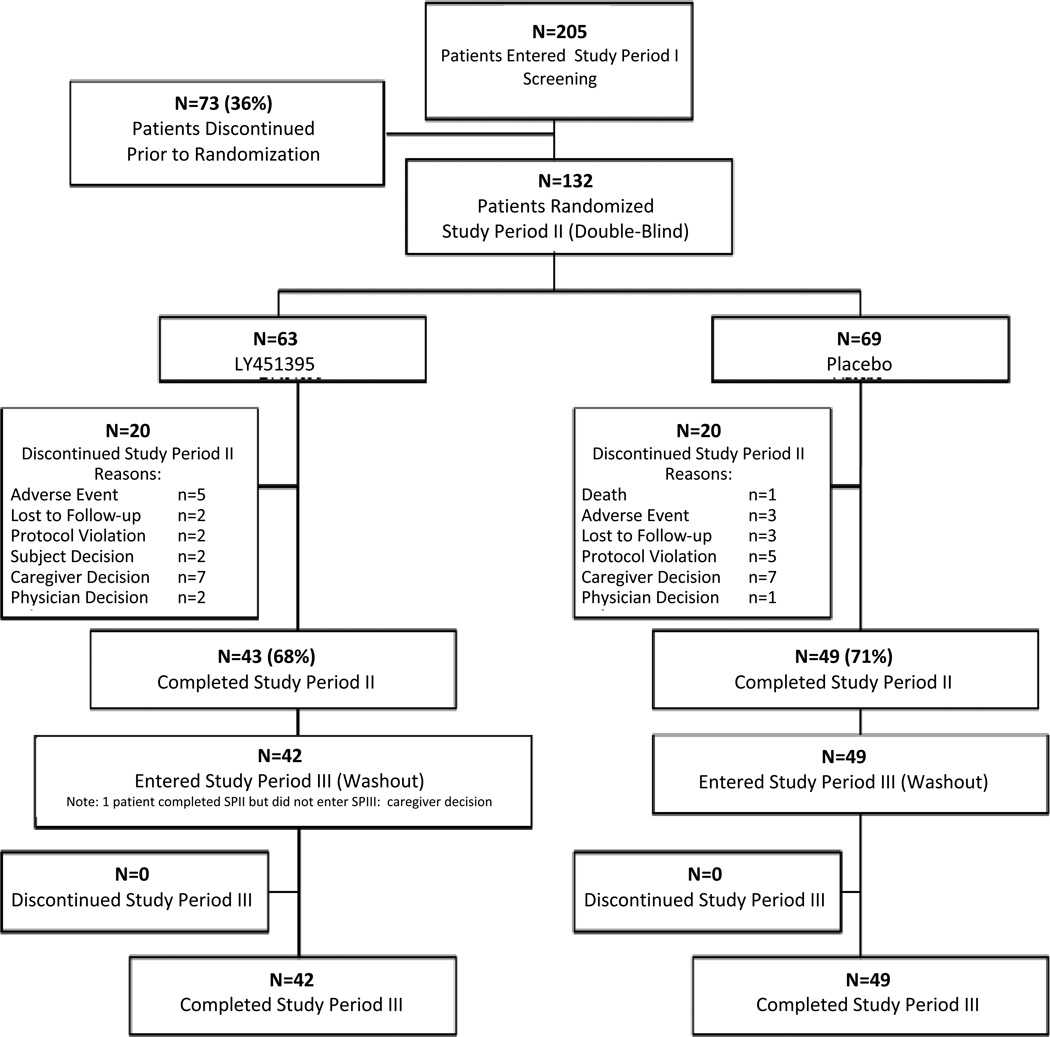
The first patient enrolled on 24 February 2009, and the last patient completed on 10 June 2011. Of 205 patients entered, 132 were randomized, 63 to mibampator and 69 to placebo. Forty-two of 63 (66.7%) mibampator patients and 49/69 (71.0%) placebo patients completed the study (Figure 1). Treatment discontinuation rates due to a TEAE were low and not significantly different, (p=.478 with 7.9% on mibampator and 4.4% on placebo).
Figure 1.
CONSORT diagram
Note: Patients were considered lost to follow-up if 3 attempts to contact the patient by telephone and sending of a certified letter to the patient resulted in no response.
Baseline patient characteristics and rating scale scores for randomized patients (Table 1) revealed that the majority were white (87.1%), and about half were female (50.8%). Age ranged from 60 to 93 years (mean 77.4 years ±7.87). Baseline demographics and severity of cognitive and noncognitive neuropsychiatric symptoms (MMSE, ADAS-Cog, NPI, CSDD, FrSBe, ADCS) were similar between groups, except the CMAI-C Score was significantly worse in the mibampator group (p=.011)
Table 1.
Baseline demographics and clinical characteristics, mean ± SD unless otherwise specified.
| Measure | Mibampator (N=63) |
Placebo (N=69) |
P-value* |
|---|---|---|---|
| Female sex, n (%) | 31 (49.2%) | 36 (52.2%) | .862 |
| Age in yrs | 77.2 (8.2) | 77.7 (7.6) | .620 |
| Range | 59–93 | 60–93 | |
| Race, n (%) | |||
| White | 53 (84.1%) | 62 (89.9%) | .411 |
| Black or African American | 9 (14.3%) | 6 (8.7%) | |
| American Indian or Alaska Native | 0 (0.0%) | 1 (1.5%) | |
| Not Provided | 1 (1.6%) | 0 (0.0%) | |
| Years of formal education | 13.4 (2.8) | 13.3 (2.9) | .809 |
| Range | 6–20 | 6–20 | |
| Level of cognitive impairment, n (%) | 63 | 69 | .074 |
| Mild (20 ≤ MMSE ≤ 26) | 21 (33.3%) | 27 (39.1%) | |
| Moderate (11 ≤ MMSE ≤ 19) | 26 (41.3%) | 35 (50.7%) | |
| Severe (6 ≤ MMSE ≤10) | 16 (25.4%) | 7 (10.1%) | |
| Apolipoprotein E4 allele, n (%) | 60 | 62 | .058 |
| Homozygous (4/4) | 14 (23.3%) | 7 (11.3%) | |
| Heterozygous (2/4, 3/4) | 16 (26.7%) | 28 (45.2%) | |
| Non-carriers (2/2, 2/3, 3/3) | 30 (50.0%) | 27 (43.6%) | |
| Depression (CSDD ≥ 7) | 60 | 69 | .859 |
| No | 27 (45.0%) | 29 (42.0%) | |
| Yes | 33 (55.0%) | 40 (58.0%) | |
| Psychosis based on NPI, n (%) | 63 | 69 | .707 |
| No (Delusions and Hallucinations < 4) | 42 (66.7%) | 49 (71.0%) | |
| Yes (Delusions ≥4 or Hallucinations ≥4) | 21 (33.3%) | 20 (29.0%) | |
| Current AChEI or memantine use, n (%) | 63 | 69 | .848 |
| No | 17 (27.0%) | 20 (29.0%) | |
| Yes | 46 (73.0%) | 49 (71.0%) | |
| Current antidepressant use, n (%) | 63 | 69 | .279 |
| No | 38 (60.3%) | 48 (69.6%) | |
| Yes | 25 (39.7%) | 21 (30.4%) | |
| Diabetic, n (%) | 63 | 69 | .016 |
| No | 60 (95.2%) | 56 (81.2%) | |
| Yes | 3 (4.8%) | 13 (18.8%) | |
| Cardiovascular disease, n (%) | 63 | 69 | .186 |
| No | 48 (76.2%) | 45 (65.2%) | |
| Yes | 15 (23.8%) | 24 (34.8%) | |
| NPI-4-A/A | 18.8 (8.7) | 18.1 (8.2) | .754 |
| NPI-10 | 31.9 (16.7) | 29.7 (13.2) | .232 |
| NPI-D-4-A/A | 8.7 (3.8) | 8.5 (3.7) | .729 |
| CMAI-C | 73.6 (22.6) | 64.7 (17.6) | .011 |
| CSDD | 8.4 (5.4) | 8.0 (4.6) | .445 |
| CGI-S-GF | 4.1 (0.9) | 4.0 (0.8) | .454 |
| CGI-S-A/A | 4.1 (0.7) | 4.1 (0.7) | .943 |
| ADAS-Cog 14-Item | 43.3 (20.4) | 40.0 (18.1) | .252 |
| ADCS-ADL | 45.9 (17.1) | 47.0 (14.3) | .576 |
| FrSBe Total T Score | 92.4 (25.5) | 89.1 (18.5) | .297 |
| MMSE | 16.0 (6.1) | 18.0 (5.3) | N/A |
| MHIS | 0.7 (0.6) | 0.8 (0.7) | N/A |
| DRS-R98 Total | 11.6 (4.0) | 10.3 (4.2) | N/A |
N’s are noted when a variable has fewer than total number per group. *,++
Efficacy
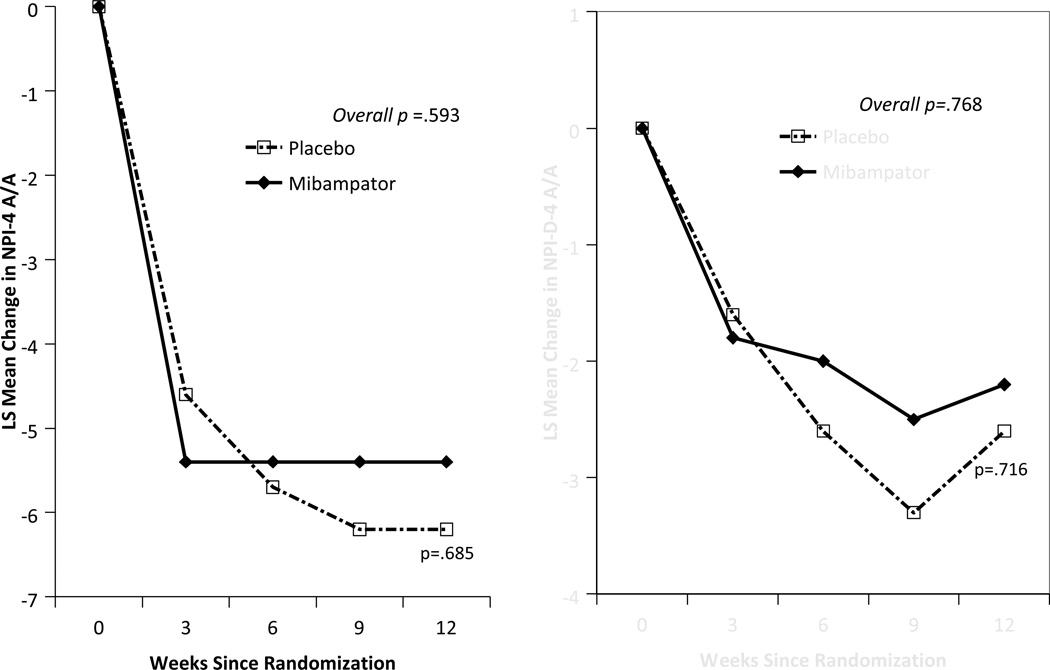
There was no significant difference between groups on the primary outcome using MMRM analysis of the NPI-4 A/A (Figure 2). Notably, both groups improved by approximately 5 points after 3 weeks on treatment and sustained that improvement throughout. Secondary analysis of the NPI-4 A/A by LOCF showed similar results. Caregiver distress, as measured by the NPI-D-4-A/A using MMRM analysis, also showed a similar pattern over time (Figure 2).
Figure 2.
NPI-4-A/A and NPI-D-4-A/A least square means change from baseline after treatment (MMRM analysis)
MMRM analyses of nearly all secondary efficacy measures showed no overall statistical separation between treatment groups. Some measures showed no change in either group over time (e.g., ADAS-Cog, ADCS-ADL) while others showed improvement in both groups, for example the NPI-10, CMAI-C, and CSDD. The exception was the FrSBe Total T-score where the mibampator group demonstrated significantly better outcomes (Figure 3) compared with placebo (p=0.007 main treatment effect). This was significant by Week 6 (mean change mibampator = −5.3 SD ± 1.88, placebo = +1.6 SD ± 1.81, [p=.004]) and sustained through Week 12 (mean change; mibampator = 2.2 SD ± 2.02, placebo = +2.3 SD ± 1.88 [p=.048]). Mibampator was significantly better than placebo on the FrSBe subscale T-scores main treatment effect for apathy (p=.040) and executive dysfunction (p=.018) and trended toward significance (p=.052) on the disinhibition subscale T-score (Figure 4). The individual NPI domain for apathy showed significant improvement in the mibampator group compared to placebo for main treatment effect (p=.039); the individual NPI domain for hallucinations comparison trended toward significance (p=.058).
There were no differences on the primary outcome between groups when analyzed by a number of subgroups including APOE-4 allele status, sex, type of caregiver, concomitant AchEIs or memantine use, functional status, certain comorbid medical conditions (cardiovascular disease, hyperlipidemia, and obesity), concomitant antidepressant use, noncognitive NPS (depression, psychosis, appetite or sleep disturbance) or demographic characteristics (education, income, occupation). Additionally, post-hoc subgroup analyses by high and low median split scores on MMSE, FrSBe, and NPI-apathy did not reveal efficacy differences.
Overall, significantly more patients on mibampator (n=23; 36.5%) compared to placebo (n=13; 18.8%) received lorazepam during the double-blind treatment period (p=.031). Mean total dosages over the course of the 12-week study period were low in both groups (mibampator=2.76 mg SD ± 2.01, max=7.5 mg; placebo=2.19 mg SD ± 1.60, max=5 mg). The by-patient distribution of total dosages among patients appeared similar between treatment groups. By-site usage of lorazepam was variable, with a few sites having proportionately higher usage. Among 6 sites randomizing 8 or more patients, the percentage of patients at each site taking at least one dose of lorazepam ranged from 7% to 54%.
Safety evaluation
Mibampator was well tolerated. There were no serious adverse events (SAEs) attributed to mibampator as deemed by blinded investigators. Incidence of TEAEs was similar in both groups (mibampator 57.14%, placebo 55.07%). There were no significant differences between groups for SAEs, discontinuation due to AEs, TEAEs, or TEAEs possibly related to study drug as deemed by the investigator. In addition, there were no TEAEs that led to a dose reduction in either treatment group. There was no evidence for withdrawal related AEs during SPIII.
There were no deaths on mibampator. The SAEs on mibampator included pancreatic pseudocyst (n=1), non-cardiac chest pain (n=1), pneumonia (n=1), transient ischemic attack (n=1), and psychotic disorder (n=1). There was one death due to intracranial hemorrhage in the placebo group. Other SAEs in the placebo group included pneumonia (n=1), diverticulitis (n=1), spinal column stenosis (n=1), and presyncope (n=1). Discontinuation reasons due to AEs in the mibampator group were back pain (n=1), myalgia (n=1), dizziness (n=1), depression (n=1), and psychotic disorder (n=1); discontinuation reasons due to AEs in the placebo group were pneumonia (n=1), spinal column stenosis (n=1), and anxiety (n=1).
Over the course of the double-blind, 12-week treatment period, no laboratory measures, vital signs or ECG recordings on drug treated patients were statistically different from placebo. There were no significant differences between treatment groups for mean change or categorical changes from baseline QT intervals regardless of correction method.
PK/PD evaluation
A one-compartment model with first-order absorption, with between-subject variability on clearance and rate of absorption, best fit the data. The model used additive and proportional error terms to describe residual unexplained variability. Body weight had a significant impact on volume of distribution. The geometric mean (%CV) for steady state estimated Cmax and AUC were 44.7 ng/mL (31.0%) and 460 ng·h/mL (33.7%). As shown in Figure 4, there was no relationship between plasma values and change scores on the primary measure (placebo group NPI-4-A/A scores are shown for comparison, with plasma exposure values=0 ng·h/mL).
DISCUSSION
Because mibampator failed to separate from placebo on the primary outcome measure in this Phase 2, double-blind, randomized evaluation of an AMPA receptor potentiator, we report no efficacy advantage of mibampator over placebo for A/A symptoms in AD. Calculations suggested our trial was adequately powered. A previous Phase 2 trial that assessed efficacy of lower doses of mibampator for cognition also failed to separate from placebo, though NPS had improved (Chappell et al., 2007). Our findings add to the large disappointing clinical trial literature for A/A symptoms in AD. Interestingly, but not unexpectedly (Lyketsos et al., 2011), there was frequent comorbidity between A/A and other NPS in our sample, with more than half exhibiting depression and almost a third with psychosis. However, subgroup analyses for a large number of medical and psychiatric indices, including risk factors like diabetes and cardiovascular conditions or the presence of depression, did not alter the efficacy findings.
Our primary outcome measure was derived from a well-validated and widely used instrument, the NPI. Composed of A/A, irritability/lability, disinhibition, and aberrant motor behavior domains, the domains included in the NPI-4-A/A were previously identified as a subscale or cluster in analyses of epidemiological and clinical samples (Aalten et al., 2007; Wood et al., 2000). This subscale previously accounted for 60% of the variance in Cohen-Mansfield Agitation Inventory total scores (Wood et al., 2000). In our cohort, we further confirmed that finding, and the concurrent validity of the NPI-4-A/A, with a significant correlation with the CMAI-C (r=0.54; p<.0001).
About one-third of patients in this study were taking stable doses of allowable concomitant antidepressants. There was no difference in efficacy by antidepressant status. Though the mibampator group used more lorazepam during the study, the total doses were low in both groups and we do not believe that this confounded efficacy analyses. We disallowed concomitant use of other sedating CNS agents prescribed to manage A/A symptoms because prior trials may have failed to separate due to sedation. Caregivers of those taking psychotropics, including antipsychotics that carry a black box FDA warning for death in this specific population, were usually reluctant to taper and discontinue. Caregiver distress levels are very high in this population (Davis et al., 2007) and it is difficult to reverse a decision to use an antipsychotic. This reluctance may have introduced a bias in recruitment.
Current drugs most often used off-label to manage A/A in AD do not have a glutamatergic mechanism of action. Memantine, indicated for cognitive impairment in moderate AD, is an N-methyl-D-aspartate (NMDA) antagonist and has a different glutamatergic mechanism of action than mibampator. A post-hoc analysis of pooled data from three randomized clinical trials with primary objectives for cognition suggested memantine had some efficacy for agitation symptoms in AD (Wilcock et al., 2008). A more recent study found no advantage of memantine over placebo for agitation in AD patients (Fox et. al., 2012).
Mibampator is specific and highly selective for AMPA receptor potentiation. Given the synaptic neurotransmission deficits and network damage in AD, it was plausible that potentiating excitatory glutamatergic neurotransmission might have improved information processing and enhanced prefrontal cortices’ regulation of limbic regions, with a resultant effect of lower A/A scores. Recent MRI analyses have implicated atrophy of fronto-insular-limbic components of the anterior salience network in A/A symptom severity in of AD. The only measure to show significant improvement in our trial was the FrSBe, measure of prefrontal cortex functioning, though this could be an artifact given the number of measures included in this trial. Of note is that mean baseline FrSBe T-scores were in the range of those seen in Frontotemporal Dementia (FTD) and higher than those reported in AD (Malloy et al., 2007). This suggests that either part of our cohort actually had FTD or that the subset with significant A/A symptoms have more prefrontal pathology (Woodward et al., 2010).
Mibampator was well-tolerated in this population, where patients were as old as 93, with no significant differences from placebo for TEAEs, SAEs or discontinuations. Despite preclinical concerns for tremor and convulsions, based on mibampator rodent studies, there were no such findings in AD patients. Our investigators conducted standardized tremor and cerebellar examinations at every office visit. Tremor was described as treatment emergent in 3/63 (4.76%) on mibampator and 2/69 (2.90%) on placebo and tended to be mild and transient, and the majority were essential tremor.
The pharmacokinetics of mibampator are well described in the present study using a population approach. Estimated steady-state plasma Cmax and AUC were consistent with expectations from previous studies, demonstrating that plasma exposure was generally higher in this study than in the previous Phase 2 study which used lower doses (Chappell et al., 2007). Previous research has shown that mibampator is able to cross the blood-brain barrier after administration of 1- and 5-mg twice daily doses, with cerebrospinal fluid (CSF) concentrations approximately 2% of that in plasma (Jhee et al., 2006). On the basis of these studies, it was expected that mibampator concentration in CSF at steady state would be approximately 1 ng/mL. However, in the absence of a human target engagement biomarker, we are unable to definitively document that mibampator concentration in the brain was sufficient to have an effect on AMPA potentiation. An analysis comparing change in NPI-4-A/A scores over the course of the study to steady-state AUC values did not suggest that subjects benefited from higher exposure.
Other possible reasons for lack of drug efficacy include caregiver, rating and drug-related issues. Primary and secondary outcomes improved in both groups except for cognitive and functional assessments. Caregivers provided information for rating scales. Given that many AD caregivers are under stress, initial ratings may have been inflated due to concerns about the patient’s behavior or desire to try non-sedating pharmacological options. Scores over time may have regressed toward the mean in both groups. Further, we provided caregivers with educational materials about AD as part of a basic standard of care, including those that described various symptoms in AD with practical suggestions for environmental adjustments (Robinson et al., 2007). It is possible that these materials may have had more than our intended impact. If support from participation in the trial had therapeutic value for caregivers, then it may have affected their perception of patients’ symptoms as improving. Drug compliance was within study parameters and comparable between groups.
It is possible that the amount of AD-related neurodegenerative damage to frontolimbic networks at the time of study entry may have been too widespread for mibampator to have selective therapeutic benefits on A/A symptoms. Given that the pathological processes leading to AD begin decades before a clinical diagnosis, perhaps drugs may need to be started much sooner in the disease process. In fact, the field is moving toward earlier diagnosis of an AD process at or before the mild cognitive impairment (MCI) stage (Sperling et al., 2011).
Future trials for A/A associated with AD pathology may need to use different methodologies to address possible shortcomings of our and others’ studies. These include placebo lead-in period before randomization, use of a continuous caregiver diary, and objective actigraphy.
ACKNOWLEDGMENTS
Research supported by Eli Lilly and Company.
CONFLICTS OF INTEREST
PTT, TK, TDF, CC, BAW, CS, EBD, and LT are employees and stockholders of Eli Lilly and Company (Indianapolis, Indiana, USA). PTT owns the copyright of the Delirium Rating Scale-Revised-98. EBD is affiliated with the Department of Psychological Sciences, Purdue University, West Lafayette, Indiana.
CL has received grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, GlaxoSmithKline, Eisai, Pfizer, Astra-Zeneca, Eli Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, and Elan. He has been consultant or advisor for Astra-Zeneca, GlaxoSmithKline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Eli Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, and Avanir. He has received honorarium or travel support from Pfizer, Forest, GlaxoSmithKline, and Health Monitor. CL is not being paid specifically for authoring this paper, although he has consulted previously on the development of mibampator.
JC has received no grant support. He has provided consultation to Abbott, Acadia, Acerra, ADAMAS, Anavex, Astellas, Avanir, Baxter, Bristol-Myers Squibb, Eisai, Elan, EnVivo, Forest, Genentech, GlaxoSmithKline, Janssen, Eli Lilly, Lundbeck, Medivation, Medtronics, Merck, Neurokos, Novartis, Pfizer, Prana, QR, Sonexa, Takeda, and Toyama pharmaceutical companies. JC had provided consultation to Bayer, Avid, GE, MedAvante, Neurotrax, and UBC. He owns stock in ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, Neurokos, and QR pharma, and he has been a speaker/lecturer at Eisai, Forest, Janssen, Novartis, Pfizer, Lundbeck. JC owns the copyright to the Neuropsychiatric Inventory.
Footnotes
DESCRIPTION OF AUTHORS’ ROLES
P. Trzepacz designed the study and wrote substantial portions of the protocol, oversight of the implementation, interpreted the data, and assisted in writing the paper. J. Cummings provided study design consultation, interpretation of the data, assisted in writing the paper. T. Konechnik assisted in designing the study and providing regulatory consultation. T. Forrester was responsible for the statistical design and oversight of the data analyses, and assisted in writing the paper. C. Chang was responsible for oversight of the safety data. E. Dennehy assisted in data interpretation and writing the paper. B. Willis was responsible for the PK/PD data collection and analysis and assisted in writing the paper. C. Shuler assisted in implementation of the study. L. Tabas assisted in writing the paper. C. Lyketsos provided study design consultation, data interpretation and assisted in writing the paper.
REFERENCES
- Aalten P, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dementia and Geriatric Cognitive Disorders. 2007;24(6):457–463. doi: 10.1159/000110738. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biological Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Ballard C, Corbett A, Chitramohan R, Aarsland D. Management of agitation and aggression associated with Alzheimer's disease: controversies and possible solutions. Current Opinion in Psychiatry. 2009;22(6):532–540. doi: 10.1097/YCO.0b013e32833111f9. [DOI] [PubMed] [Google Scholar]
- Blanchard BJ, Chen A, Rozeboom LM, Stafford KA, Weigele P, Ingram VM. Efficient reversal of Alzheimer's disease fibril formation and elimination of neurotoxicity by a small molecule. Proceedings of the National Academy of Sciences USA. 2004;101(40):14326–14332. doi: 10.1073/pnas.0405941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology. 2007;68(13):1008–1012. doi: 10.1212/01.wnl.0000260240.46070.7c. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Werner P, Watson V, Pasis S. Agitation among elderly persons at adult day-care centers: the experiences of relatives and staff members. Int Psychogeriatrics. 1995;7(3):447–458. doi: 10.1017/s1041610295002195. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davis JD, Tremont G. Impact of frontal systems behavioral functioning in dementia on caregiver burden. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19(1):43–49. doi: 10.1176/appi.neuropsych.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy EB, Kahle-Wrobleski K, Sarsour K, Milton DR. Derivation of a brief measure of agitation and aggression in Alzheimer’s disease. International Journal of Geriatric Psychiatry. doi: 10.1002/gps.3807. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Doody RS, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 'Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox C, et al. Efficacy of Memantine for Agitation in Alzheimer's Dementia: a Randomised Double-Blind Placebo Controlled Trial. PLoS One. 2012;7(5):e35185. doi: 10.1371/journal.pone.0035185. Epub 2012 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer’s disease. Journal of American Geriatrics Society. 2004;52(7):1070–1076. doi: 10.1111/j.1532-5415.2004.52303.x. [DOI] [PubMed] [Google Scholar]
- Gates M, Ogden A, Bleakman D. Pharmacological effects of AMPA receptor potentiators LY392098 and LY404187 on rat neuronal AMPA receptors in vitro. Neuropharmacology. 2001;40(8):984–991. doi: 10.1016/s0028-3908(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Gilley DW, Bienias JL, Wilson RS, Bennett DA, Beck TL, Evans DA. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer's disease. Psychological Medicine. 2004;34(6):1129–1135. doi: 10.1017/s0033291703001831. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy PF. Frontal Systems Behavior Scale (FrSBe): professional manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology, revised. Bethesda (MD): US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- Herrmann N, Lanctôt KL, Khan LR. The role of norepinephrine in the behavioral and psychological symptoms of dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):261–276. doi: 10.1176/jnp.16.3.261. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Lanctôt KL. Pharmacologic management of neuropsychiatric symptoms of Alzheimer disease. Canadian Journal of Psychiatry. 2007;52(10):630–646. doi: 10.1177/070674370705201004. [DOI] [PubMed] [Google Scholar]
- Hirono N, Mega MS, Dinov ID, Mishkin F, Cummings JL. Left frontotemporal hypoperfusion is associated with aggression in patients with dementia. Archives of Neurology. 2000;57(6):861–866. doi: 10.1001/archneur.57.6.861. [DOI] [PubMed] [Google Scholar]
- Holmes C, et al. Psychosis and aggression in Alzheimer’s disease: the effect of dopamine receptor gene variation. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:777–779. doi: 10.1136/jnnp.71.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhee SS, et al. Multiple-dose plasma pharmacokinetic and safety study of LY450108 and LY451395 (AMPA receptor potentiators) and their concentration in cerebrospinal fluid in healthy human subjects. Journal of Clinical Pharmacology. 2006;46(4):424–432. doi: 10.1177/0091270006286899. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kaufer D, Reiter CT, Carra J, DeKosky ST, Palmer AM. Relationship between CSF neurotransmitter metabolites and aggressive behavior in Alzheimer’s disease. European Journal of Neurology. 1996;3:153–155. [Google Scholar]
- Lyketsos CG, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers & Dementia. 2011;7(5):532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. AMPA receptor modulators as cognitive enhancers. Current Opinion in Pharmacology. 2004;4(1):4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43(1):1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Madsen U, Ebert B, Krogsgaard-Larsen P. Modulation of AMPA receptor function in relation to glutamatergic abnormalities in Alzheimer's disease. Biomedicine & Pharmacotherapy. 1994;48(7):305–311. doi: 10.1016/0753-3322(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Malloy P, Tremont G, Grace J, Frakey L. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer's disease. Alzheimers & Dementia. 2007;3(3):200–203. doi: 10.1016/j.jalz.2007.04.374. [DOI] [PubMed] [Google Scholar]
- Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59(11):1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ. SMi 4th Annual Conference on Neurodegenerative Disorders: a focus on Alzheimer's and Parkinson's disease. Expert Opinion on Investigational Drugs. 2004;13(10):1369–1373. doi: 10.1517/13543784.13.10.1369. http://informahealthcare.com/doi/pdf/10.1517/13543784.13.10.1369. [DOI] [PubMed] [Google Scholar]
- Reinikainen KJ, Soininen H, Riekkinen PJ. Neurotransmitter changes in Alzheimer's disease: implications to diagnostics and therapy. Journal of Neuroscience Research. 1990;27(4):576–586. doi: 10.1002/jnr.490270419. [DOI] [PubMed] [Google Scholar]
- Robinson A, Spencer B, White L. Understanding Difficult Behaviors: Practical Suggestions for Coping with Alzheimer's Disease and Related Illnesses. Ypsilanti, MI: Eastern Michigan University Alzheimer's Education Program, published by Eastern Michigan University; 2007. http://www.amazon.com/Understanding-Difficult-Behaviors-suggestionsAlzheimers/dp/0978902009. [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Rosenheck RA, et al. Cost-benefit analysis of second-generation antipsychotics and placebo in a randomized trial of the treatment of psychosis and aggression in Alzheimer disease. Archives of General Psychiatry. 2007;64(11):1259–1268. doi: 10.1001/archpsyc.64.11.1259. [DOI] [PubMed] [Google Scholar]
- Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. American Journal of Geriatric Psychiatry. 2005;13(6):469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Science Translational Medicine. 2011;3(111):111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukonick DL, et al. The 5-HTTPR*S/*L polymorphism and aggressive behavior in Alzheimer disease. Archives of Neurology. 2001;58(9):1425–1428. doi: 10.1001/archneur.58.9.1425. [DOI] [PubMed] [Google Scholar]
- Sultzer DL, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. American Journal of Psychiatry. 2008;165(7):844–854. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, et al. The 5-HTTPR polymorphism confers liability to a combined phenotype of psychotic and aggressive behavior in Alzheimer disease. International Psychogeriatrics. 2001;13(4):401–409. doi: 10.1017/s1041610201007827. [DOI] [PubMed] [Google Scholar]
- Tekin S. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Annals of Neurology. 2001;49(3):355–361. [PubMed] [Google Scholar]
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. Journal of the American Medical Association. 2003;289(2):210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. The Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(2):229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Huff K, Bond A, Lodge D. Potentiation of responses to AMPA on central neurones by LY392098 and LY404187 in vivo. Neuropharmacology. 2001;40(8):1003–1009. doi: 10.1016/s0028-3908(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Walton HS, Dodd PR. Glutamate-glutamine cycling in Alzheimer’s disease. Neurochemistry International. 2007;50:1052–1066. doi: 10.1016/j.neuint.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Weiner MF, et al. Quantifying behavioral disturbance in Alzheimer’s disease patients. Journal of Psychiatric Research. 2000;34(2):163–167. doi: 10.1016/s0022-3956(99)00042-4. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer's disease: a pooled analysis of 3 studies. J Clin Psychiatry. 2008;69(3):341–348. doi: 10.4088/jcp.v69n0302. [DOI] [PubMed] [Google Scholar]
- Wood S, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. American Journal of Geriatric Psychiatry. 2000;8(1):75–83. doi: 10.1097/00019442-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Woodward M, Jacova C, Black SE, Kertesz A, Mackenzie IR, Feldman H the ACCORD investigator group. Differentiating the frontal variant of Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2010;25(7):732–738. doi: 10.1002/gps.2415. [DOI] [PubMed] [Google Scholar]
- Wu X, et al. AMPA protects cultured neurons against glutamate excitotoxicity through a phosphatidylinositol 3-kinase-dependent activation in extracellular signal-regulated kinase to upregulate BDNF gene expression. Journal of Neurochemistry. 2004;90(4):807–818. doi: 10.1111/j.1471-4159.2004.02526.x. [DOI] [PubMed] [Google Scholar]
- Yamada KA. Therapeutic potential of positive AMPA receptor modulators in the treatment of neurological disease. Expert Opinion on Investigational Drugs. 2000;9(4):765–778. doi: 10.1517/13543784.9.4.765. [DOI] [PubMed] [Google Scholar]
- Zhao P, Ignacio S, Beattie EC, Abood ME. Altered presymptomatic AMPA and cannabinoid receptor trafficking in motor neurons of ALS model mice: implications for excitotoxicity. European Journal of Neuroscience. 2008;27(3):572–579. doi: 10.1111/j.1460-9568.2008.06041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]