Enzyme activities in central metabolism of tomato fruits are strongly influenced by developmental stage but only weakly by environment.
Abstract
To assess the influence of the environment on fruit metabolism, tomato (Solanum lycopersicum ‘Moneymaker’) plants were grown under contrasting conditions (optimal for commercial, water limited, or shaded production) and locations. Samples were harvested at nine stages of development, and 36 enzyme activities of central metabolism were measured as well as protein, starch, and major metabolites, such as hexoses, sucrose, organic acids, and amino acids. The most remarkable result was the high reproducibility of enzyme activities throughout development, irrespective of conditions or location. Hierarchical clustering of enzyme activities also revealed tight relationships between metabolic pathways and phases of development. Thus, cell division was characterized by high activities of fructokinase, glucokinase, pyruvate kinase, and tricarboxylic acid cycle enzymes, indicating ATP production as a priority, whereas cell expansion was characterized by enzymes involved in the lower part of glycolysis, suggesting a metabolic reprogramming to anaplerosis. As expected, enzymes involved in the accumulation of sugars, citrate, and glutamate were strongly increased during ripening. However, a group of enzymes involved in ATP production, which is probably fueled by starch degradation, was also increased. Metabolites levels seemed more sensitive than enzymes to the environment, although such differences tended to decrease at ripening. The integration of enzyme and metabolite data obtained under contrasting growth conditions using principal component analysis suggests that, with the exceptions of alanine amino transferase and glutamate and malate dehydrogenase and malate, there are no links between single enzyme activities and metabolite time courses or levels.
Tomato (Solanum lycopersicum) is ranked number one among fruits and vegetables, with 14% of the total production worldwide. Some 4 million ha are in production, yielding 18 kg per habitant (Food and Agriculture Organization of the United Nations; http://faostat.fao.org). Tomato is also the most studied fleshy fruit (Giovannoni, 2001; Klee and Giovannoni, 2011) thanks to ease of cultivation, short generation times, a relatively small-sized diploid genome, and good tolerance of interspecific crosses, inbreeding, high-density mutagenesis, and transformation. Numerous introgression lines (Eshed and Zamir, 1995; Tanksley et al., 1996), mutants (Saito et al., 2011), and transformants (Smith et al., 1988; Klee et al., 1991; Carrari et al., 2003) have been generated. Its genome has been sequenced and annotated recently (Tomato Genome Consortium, 2012), and a number of postgenomic approaches have been used to gain insights into molecular networks controlling fruit development and ripening. They include analyses of fruit transcriptomes (Alba et al., 2005; Lemaire-Chamley et al., 2005), proteomes (Rocco et al., 2006; Faurobert et al., 2007), and metabolomes (Roessner-Tunali et al., 2003; Schauer et al., 2006) as well as multilevel studies integrating transcriptomics and metabolomics (Carrari et al., 2006; Mounet et al., 2009), transcriptomics and enzyme profiles (Steinhauser et al., 2010), or transcriptomics, proteomics, and metabolomics (Osorio et al., 2011). These efforts, mostly focused on the cellular level, are leading to an increasingly broad understanding of organogenesis, development, and maturation of fleshy fruits. However, such approaches are usually conducted under standard growth conditions and do not take into account environmental factors known to affect yield quality and quantity.
Metabolite composition, which is an important component of fruit quality, results from numerous metabolic pathways that undergo profound reprogramming throughout fruit growth and ripening (Carrari et al., 2006). Although such reprogramming strongly depends on the genotype (Sinesio et al., 2010), producers and gardeners know very well that it is also modulated by the environment (Beckles, 2012). The main limiting resources for fruit growth and quality are water and carbon coming from the plant (Prudent et al., 2010). The main abiotic variables affecting these resources are light, water, and temperature. Thus, shading leads to a reduction in fruit fresh weight, size, and quality (Cockshull et al., 1992). Conversely, high temperature and light intensity have been found to increase hexoses in cherry tomato fruits (S. lycopersicum cv. Naomi; Rosales et al., 2007). It is also well-known that salinity (Cuartero and Fernández-Muñoz, 1999) and increased electrical conductivity of the growth medium (Fanasca et al., 2007) both lead to various metabolic responses in fruits, in particular to increased sugar and organic acid contents. More generally, it has been shown that interactions between genotype and the environment result in high variability for a wide range of primary and secondary metabolites (Schauer et al., 2008). Finally, fruit load (Do et al., 2010) and fruit position also influence quality. Tomato fruits are known to compete within a truss (Gautier et al., 2005), and greenhouse tomato producers usually prune trusses to maximize fruit growth and quality. The position of the truss on the plant has also been found to influence various metabolites in the fruits (Bénard et al., 2009), including lycopene and β-carotene (Fanasca et al., 2007), as well as seed quality (Dias et al., 2006). Such variations are probably caused by a combination of factors, especially in indeterminate varieties, where new trusses are initiated while the plant continues to grow, which implies different microclimatic or macroclimatic conditions for each truss.
The study of metabolic fluxes and enzyme activities provides critical information for a better understanding of metabolic control in fruits (Beckles et al., 2012). Accordingly, several reports show that modifications of enzyme properties can impact fruit metabolite composition. For example, a 30% decrease in aconitase activity in transgenic tomato fruits expressing an antisense gene construct led to a strong increase in organic acids at maturity (Morgan et al., 2013). Similarly, introgression of the gene encoding the regulatory subunit of ADP-Glc pyrophosphorylase from Solanum hirsutum into the cultivated tomato resulted in higher starch levels in the developing fruit and then, higher Brix in mature fruits (Schaffer et al., 2000). Finally, introgression of a gene-encoding cell wall invertase from Solanum pennellii, with higher affinity for Suc, also resulted in fruits with higher Brix (Fridman et al., 2004). However, intensive investigation of a small set of enzymes from Suc and starch metabolism pinpointed significant differences between cultivars and related species and eventually brought about better understanding of carbon source sink relationships in fruits (Miron and Schaffer, 1991; Wang et al., 1993; Demnitz-King et al., 1997; Schaffer and Petreikov, 1997). For example, it was shown that a strong increase in acidic invertase at ripening is responsible for the accumulation of hexoses at the expense of Suc in the cultivated tomato (Yelle et al., 1991). More recently, the profiling of 22 enzymes revealed subtle but significant differences between two tomato varieties (Steinhauser et al., 2010), confirming that the genetic component of metabolite composition includes programming of pathway enzymes.
Conversely, very little is known about the influence of the environment on fruit metabolic pathways. In particular, it is still unclear to what extent metabolic responses to factors such as light intensity or water supply are caused by a reprogramming of fruit central metabolism or simply alterations in assimilate supply from the mother plant. Profiling enzyme activities from various pathways in fruits harvested at various developmental stages and grown under contrasting growth conditions, therefore, seems a rational step. Indeed, even when measured in vitro, enzymes provide integrated information about gene expression and posttranslational regulation (Gibon et al., 2004). Furthermore, the recent development of robotized enzyme profiling now enables the profiling of tens of activities in large numbers of samples at relatively moderate costs (Rogers and Gibon, 2009), which enables the performance of detailed experiments.
The aim of the study was to assess the effect of environment on fruit metabolism by comparing enzyme activity profiles obtained at various developmental stages in fruits from different trusses and/or plants grown under contrasting growth conditions. The integration of this information with metabolite levels provides a better understanding about relationships between metabolism and fruit development and maturation.
RESULTS
All experiments were performed with the cv ‘Moneymaker,’ which is an indeterminate greenhouse variety that was bred in the early 20th century by F. Stonor and Sons in Southampton, United Kingdom. This cultivar, which is still available on the market (essentially for gardeners and organic farmers), has been used in many studies focused on metabolism (Holtzapffel et al., 2002; Carrari et al., 2006; Luengwilai et al., 2010).
Plant and Fruit Development under Optimal Conditions, Shading, and Water Limitation
About 580 tomato plants were grown in a greenhouse in the southwest of France (Sainte-Livrade sur Lot) during the summer of 2010 according to usual production practices. To avoid competition for assimilates between fruits of a given truss, fruit number was limited to six per truss by pruning.
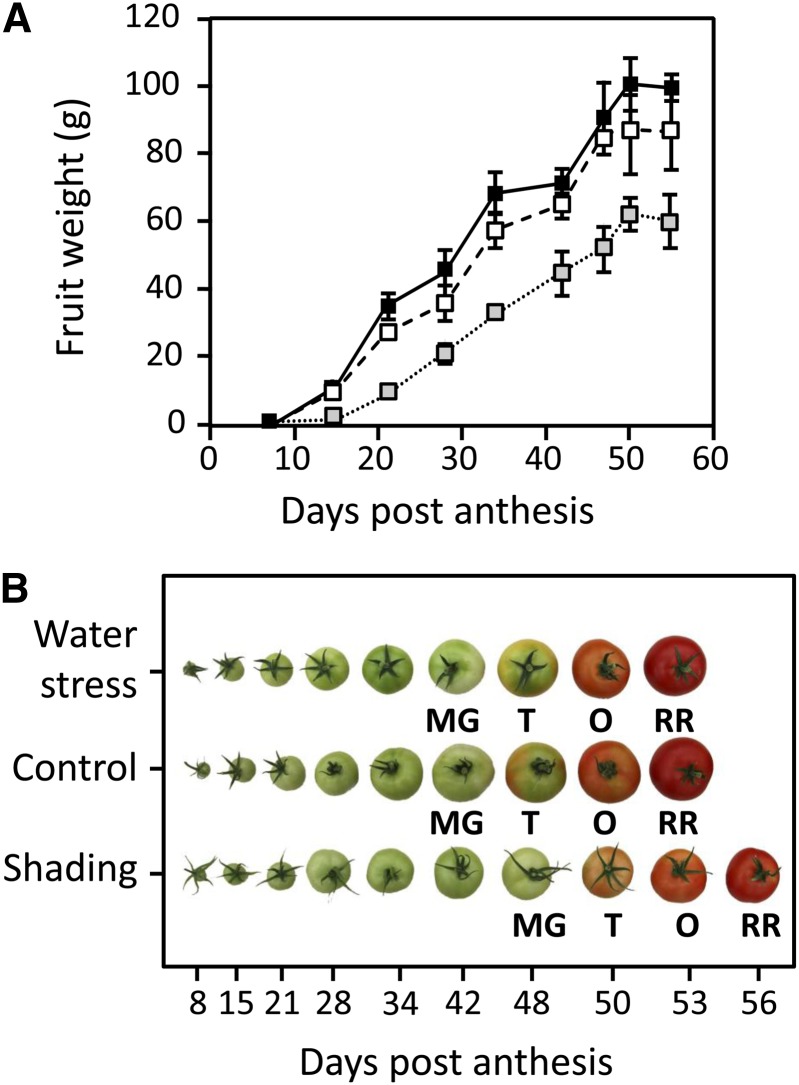
To assess the effects of a decrease in light level, a mesh was installed above the plants to remove 60% of the photosynthetically active radiation. To assess the effect of water limitation, the supply of the nutrient solution was decreased by 50%. This treatment provoked a global increase in nutrients in the draining solution but only marginally affected their relative concentrations, indicating that there was no nutritive stress (data not shown). Both treatments, which were started at 75 d after sowing, affected plant growth. Thus, compared with the control plants (average size at the end of the culture: 295 ± 15.2 cm, n = 9), shaded plants were etiolated and taller (346 ± 15.4 cm, n = 10), and plants under water shortage were slightly smaller (266 ± 11 cm, n = 9). Trusses 1–4 were already growing on the plants at the onset of treatment. Whereas water shortage did not provoke any fruit abortion, shading resulted in the abortion of about 90% of the flowers and young fruits. Later, the proportion of abortion decreased in shaded plants, resulting in three to five fruits per truss, indicating that source sink relationships eventually improved. Fruits were harvested on three different trusses (trusses 5–7) and at nine stages of development (i.e. 8, 15, 21, 28, and 34 DPA, Mature Green [about 42 DPA], Turning [about 47 DPA], Orange [about 50 DPA], and Red Ripe [about 55 DPA]). Figure 1A presents the fresh weight of fruits throughout development grown under the three conditions. At the Red Ripe stage, fruits were the largest under control conditions, with an average weight of 99.6 ± 3.5 g, whereas fruits weighed 86.7 ± 11.6 g under water shortage and only 60.3 ± 8.3 g under shaded conditions. Ripening of fruits of shaded plants was also delayed, which is illustrated in Figure 1B, and 60–63 d were necessary to reach the Red Ripe stage instead of 55 d for control and water shortage conditions.
Figure 1.
Growth of fruits of S. lycopersicum ‘Moneymaker’ obtained under different environmental conditions. A, Fruit weight is expressed in grams ± sd (n = 9) at various development stages under optimal conditions (black line), with 50% water shortage (dashed line), and under a net removing 60% of the photosynthetically active radiation (dotted line). B, Illustration of the nine developmental stages harvested (8, 15, 21, 28, and 34 DPA, Mature Green [MG], Turning [T], Orange [O], and Red Ripe [RR]). Fruit ripening under shading was delayed by 5–8 d.
Developmental Changes in Enzyme Activities of Fruits Grown under Optimal Growth Conditions
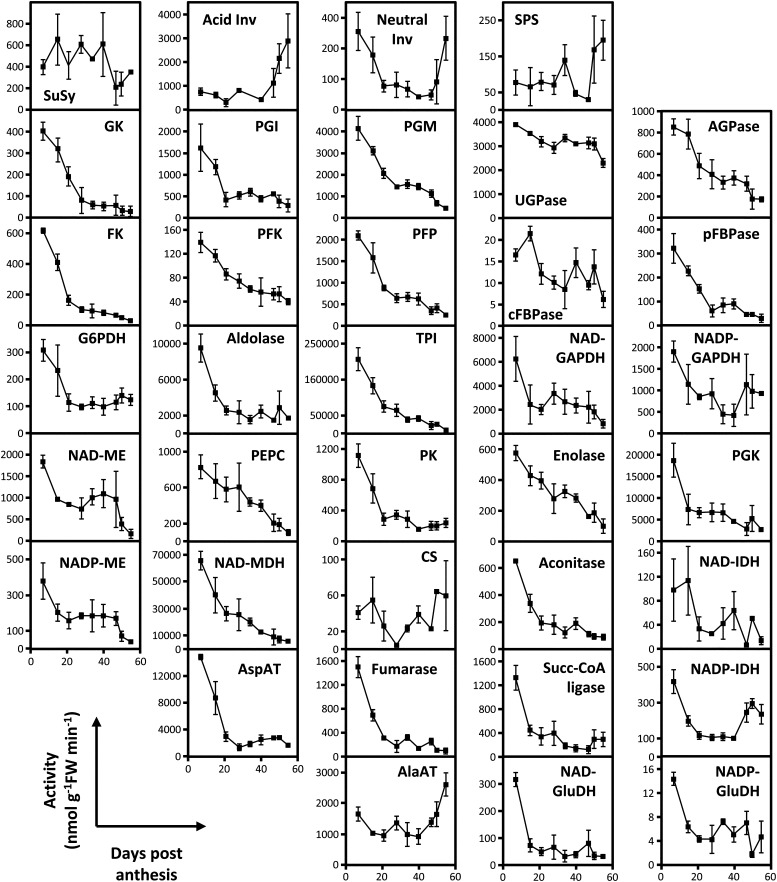
The activities of 36 enzymes involved in carbohydrate metabolism, glycolysis, metabolism of organic acids, and the Calvin–Benson cycle were measured at nine stages of development. Figure 2 presents the results obtained for the seventh truss (Supplemental Table S1). To enable comparison with previous work (Steinhauser et al., 2010), we first expressed them on a fresh weight basis. Most enzyme activities were highest in the youngest stage and decreased sharply during the first 15–21 DPA, tending to a plateau until the end of fruit development and maturation. This trend was particularly marked for enzymes involved in carbohydrate metabolism (i.e. glucokinase [EC 2.7.1.2] and fructokinase [EC 2.7.1.4], which are involved in the phosphorylation of hexoses, ATP phosphofructokinase [PFK; EC 2.7.1.11] and inorganic pyrophosphate phosphofructokinase [PFP; EC 2.7.1.90], which are involved in the phosphorylation of Fru-6P, and phosphoglucose isomerase [PGI; EC 5.3.1.9] and phosphoglucomutase [PGM; EC 5.4.2.2], which catalyze interconversions of hexose-P). In contrast, enzymes involved in Suc degradation (Suc synthase [EC 2.4.1.13] and invertases [EC 3.2.1.26]) and synthesis (Suc phosphate synthase [EC 2.4.1.14]) showed less marked changes, with the exception of acid invertase, which dramatically increased at maturation, increasing fourfold within a few days. UDP-Glc pyrophosphorylase (UGPase; EC 2.7.7.9), which is involved in Suc synthesis as well as the synthesis of cell wall components, was particularly stable throughout fruit development. Stromal Fru-1,6-bisphosphatase (EC 3.1.3.11), which is exclusively located in the plastid, but also, aldolase (EC 4.1.2.13), triose-P isomerase (EC 5.3.1.1), NADP-glyceraldehyde-3P dehydrogenase (EC 1.2.1.9), and phosphoglycerokinase (EC 2.7.2.3), which are also partly located in the plastids, showed a sharp decrease in activity between 8 and 20 DPA. NADP-malic enzyme (NADP-ME; EC 1.1.1.40) initially showed a similar pattern (sharp decrease between 8 and 21 DPA followed by stabilization) but then dropped significantly and abruptly at the beginning of ripening. Also, NAD-ME (EC 1.1.1.38) and pyruvate kinase (PK; EC 2.7.1.40) as well as most enzymes involved in the TCA cycle, the main aminotransferases (ATs), and both NAD+-Glu dehydrogenase and NADP-Glu dehydrogenase showed an initial decrease followed by stabilization. Among them, NADP-isocitrate dehydrogenase (NADP-IDH; EC 1.1.1.42), citrate synthase (EC 2.3.3.1), and Ala-AT (EC 2.6.1.2) increased quite dramatically during ripening. Finally, phosphoenolpyruvate carboxylase (PEPC; EC 4.1.1.31), enolase (EC 4.2.1.11), and NAD+ malate dehydrogenase (MDH; EC 1.1.1.37) showed a different pattern, because their activities decreased in a quasilinear fashion throughout fruit development.
Figure 2.
Maximal activities of 36 enzymes of central metabolism throughout tomato fruit development. Activities were measured in fruit pericarps harvested between 8 and 55 DPA at substrate saturation and 25°C, and they are expressed as nanomoles per minute per gram fresh weight ± sd (n = 5). Acid Inv, Acid invertase; Ald, aldolase; cFBPase, cytosolic Fru-1,6-bisphosphatase; CS, citrate synthase; FK, fructokinase; GK, glucokinase; Neutral Inv, neutral invertase; pFBPase, plastidial Fru-1,6-bisphosphatase; Succ-CoA, succinyl-CoA ligase; SuSy, Suc synthase.
These data are in agreement with previously published data (Steinhauser et al., 2010). For example, the maintenance of high Suc phosphate synthase (SPS) activity at ripening is confirmed. However, this dataset covers earlier developmental stages (8, 15, and 21 DPA) corresponding to cell division and early cell expansion, at which time most of the activities were much higher than later stages.
Expressing Enzyme Activities per Protein Content Minimizes the Influence of Vacuolar Expansion
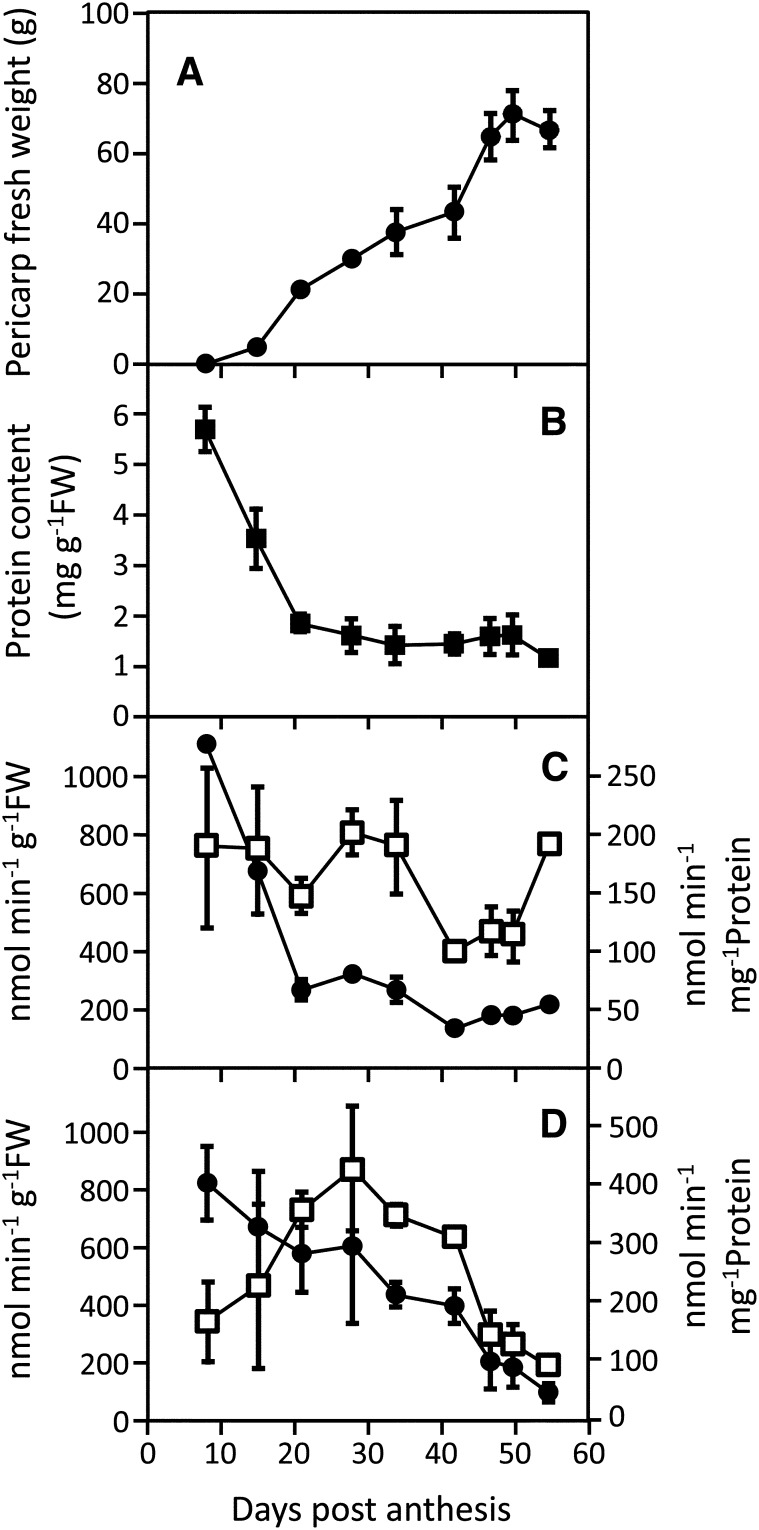
Expansion of the vacuole in fruit pericarp cells (Amemiya et al., 2006) probably explains a large proportion of the variations during development for enzyme activities expressed on a fresh weight basis (Steinhauser et al., 2010), especially during the first 20 d. Consistently, although fruit pericarp biomass increased (Fig. 3A), protein content decreased sharply from 8 to 21 DPA, and then, it tended to stabilize until ripening (Fig. 3B). Figure 3, C and D compares developmental changes expressed on fresh weight and protein basis for fructokinase and PEPC, respectively. Whereas fructokinase decreased in both cases, PEPC showed very different patterns. Indeed, when expressed on fresh weight basis (nanomoles per minute per gram fresh weight), it decreased throughout fruit development, but when expressed on protein basis (nanomoles per minute per milligram protein), it increased during early stages, peaked at 28 DPA, and then, decreased until ripeness. Strikingly, SlPPC2 (for Solanum lycopersicum sérine/thréonine protéine phosphatase 2C) transcripts, which encode a fruit-specific PEPC, have also been shown to peak during cell expansion (Guillet et al., 2002). Thus, assuming that the dilution effect caused by vacuole expansion would be confounding for the interpretation of a large proportion of data, all values have been expressed per unit of protein from this point.
Figure 3.
Pericarp weight (A), protein content (B), fructokinase (C), and phosphoenolpyruvate carboxylase (D) activity during tomato fruit development. Fruit age is expressed as DPA, pericarp weight is expressed in grams ± sd (n = 9), and enzyme activities are expressed in nanomoles per minute pergram fresh weight− (black circles) and nanomoles per minute per milligram protein− ± sd (white squares; n = 5).
Hierarchical Clustering Reveals Tight Associations between Enzymes Activities and Developmental Phase
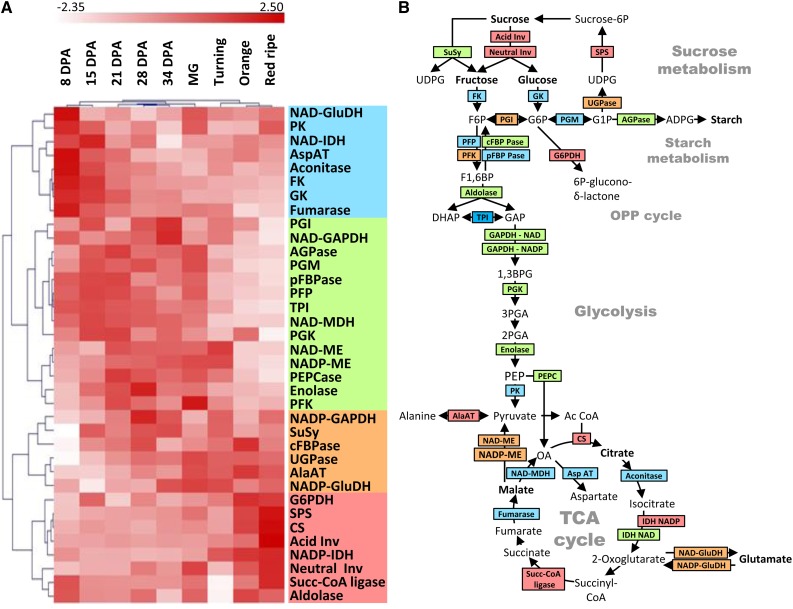
Classically, fruit development is divided in three main phases: cell division (roughly corresponding to the 8- and 15-DPA samples), cell expansion (corresponding to 21-, 27-, and 34-DPA and Mature Green samples), and ripening (corresponding to Turning, Orange, and Red Ripe samples). To investigate whether changes in enzyme activities could be assigned to these three phases, a hierarchical clustering analysis based on Pearson’s correlations was performed on mean-centered data obtained for trusses 5–7 scaled to unit variance and displayed as a heat map; white corresponds to the lowest activity (but not absence of activity), and dark red corresponds to the highest activity (Fig. 4A). It first appeared that the unsupervised clustering analysis was always able to classify the nine developmental stages based on enzyme activities. Thus, 8 and 15 DPA (cell division), 21, 28, and 34 DPA and Mature Green stage (cell expansion), and Turning, Orange, and Red Ripe stages (ripening) were found in three well-separated clusters. The clustering of enzymes highlighted four main clusters.
Figure 4.
Hierarchical clustering analysis of enzyme activity profiles throughout development and ripening of fruit obtained under optimal growth conditions. A, The clustering analysis was performed on activities expressed on a protein basis by Pearson’s correlation, mean centered, and scaled to unit data. Columns correspond to nine developmental stages, and rows correspond to enzyme activities. The four main enzyme clusters are highlighted with a colored bar on the left. B, Simplified drawing of central metabolism in plant. The color code corresponds to the clusters selected in A. Blue, activities highest during cell division and beginning of cell expansion; green, activities highest during cell expansion; orange, activity peaking at late expansion; red, activities highest at ripening. Abbreviations for enzymes are the same as in Figure 2.
The first cluster groups enzymes with high capacities during cell division (blue cluster) and to a lesser extent, at the beginning of cell expansion (until 21 DPA) followed by a slow decrease (with the exception of NAD-Glu dehydrogenase [NAD-GluDH] and PK, which show an increase at Mature Green stage and during ripening).
Enzymes of the second cluster presented a clearly different pattern (green cluster), with lower activity during cell division (8 and/or 15 DPA) followed by an increase to reach a plateau between 21 DPA and Mature Green stage. Their activities then decreased more or less gradually until the end of ripening. Thus, this cluster characterizes cell expansion.
The pattern of the third cluster (orange cluster) was similar to the second one, with activities increasing during cell expansion and maxima reached close to the Mature Green and/or Turning stages followed by a plateau or even a slight decrease during ripening.
The fourth cluster (red cluster) contained enzymes with large increases in activity during ripening between Turning and Orange and/or Red Ripe stage, with mainly enzymes related to hexoses and Suc (Suc phosphate synthase; both acidic and neutral invertase) or citrate synthase (EC 2.3.3.1).
As shown in Figure 4B, the four clusters not only match developmental phases but also, correspond to pathways or subpathways in central metabolism. For instance, the first cluster associated to cell division contained glucokinase and fructokinase as well as most enzymes of the TCA cycle. The cell expansion cluster could be clearly assigned to glycolysis and chloroplastic pathways (starch synthesis and the Calvin–Benson cycle). The orange and red clusters comprised enzymes that are directly involved in reactions leading to metabolites accumulated during ripening, particularly invertase and citrate synthase.
Principal Component Analysis of Enzyme Activities Throughout Fruit Development
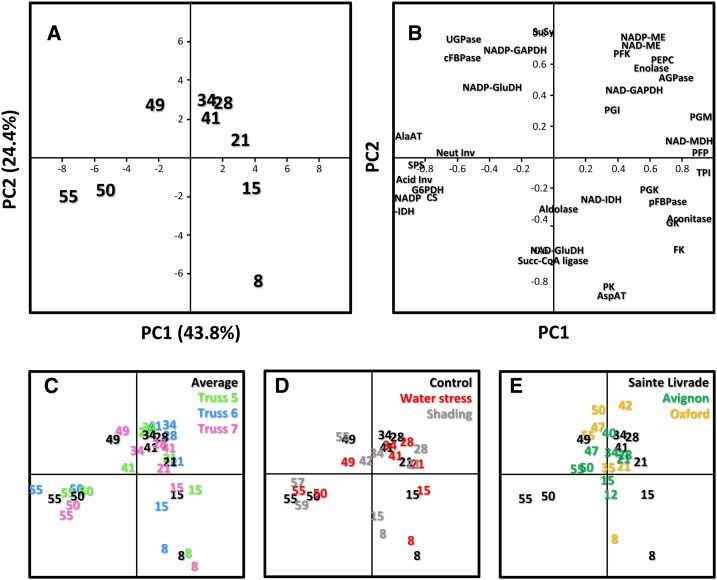
Given the large number of variables, principal component analysis (PCA) was performed to facilitate the comparison between fruits obtained under various conditions. For this comparison, a PCA was first performed on averaged data obtained from all fruits grown under optimal conditions. The score plot (Fig. 5A) obtained for fruits grown under control conditions clearly distinguishes the nine developmental stages. The first principal component (PC1), which explains 43.8% of total variance, separates all green stages (from cell division to Mature Green; Fig. 5A, right side) from ripening stages (from Turning to Red Ripe; Fig. 5A, left side). Because, for all stages except 8 DPA, the score on PC1 decreases when DPA increases, PC1 can be interpreted as expressing time. In contrast, the second principal component (PC2), which explains 24.4% of total variance, clearly separates the cell expansion phase (from 21 DPA to Turning) from the two other phases. Thus, PC2 can be interpreted as expressing transitions between the three phases of fruit development.
Figure 5.
Comparison of enzyme activity profiles obtained in fruits grown on different trusses under optimal or suboptimal growth conditions and at different locations by PCA. Numbers indicating the coordinates correspond to fruit age. A, Scores plot obtained for the reference culture conducted in Sainte-Livrade, France (numbers in black) and subsequently used for comparison. B, Corresponding loadings plot. C, Scores plots obtained by adding data obtained for trusses 5 (numbers in green), 6 (numbers in blue), and 7 (numbers in pink). D, Scores plots obtained by adding data obtained under water shortage (numbers in red) and shading (numbers in gray). E, Scores plots obtained by adding data obtained from fruits grown in Avignon, France (numbers in green) and Oxford, United Kingdom (numbers in orange). Abbreviations for enzymes are the same as in Figure 2.
The loading plot (Fig. 5B) indicates that all enzymes participate in a balanced way in the separation of the developmental stages, because the loadings are relatively high for all of them. Positions of enzymes, thus, correspond to the stages at which they are highest.
PCA analysis was next used to compare developmental changes in enzyme activities of fruits grown on different trusses and under different conditions. To facilitate interpretation, loadings and scores of controls were maintained, and data obtained under other growth conditions were added as supplementary individuals (Lê et al., 2008). Thus, the closer to the control that they are, the smaller the distance on the PCA plot.
High Reproducibility of Enzyme Time Courses from Truss to Truss
As mentioned in the introduction, a range of environmental and physiological variables influencing fruit development are likely to vary from truss to truss, and one well-known consequence is a variation in fruit metabolite composition (Winsor, 1979). To assess whether seasonal variations would be associated with changes at the level of the activome, a PCA was performed for trusses 5–7, which were obtained between July and the end of August (Fig. 5C). It suggests that differences between the three trusses were very limited. This suggestion was confirmed by ANOVA and Tukey’s grouping test (Supplemental Table S2). Considering each enzyme activity throughout fruit development, no significant difference (P < 0.05) was found between the three trusses (data not shown). A few significant differences appeared when grouping time points according to the stage of development. Thus, during cell division (8 and 15 DPA), stromal Fru-1,6-bisphosphatase, PFP, and NAD–glyceraldehyde-P dehydrogenase (NAD-GAPDH) were significantly different (P = 0.04, 6E-3, and 0.03, respectively), although the coefficient of variation (CV) was below 15% for Fru-1,6-bisphosphatase and PFP and below 21% for NAD-GAPDH. During cell expansion, significant differences were found for aconitase (P = 0.05, CV = 20%) and NAD-GAPDH (P = 2E-3, CV = 22%). Finally, during ripening, no significant difference was found for any of 36 enzymes. The fact that differences observed between trusses were very limited might be explained by growth conditions being optimal. Assuming that light intensity (Guan and Janes, 1991) and watering (Mitchell et al., 1991) are among the variables exerting the strongest influence on tomato fruit growth and quality, we next investigated their effects by profiling enzyme activities in fruits obtained under shading or water limitation.
Comparison of Developmental Changes in Enzyme Activities of Fruits Grown under Optimal Conditions, Water Shortage, and Shading
For fruits obtained under water shortage, the PCA plot indicates that there were very few differences with control fruits (Fig. 5D; Supplemental Table S3), and most of the differences found were not significant, independent of the growth phase (Supplemental Table S4). Exceptions were aconitase (P = 0.02, CV = 12%), stromal Fru-1,6-bisphosphatase (P = 6E-5, CV = 21%), and NAD-IDH (P = 2E-5, CV = 35%) during cell division, aconitase (P = 0.04, CV = 11%) during cell expansion, and finally, Ala-AT (EC 2.6.1.2; P = 6E-4, CV = 23%) and phosphoglycerokinase (P = 1E-6, CV = 11%) in Red Ripe fruits.
For fruits obtained under shading, the PCA plot (Fig. 5D) indicates that, at 8 and 15 DPA, fruits were clearly divergent from the control fruits. Indeed, during cell division, seven of the measured activities were significantly different (i.e. aldolase [P = 5E-3, CV = 21%], stromal Fru-1,6-bisphosphatase [P = 6E-5, CV = 15%], NAD-GAPDH [P = 5E-3, CV = 22%], glucokinase [P = 0.02, CV = 23%], NAD-IDH [P = 2E-5, CV = 57%], PFK [P = 9E-3, CV = 20%], and PFP [P = 1E-3, CV = 19%]). During cell expansion, there were fewer differences. Only PEPC showed a significantly lower activity in shaded fruits (P = 9E-4, CV = 15%), whereas glucokinase (P = 0.03, CV = 21%) and UGPase (P = 2E-7, CV = 11%) activities were higher. During ripening, most differences were not significant, and only 11 enzymes (aconitase, Asp-AT, FBP-aldolase, cytosolic Fru-1,6-bisphosphatase, Glc-6-P dehydrogenase [G6PDH], PEPC, PFP, phosphoglycerokinase [PGK], PK, triose-P isomerase, and UGPase) were significantly different (but with an average CV of less than 21%).
Comparison of Developmental Changes in Enzyme Activities of Fruits Grown at Three Locations
To increase heterogeneity in growth conditions, fruits obtained from two other batches of plants grown in glasshouses in Oxford (United Kingdom) during early spring and Avignon (France) during late spring were analyzed, and data were compared with the previous data (controls of Sainte-Livrade culture). It is worth mentioning that, in addition to different light and temperature regimes, Oxford and Avignon used pots and soil, whereas plants were grown in Sainte-Livrade hydroponically. Figure 5E presents the PCA score plot obtained for Oxford and Avignon samples (Supplemental Table S5). Contrary to the Sainte-Livrade data, these fruit were staged according to DPA (“Materials and Methods”) and not standards of the Organisation for Economic Co-operation and Development (http://www.oecd.org). Globally, the PCA did not clearly separate the experiments, confirming that enzyme activities tend to follow a unique trajectory throughout fruit development. However, samples collected at 8 DPA in Oxford behaved differently, appearing very similar to those samples collected under shading at the same stage in Sainte-Livrade. At the latest harvested stages (55 d), Oxford and Avignon samples were very similar to samples from Sainte-Livrade collected at 42 DPA or Mature Green, which suggests that they were delayed in their development compared with Saint-Livrade. ANOVA and Tukey’s test performed for cell division, cell expansion, and ripening stages confirmed such differences between the experiments (Supplemental Table S6). Thus, during cell division, 30 enzymes were significantly different between the three locations, and the average of the variation coefficients calculated for each enzyme was 38%. During cell expansion, variability decreased, with 22 enzymes being significantly different and an average of variation coefficients of less than 18%. During ripening, differences were again higher, with 27 enzymes statistically different and an average CV of 27%. It was noticeable that enzymes responsible for metabolite accumulation (acid invertase and citrate synthase) remained much lower in Oxford and to a lesser extent, Avignon samples than Sainte-Livrade experiment, even in shaded fruits, again suggesting that fruits of these experiments were delayed in their ripening. The fact that, at young stages, Oxford samples resembled those samples obtained under shading suggests that the plants were carbon limited. Such limitation would also explain their slow development. This finding suggests that most differences found for activities were a consequence of differences in rates of fruit development. It is worth mentioning that differences were much lower when expressing enzyme activities on a fresh weight basis (data not shown).
We conclude that, throughout fruit development and with only a few exceptions, activities of enzymes of central metabolism follow a pattern that is only weakly influenced by the environment.
Metabolites Are More Sensitive to Growth Conditions Than Enzyme Activities
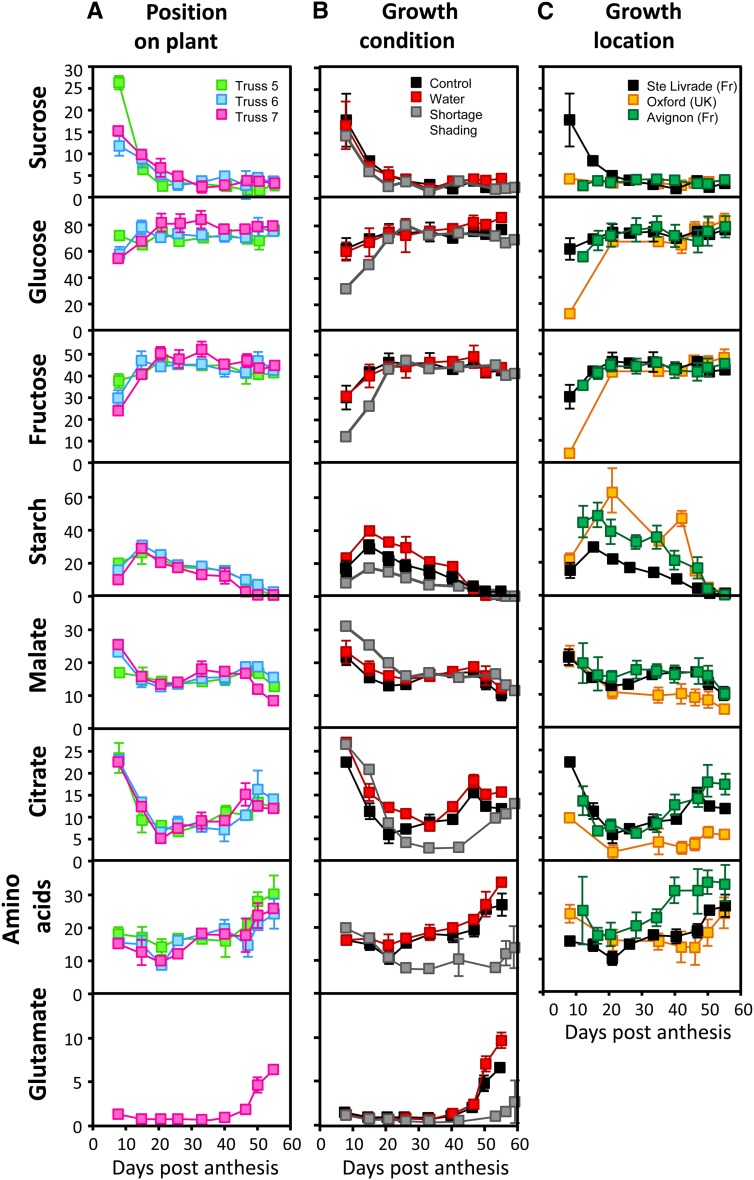
Major metabolites, such as carbohydrates (Glc, Fru, Suc, and starch), organic acids (citrate and malate), and also, total amino acids, as well as protein content were measured in the pericarp during fruit development. Figure 6A presents the metabolite content depending on the truss position on the control plants (Supplemental Table S7). The ANOVA and Tukey’s test showed that most metabolite concentrations were not statistically different from truss to truss (Supplemental Table S8) or when significant, that differences were relatively low (average CV = 12.3%). The largest changes in metabolites occurred during the first 15–20 d of development. Protein content (Fig. 3) and Suc (Fig. 6A) decreased from 8 to 21 DPA and then, reached a plateau until the end of ripening. In contrast, Glc and Fru concentrations increased between 8 and 15 DPA and then, remained constant. Starch increased from 8 to 15 DPA, was highest at 15 DPA, and decreased until the Red Ripe stage. Citrate and malate presented a similar profile from 8 DPA to Mature Green stage (strong decrease during cell division followed by a plateau or slight increase), and then, citrate was quickly accumulated while malate was decreasing. Total amino acids slightly increased until Mature Green and were accumulated much stronger during ripening. Most of the amino acids followed the same pattern and tended to increase or reach a plateau during fruit development and ripening, with a few exceptions (Supplemental Table S9). Thus, γ-aminobutyrate decreased slightly from 8 DPA to Red Ripe stage, Pro decreased sharply from 8 to 21 DPA and then, remained constant until Red Ripe, and Tyr increased during cell division, remained constant during cell expansion, and decreased during ripening. Finally, the most striking fact was that Glu increased sharply during maturation, representing up to 50% of the total amino acids. Figure 6B compares metabolite profiles in fruits of control and stressed plants. The protein content was never significantly different between water shortage and control conditions (Supplemental Table S10). The difference between shading and control conditions was always below 17%, even if significant at 15 DPA, Mature Green, Orange, and Red Ripe stages. Suc content was not different between the treatments, except at the Red Ripe stage. Glc and Fru contents were strongly affected by shading but mainly, during early fruit development. Differences were also found during ripening for Glc, which was lower under shading and higher under water shortage. Starch content presented the biggest differences between growth conditions, with a CV ranging from 27% to 45%. Starch was always highest under water shortage and lowest under shading. Few differences were observed in organic acid contents between control and water shortage fruits, where they were slightly higher. Under shading, malate was higher during the first 15 d of development. In contrast, citrate was not different during cell division, became lower during cell expansion, and strikingly, recovered at ripening. Similar to organic acids, amino acids were slightly higher under water shortage than control conditions. Under shading, amino acids were not different during cell division but then, decreased strongly compared with the two other treatments during cell expansion and ripening (see also Supplemental Table S11).
Figure 6.
Changes in major metabolites throughout tomato fruit development. Fruit age is given in DPA. Total amino acids, Glc, Fru, malate, and citrate are expressed in micromoles per gram fresh weight− ± sd, and Suc and starch are expressed in Glc equivalents (micromoles glucose per gram fresh weight− ± sd). A, Data obtained from fruits grown in Sainte-Livrade under optimal growth conditions on trusses 5–7 (n = 5). B, Data from fruits grown under control (means for trusses 5–7), water-limited (n = 5), and shaded (n = 5) growth conditions. C, Data obtained from fruits grown in Sainte-Livrade (same as in B), Avignon (n = 5), and Oxford (n = 5).
Figure 6C presents the results obtained for the three growth locations. Overall, larger differences were observed between growth locations than between treatments. Fruits from Avignon and Sainte-Livrade cultures had similar profiles for proteins (Supplemental Table S12), hexoses, Suc (except at 8 DPA), and organic acids. The main differences were the amino acids and starch, which were both higher in Avignon than Sainte-Livrade. In fruits collected in Oxford, each metabolite profile was different, especially hexoses at 8 DPA. Profiles of the latter were very similar to those profiles found under shading in Saint-Livrade fruits, suggesting the occurrence of carbon limitation during the first days of development. Later, starch content also became higher in Oxford fruits. In contrast, Oxford fruits contained lower amounts of organic acids. Malate content was, nevertheless, not significantly different for the first 20 d of fruit development, whereas citrate content was always statistically lower than in Sainte-Livrade fruits (Supplemental Table S13).
To summarize these results, metabolite levels were more susceptible to environmental changes than enzyme activities, but the position of the fruit on the plant did not seem to be a major source of variability in metabolite content (results were also obtained with a PCA analysis in Supplemental Fig. S1, A to C). Comparing growth conditions, major differences were observed for starch, amino acids, and organic acids. In contrast (already observed in the cv ‘Cervil’; Gautier et al., 2008), hexoses and Suc seemed to be very stable after cell division was achieved. Glc and Fru concentrations measured during cell expansion were particularly reproducible between trusses, treatments, and growth locations.
Metabolic Responses to the Environment Tend to Decrease at Ripening
As shown above (Fig. 5), most significant differences in enzyme activities were found for the youngest stages of fruit development. For major metabolites, the biggest differences were also observed at the youngest stages (i.e. during cell division and cell expansion until Mature Green stage; Fig. 6). However, as already mentioned, differences were bigger for metabolites than for enzymes and persisted for a longer time. After fruits had attained their final size and underwent ripening, differences between growth conditions or locations tended to decrease. This trend is also visible on the PCA plots (Supplemental Fig. S1, B and C), where the distance between fruits of the same age decreases to ripening. A similar observation has been made with tomato fruits overexpressing hexokinase, where the influence of the genetic manipulation on metabolites decreased during ripening (Roessner-Tunali et al., 2003). Paradoxically, whereas during cell expansion, enzyme profiles were very similar under the various growth conditions, metabolites showed strong differences. Furthermore, the opposite was observed at ripening, because enzymes tended to diverge, whereas metabolites converged. Taken together, these results suggest that the final reprogramming of metabolism occurring at ripening, which includes steps involved in Suc and organic acid metabolism, results in a rather standard composition of fruit flesh.
Expressing Metabolite Content on Fruit Basis Reveals Hidden Events
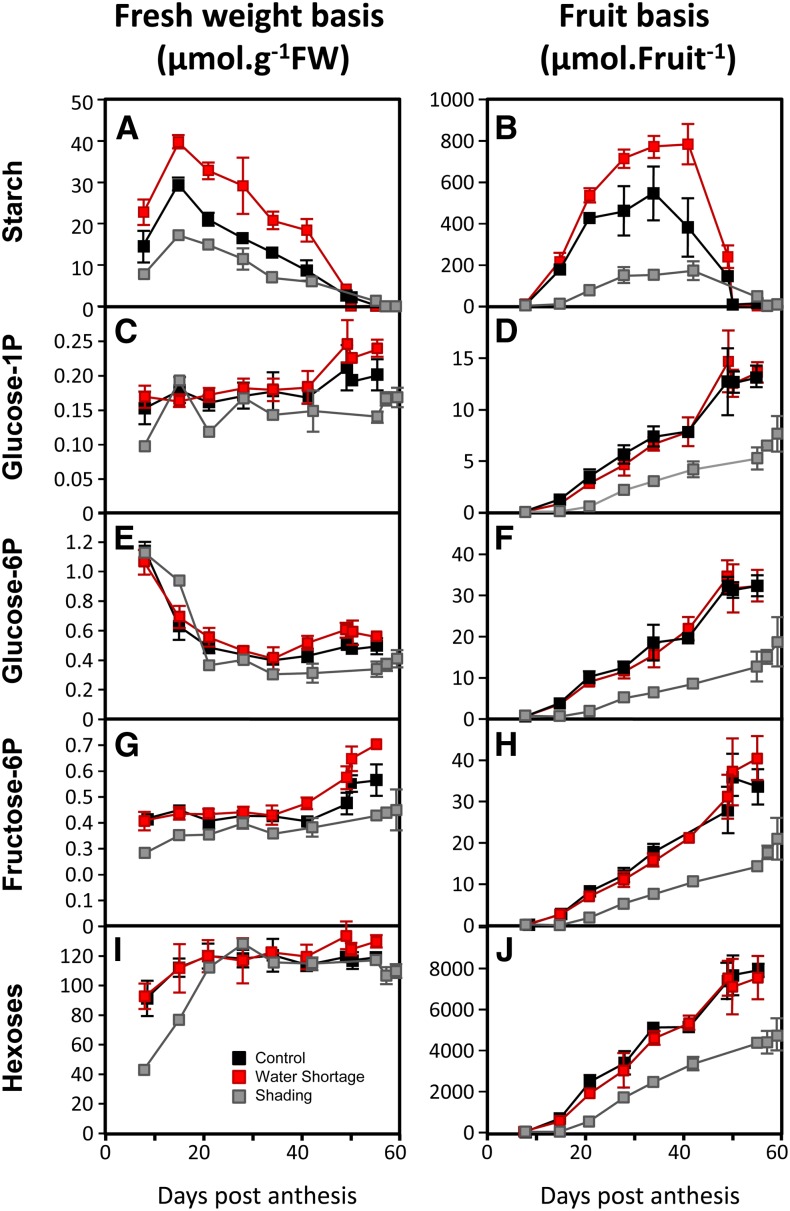
As previously shown, starch was more variable than most metabolites analyzed. When expressed on a fresh weight basis (Fig. 7A), the starch content increased from fruit set to 15 DPA and then decreased until ripeness. When expressed on whole-fruit basis (micromoles Glc equivalents per fruit pericarp; Fig. 7B), starch accumulated until the Mature Green stage and degraded very quickly at the beginning of ripening (between Mature Green and Turning stages, which roughly corresponds to 5 d; Luengwilai and Beckles, 2009). Starch degradation operates through different pathways, leading to maltose or Glc accumulation but also, Glc-1-P as an intermediate by the activity of α-glucan phosphorylase (Zeeman et al., 2007). The hexoses-P Glc-1-P, Glc-6-P, and Fru-6-P were measured in the same samples and expressed on a fresh weight basis (Fig. 7, C, E, and G). Although Glc-1-P was negatively correlated with starch, no relationship was apparent for Glc-6-P and Fru-6-P. When their amounts were calculated on a per-fruit basis (Fig. 7, D, F, and H), it was clear that all three hexoses-P, which probably tend to equilibrate through the activities of PGI and PGM, were strongly increased at the very moment of starch degradation. Furthermore, this increase in hexoses-P, particularly Glc-1-P, seems to be roughly correlated with the amount of starch in the fruit. Indeed, fruits obtained under shading, which had the lowest starch content, also showed the smallest increase in hexoses-P. Taken together, these results suggest that, in maturing fruits, starch degradation results in a significant increase in hexoses-P that are then readily available for metabolic shifts associated with ripening.
Figure 7.
Comparison of changes throughout fruit development in starch, hexoses phosphate, and hexoses when expressed on a fresh or whole-fruit pericarp basis. Fruit age is given in DPA. A, C, E, G, and I, Data are expressed on a fresh weight basis (moles per gram fresh weight [FW]). B, D, F, H, and J, Data are expressed on a fruit basis (micromoles per fruit per pericarp).
When expressed on a fresh weight basis, hexose levels (Fig. 7, I and J) were almost constant during cell expansion and ripening and did not differ between the three treatments. In contrast, when expressed on fruit basis, differences were apparent. In particular and similarly to hexoses-P, the rate of increase in hexoses slowed just before the Mature Green stage but strongly accelerated until the Turning stage. However, the amount of hexoses accumulated during this period represented about 2,500 µmol per fruit in the controls, whereas the remobilization of starch corresponded to only 600–800 µmol in Glc equivalent per fruit. Thus, the strong increase in hexoses was probably, for the largest part, caused by an increase in net sugar import, which has already been suggested (Balibrea et al., 2006). Indeed, starch degradation only represents 25%–30% of hexoses accumulated during ripening and 10% of the hexose content in ripe fruits.
Integration of Changes in Enzyme Activities and Metabolite Levels
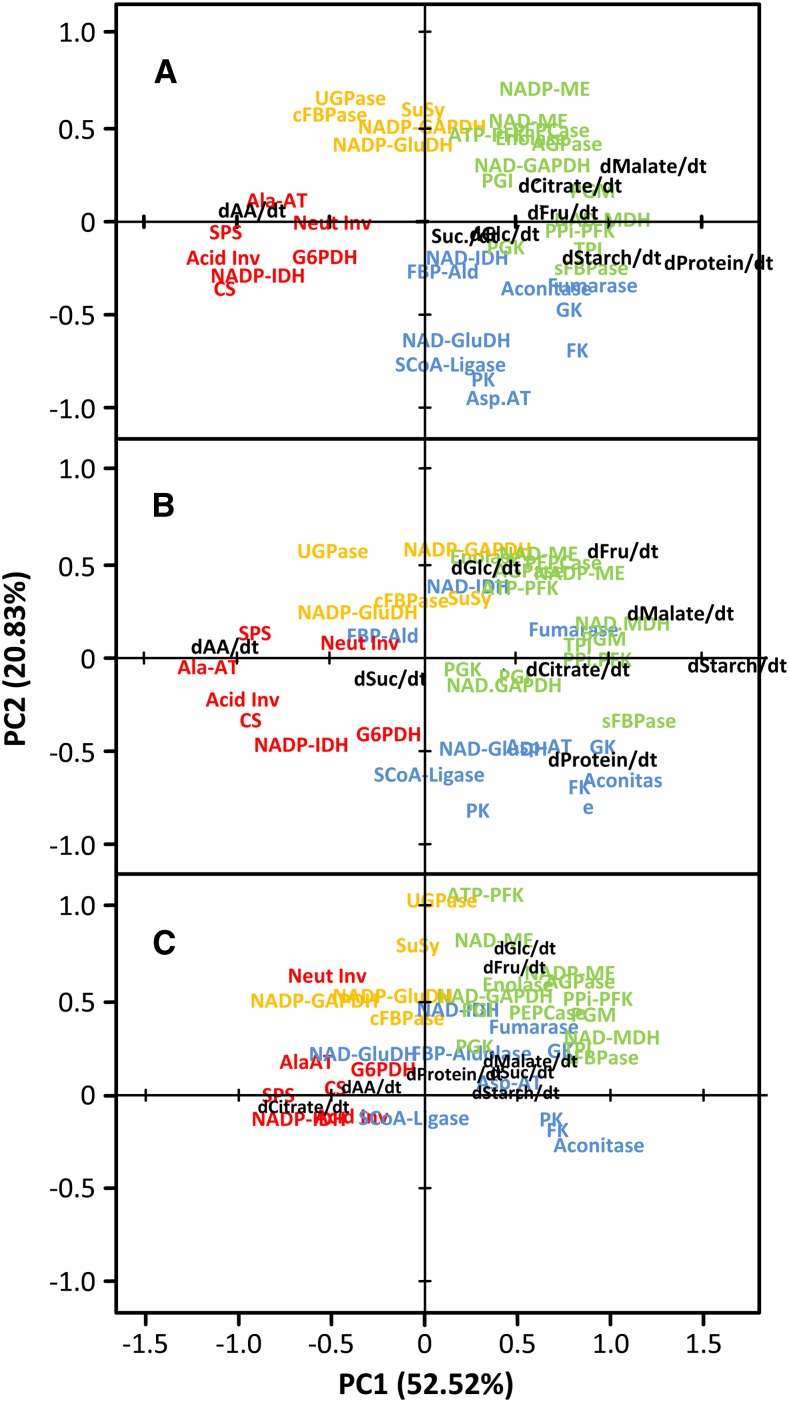
The integration of multilevel metabolic data strongly depends on how the data are expressed. We assume that enzymes can control both metabolite concentrations and fluxes. Among all the possible ways of expressing enzyme activities and metabolite contents or net accumulation rates (per gram fresh weight, per protein, and per fruit), results with the largest scattering of metabolites were obtained for enzyme activities per protein and net accumulation rates of metabolites per fruit (Fig. 8A; Supplemental Figs. S2, S3, S4, and S5 show the complete set). PCA was used to integrate enzyme and metabolite data. Variables have been normalized over the three treatments (control, water limitation, and shading) to enable comparison. Loadings have first been computed with the control dataset (Fig. 8A), and data corresponding to water limitation (Fig. 8B) and shading (Fig. 8C) have then been added to the PCA as additional variables.
Figure 8.
Integration of enzyme activities and metabolites accumulation rates in tomato fruits during development and ripening using PCA. Enzyme activities were expressed on a protein basis, and data for metabolite rates were expressed as per fruit pericarp and per day. Data were centered and scaled before PCA. A, Loadings plot obtained with control data (fruits grown under optimal conditions). B, Loadings plot for additional data obtained under water shortage. C, Loadings plot for additional data obtained under shading. Coordinates for each enzyme activity or metabolite are indicated by text. Text colors indicate the cluster to which enzymes have been assigned using hierarchical clustering (blue, cell division; green, cell early expansion; orange, late expansion; red, ripening; Fig. 4). Abbreviations for enzymes are the same as in Figure 2.
In control fruits, the loadings plot obtained with enzyme activities and net accumulation rates of metabolites was similar to the plot obtained with enzymes only (Fig. 5). Thus, enzymes that have been associated with each developmental phase (Figs. 4 and 5) were still clearly separated. Interestingly, the highest loadings (mainly on PC1) for metabolites and protein content, which correspond to their highest net accumulation rates, colocalized with enzymes peaking at early expansion. The only exception was amino acids, which were associated with enzymes rising at ripening. Strikingly, malate and citrate net accumulation rates were coincident with enzymes involved in interconversions between C3 and C4 organic acids. In contrast, Suc and hexoses were not associated with enzymes involved in Suc metabolism (Fig. 8A). In fruits grown under water limitation, most enzyme loadings were similar to those enzyme loadings obtained with control fruits, whereas metabolites appeared more scattered. For both groups of analytes, differences were more marked on PC2, which suggests the occurrence of shifts in transitions between developmental phases (Fig. 8B). As expected, the loadings plot obtained with shaded fruits was the most divergent. For enzymes, this result was mainly caused by the cluster associated to cell division. For metabolites, differences in loadings were much more marked at both PC1 and PC2 (Fig. 8C).
Considering all three treatments, only two statistical associations were found between a metabolite net flux and an enzyme activity. The closest association was between total amino acids and Ala-AT, which suggests that the strong accumulation of Glu at ripening (by far the largest pool among accumulating amino acids) involves this activity. One additional interesting, although looser, interaction was found for malate and MDH. Other metabolites were associated to groups of enzymes or totally disconnected.
DISCUSSION
This work addresses the effect of the environment on the programming of metabolism in tomato fruits. Surprisingly, it seemed that, within the range of conditions used, enzyme activities were only marginally affected by the environment, whereas time courses of metabolites underwent larger changes in response to growth conditions, especially for starch and amino acids. These changes were attenuated during ripening and not associated with specific reprogramming of enzyme activities.
Reprogramming of Central Metabolism during Developmental Transitions Is Mainly Driven by De Novo Synthesis of Enzyme Clusters
Although statistical analyses (ANOVA and Tukey’s grouping test) indicate that there were significant differences in given activities and stages of development, no atypical trajectories were found for individual enzymes. PCA score plots (Fig. 5A) suggest that the environment was mainly affecting changes over time (on PC1; for example, by slowing down the changes in enzyme activities) without affecting the trajectories. Such shifts in rates of developmental changes could be explained by differences in, for example, temperature or carbon availability.
This analysis reveals that the beginning of each of the three phases of fruit development was characterized by high activities for specific groups of enzymes. Thus, PC2 might be explained by a pronounced de novo synthesis of enzymes (Fig. 5). Then, except for enzymes peaking at maturity, a gradual decrease in activity was observed (Fig. 4), revealing that enzymes were decaying slower than they were made. Slow decay of enzymes might be explained, in part, by most enzymes having a slow turnover, which was suggested for another plant model system (Piques et al., 2009). Another point is that there was obviously no induction of specific enzyme degradation that was associated with developmental shifts. The only exception was NADP-ME, which peaked at Turning stage and almost vanished at Orange stage (i.e. within a few days). We compared our data with transcriptome data available from the literature for comparable developmental series (Carrari et al., 2006; Osorio et al., 2011) but could not find any straightforward relationship between changes in transcripts and the activities of the encoded enzymes, which was also concluded by Steinhauser et al., 2010. In contrast, it is striking that the moments (20–28 and 48–53 DPA in controls) at which changes occurred for enzyme activities almost perfectly match those moments found for more than 80 metabolites from various pathways in another study performed with cv ‘Moneymaker’ fruits (Carrari et al., 2006). This finding suggests that coordinated changes in enzyme capacities, which integrate various levels of regulation, effectively impact the metabolome.
The analysis also reveals that the programming of metabolism throughout fruit development operates at a pathway level (Fig. 4B). Each of four clusters was associated with both a developmental phase and relatively well-defined metabolic sectors. In particular, although cluster 1 contains enzymes involved in the upper part of glycolysis and the TCA cycle, cluster 2 is characterized by the lower part of glycolysis.
Cell Division Is Characterized by a Turbo Design
Cluster 1 contains enzymes involved in glycolysis, especially glucokinase and fructokinase, and their activities were particularly high during cell division. High levels of Glc-6-P were also observed during that phase. The occurrence of high ATP-consuming activities suggests that dividing fruit cells use a turbo design to drive glycolysis (Teusink et al., 1998). In brief, maintaining a low ATP to ADP ratio and high hexose-P levels probably results in high flux through glycolysis. This hypothesis is in agreement with the results by Liu et al., 2007, indicating that the demand in Suc is highest during cell division in tomato fruits. It is also in agreement with previous results obtained with discs of tomato pericarp collected at 21, 35, and 49 DPA, indicating that the highest glycolytic flux is observed in the youngest fruits (Carrari et al., 2006). The fact that PK and TCA cycle enzymes were also high (aconitase, succinyl CoA ligase, and fumarase) reinforces the idea that a fine adjustment of the ATP production to the growth-linked ATP demand is a major issue during cell division (Takahashi et al., 2011).
Logically, fruits are particularly sensitive to carbon availability during cell division, and carbon starvation leads to their abortion (Ruan et al., 2012). Fructokinase, which showed one of the highest variability at 8 DPA, has been shown to play a crucial role in floral initiation and abortion (Carrari and Fernie, 2006). The biggest differences found for sugars between treatments were at this developmental phase, especially for hexoses, which were strongly decreased under shading.
Early Cell Expansion Is Characterized by Anaplerosis
As shown in Figure 4, cell expansion can be subdivided in two steps, which have been assigned to early and late expansion. Cluster 2, which characterizes early cell expansion, includes a group of enzymes involved in the middle part of glycolysis (NAD-GAPDH, PGK, and enolase) as well as PEPC. The strong correlation between these enzymes suggests that they operate together to adjust the level of oxaloacetate available for the TCA cycle, an anaplerotic process that has been proposed as a way to compensate for the loss of carbon induced by the numerous syntheses needed for cell expansion (O’Leary et al., 2011), particularly the accumulation of organic and amino acids, to provide the osmotic driving force (Fig. 6). The PK profile actually strengthens this hypothesis, because when PK was high, PEPC was low and vice versa. Another enzyme associated with cell expansion is ADP-Glc pyrophosphorylase (AGPase; EC 2.7.7.27) which catalyzes the production of ADP-Glc for starch synthesis. Its activity was highest at the beginning of cell expansion, remained high until the Mature Green stage, and then, decreased sharply between the Mature Green and Turning stages, thus mirroring starch accumulation and breakdown. Suc synthase, which reached its highest level during that phase, has been shown to control sink strength in growing tomato fruit (Wang et al., 1993) and could be involved in the synthesis of cellulose and other cell wall components, which was suggested for other model systems (Winter et al., 1997). The activity of G6PDH peaked reproducibly at the beginning of cell expansion. This finding might reflect an activation of the pentose phosphate pathway for the biosynthesis of nucleotides. It is, indeed, striking that the G6PDH peak coincides with the strong increase in ploidy resulting from an increased rate of endoreduplication, which is observed in developing tomato fruit at the beginning of cell expansion (Nafati et al., 2011). NADPH produced by the oxidative pentose phosphate cycle may also be used for lipid synthesis that is required for cell expansion.
Cell Expansion Is Driven by Hexose Content
In plants experiencing mild water shortage, growth is usually affected earlier than photosynthesis, leading to carbon excess (Muller et al., 2011). It is likely that more carbon was available to fruits grown on water-limited plants, but they did not increase their concentration in soluble sugars during expansion. Instead, more starch was accumulated, perhaps in relation with the sugar-dependent posttranslational activation of AGPase, which was shown for potato tubers (Tiessen et al., 2002) and leaves of several species (Hendriks et al., 2003). Consistently, starch accumulation was weak in fruits grown under shading. Although these observations confirm that net starch accumulation is triggered by the amount of available carbon (N'tchobo et al., 1999), there was no correlation between starch and soluble sugars during cell expansion, probably because soluble sugars are mainly located in the vacuole. Intriguingly, during cell expansion, concentrations in hexoses and Suc were nearly identical across all growth conditions tested here. Hexoses are, by far, the most abundant metabolites in the pericarp of developing fruits. Their contribution to the osmotic potential can be estimated at −0.4 MPa (150 mosmol). Strikingly, a previous study reported an identical value in growing fruits of Solanum lycopersicum obtained under optimal conditions but also, salt stress (Bolarin et al., 2001). Such osmotic potential represents at least 50% of the fruit osmotic potential, which suggests that hexoses are key drivers of cell expansion. Then, the apparent stability of hexoses may simply result from an adjustment of cell expansion, because fruits of shaded and water-limited plants were smaller. In the case of shaded plants, sugar supply to the fruits may limit cell expansion, whereas under water limitation, at equal sugar concentrations, turgor potential would be less because of a decreased water potential. Thus, it can be concluded that, in this model system, a mechanism determining vacuolar hexose concentrations plays a major role in cell expansion.
Enzyme Programming during Late Expansion Anticipates Ripening
In tomato, the transition between the end of cell expansion and ripening takes place within a few days at the end of the Mature Green stage. It involves highly coordinated processes involving ethylene-sensitive and ethylene-insensitive pathways (Giovannoni, 2001). Interestingly, several enzymes, grouped in cluster 3 (Fig. 4), increased gradually during cell expansion to eventually peak just before the beginning of ripening, suggesting the anticipation of ripening. They include PGI, PFK, and UGPase, which are probably involved in the recycling of the large amounts of hexoses-P released by starch degradation. This cluster also contains both MEs. In tomato fruits and grape berries, NADP-ME has been shown to be involved in respiration during ripening, providing pyruvate and NADPH as substrates for respiration (Drincovich et al., 2001). Furthermore, it has recently been shown that fruits of transgenic tomato plants with NADPH-ME activity had lower starch levels at the breaker stage in connection with a decrease in the redox activation of AGPase (Osorio et al., 2011). The fact that this enzyme shows a sharp peak at the Mature Green stage might be related to its involvement in the forthcoming climacteric crisis, which will result in a strong increase in respiration (Hill and ap Rees, 1994) and initiate the beginning of ripening. The mitochondrial enzyme NAD-ME has been proposed to produce pyruvate from malate to maintain the TCA cycle when MDH is inhibited by high levels of NADH and oxaloacetate (Wedding, 1989). The cluster also contains NADP-GluDH, an enzyme with a role that remains unknown, and it has been proposed to be located in plastids (Miyashita and Good, 2008). Our results confirm that it is not the same enzyme as NAD-GluDH, because their activity profiles were different. Like NAD-GluDH, NADP-GluDH did not respond to carbon limitation (Supplemental Table S2, nonsignificant difference between shaded and control plants), but both activities increased strongly between expansion and ripening, when Glu, an important component of tomato fruit quality (Carrari et al., 2007), started to accumulate. Although it is assumed that NAD-GluDH catalyses the oxidation of Glu in the mitochondrion (Labboun et al., 2009), the opposite reaction could take place in the plastid (or the cytosol) by NADP-GluDH, suggesting that, at ripening, these activities could be involved in different processes or participate in a Glu shuttle between both compartments. Strikingly, the constitutive overexpression of a fungal NADP-GluDH in tomato has been shown to result in a twofold to threefold increase in Glu content in fruits (Kisaka and Kida, 2003).
Ripening Involves Both Catabolism and Accumulation of Key Metabolites
Enzymes associated with ripening are essentially grouped in cluster 4 (red), although several enzymes found in cluster 1 (blue) also increased during that phase (Fig. 4). All in all, about one-third of the enzymes studied here significantly increased during ripening, and some increased dramatically.
Concomitantly, fruit biomass accumulation rate was increased, whereas protein content was maintained or even slightly increased (Fig. 2), which suggests a global rise in protein synthesis, like in banana (Brady et al., 1970). Concomitantly, there was a peak in the accumulation rate of hexoses, which are likely to be stored in the vacuole. Clearly, both protein synthesis and hexose accumulation in the vacuole are processes that imply an increase in energy demand. This finding is in line with the fact that activities of enzymes involved in glycolysis (FBP-aldolase and PK) and the TCA cycle (citrate synthase and succinyl CoA ligase) were strongly increased. However, glucokinase and fructokinase went on decreasing to reach very low activities. Starch degradation can, therefore, be seen as an alternative source of hexose-P as substrates for respiration that can be remobilized within a short period of time. The concomitant increases in Glu-1-P and to a lesser extent, Glu-6-P and Fru-6-P, when expressed on a fruit basis, support this view.
Metabolic intermediates were not only used for respiration, because ripening also resulted in the accumulation of citrate, which is consistent with high citrate synthase activity, and amino acids, particularly Glu. The increases in NADP-IDH and Ala-AT could be associated with the massive accumulation of Glu through the synthesis of 2-oxoglutarate in the cytosol (Sulpice et al., 2010) and its subsequent conversion into Glu. This hypothesis is supported by the strong correlation found between amino acids (and thus, Glu) and Ala-AT.
The strong increase in invertase activity has been proposed to account for the accumulation of hexoses in cultivated tomato (Yelle et al., 1991). The fact that the activity of SPS also strongly increased at ripening seems contradictory at first sight, because the latter enzyme is involved in the synthesis of Suc, which is a substrate of invertases. However, in other species related to tomato, ripening fruits do not enhance their invertase activity and accumulate Suc instead of hexoses (Yelle et al., 1991). Furthermore, it has been shown that SPS dramatically increases in maturing fruits of S. pennellii (Steinhauser et al., 2010). Also, increasing the capacity of Suc synthesis at ripening can be associated with the increase in carbon import and remobilization of starch, both processes that lead to hexoses and hexose-P.
Fruit Quality Depends for a Large Part on Carbon Import During Ripening
During ripening, concentrations in metabolites of tomato fruits grown under contrasted environments were converging, thus tending to generate a reproducible metabolic phenotype, despite differences in fruit size and morphology. One interpretation is that final reprogramming of metabolism participates in generating a reproducible fruit quality as a guarantee to attract the consumer, who will eventually disseminate the seeds. However, it is important to notice that sugar import during ripening might be essential to achieve such standard fruit quality. Indeed, starch would represent only 10% of the final sugar content if totally converted into hexoses, whereas late carbon import would represent 20%. In other words, harvesting such fruits at the Turning stage (followed by shelf maturing) would inevitably result in poorer quality, even when they are grown under optimal conditions (Beckles, 2012). It is worth mentioning that this finding probably explains, for a large part, why garden tomatoes are usually considered better than commercial fruits.
CONCLUSION
The surprisingly high reproducibility and consistency of enzyme time courses monitored in fruits of S. lycopersicum ‘Moneymaker’ throughout their development suggest that the environment exerts only a minor influence on the programming of fruit metabolism. Whereas enzyme profiles of young fruits are the most variable, they tend to converge—sooner or later—in expanding and maturing fruits. This result is reflected by the rather reproducible composition in metabolites at maturity. One hypothesis is that fruit metabolism would have evolved (or been selected) to guarantee reproducibility of the edible character, essential for seed dispersion. It will be important to investigate enzyme profiles of other genotypes, particularly wild relatives, to verify if this finding can be generalized. Then, knowing that each developmental phase is characterized by a reproducible and consistent enzyme profile provides new opportunities. A minimal set of enzymes could be selected to diagnose developmental stages in fruits, which could, for instance, be useful when studying mutants impaired in visual traits. It will also be useful to search for the molecular bases of the metabolic shifts that accompany developmental changes. Metabolic pathways could also be modeled for each developmental phase to get a better understanding of how metabolism participates in fruit growth and quality. Importantly, the parameterization of such models with enzyme data will be much easier than initially thought. It can be expected that such an approach would ultimately pinpoint future targets, including maximal activities, kinetic properties, or posttranslational mechanisms, for fruit improvement.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of the Solanum lycopersicum ‘Moneymaker’ variety were provided by Alisdair Fernie (Max Planck Institute of Molecular Plant Physiology). They have been used in three distinct experiments, which took place in Sainte-Livrade (France), Avignon (France), and Oxford (United Kingdom). All seeds were from a common lot.
In the Sainte-Livrade experiment (southwest of France, 44° 23′ 56″N and 0° 35′ 25″E), plants were cultivated under realistic production conditions between June and October of 2010. Seeds were germinated in a nursery on April 30, 2010. Seedlings were transferred to rockwool blocks on June 4, 2010 at a density of 2 plants m−2 in a 350-m2 greenhouse. The nutrient solutions were supplied with a drip system, and the volume of water supply was adjusted to the climate. Three growing scenarios have been performed: (1) control with commercial production practices (276 plants), (2) water shortage by providing only one-half of the volume of nutritive solution compared with the control plant (138 plants), and (3) low light treatment by hanging a shadow net in a part of the greenhouse (the shadow net stopped 60% of the photosynthetically active radiation; 138 plants). Stresses were applied at the flowering of the fourth truss. Environmental variables (temperature, relative humidity, and photosynthetically active radiation and light intensity) were recorded on data loggers (Delta-T Devices). Lateral stems were systematically removed. Each flower anthesis was recorded, and trusses were pruned at six developed fruits to limit fruit size heterogeneity.
In the Avignon experiment (south of France, 43° 57′ 00″N and 4° 49′ 01″E), on January 31, 2011, seeds were sown in petri dishes containing Murashige and Skoog medium (Murashige and Skoog, 1962). Plants with five growing leaves were transplanted into 5-L pots containing potting soil (H21 Tref; Tref EGO Substrates B.V.) at a density of 1.8 plants m−2 in a glasshouse. Plant nutrition and disease control were in accordance with the commercial practices. Water was supplied with a drip irrigation system to maintain 20%–30% drainage. Flowers were mechanically pollinated three times a week. Each flower anthesis was recorded, and trusses were pruned at five (rather than six given that less light was available than in the other experiments) developed fruits to limit fruit size heterogeneity.
In the Oxford experiment (southeast of England, 51° 45′ 07″N and 1° 15′ 28″W), seeds were germinated on John Innes potting compost no. 3 (The John Innes Manufacturer Association) and grown for 30 d before potting into 10-L pots in the same compost with the addition of slow-release fertilizer grains (Osmocote Exact Standard by Scotts with 5-month release time). Plants were grown in a temperature-controlled and light-controlled greenhouse (16 h of light from 6 am to 10 pm and 8 h of dark) under high-pressure sodium and metal halide lamps at a density of 4 plants m−2. Environmental conditions were recorded using a Hobo U12 data logger (onsetcomp.com) set to one recording per hour. On average, the light intensity was 200 μmol m−2 s−1 during the illumination period, with an average temperature of 25.7°C and 32% relative humidity. During darkness, the average temperature was 19°C, with 42% relative humidity. Plants were watered daily from the top, with the addition of liquid fertilizer (Levington Tomorite) one time per week when the plants started flowering. Fruit samples were collected between March 2 and May 4, 2011 from plants sown on November 26, 2010 and January 4, 2011. Trusses were limited to six per plant, each flower anthesis was recorded, and trusses were pruned at six developed fruits to limit fruit size heterogeneity.
Harvest and Sample Processing
In the Sainte-Livrade experiment, nine developmental stages from 8 DPA to Red Ripe stage were harvested on three different trusses (trusses 5–7). Six stages were based on the age of the fruit (8, 15, 21, 28, 34, and 42 DPA [Mature Green]). Three stages were based on the fruit color according to OECD color gauge: Turning (grade 4), Orange (grade 8), and Red Ripe (grades 11 and 12), which roughly correspond to 47, 50, and 55 DPA, respectively. For each sample, three biological replications were prepared, with a minimum of four fruits per replication. In the Oxford experiment, seven developmental stages were harvested (7, 21, and 35 DPA, Mature Green, Turning, Orange, and Red Ripe), with a minimum of four biological replications. In the Avignon experiment, fruits were harvested on trusses 4 and 5 at 12, 15, 21, 28, 34, 42 (Mature Green), 47 (Turning), 50 (Orange), and 55 DPA (Red Ripe).
For the sample preparation, fruits were cut; seeds, jelly, and placenta were removed, and small pieces (approximately 1 × 0.2 cm) of pericarp were immediately deep frozen in liquid nitrogen in less than 1 min from the fruit harvest to the frozen piece of tissue. Frozen samples were then ground into fine powder with liquid nitrogen. Aliquots of about 20 mg were then weighted in 1.1-mL Micronic tubes and stored at −80°C until additional analysis.
Chemicals
All chemicals and substrates were purchased from Sigma-Aldrich Ltd., except acetyl CoA, ATP, dithiothreitol, leupeptin, NAD, NADH, NADP, NADPH, and phosphoenolpyruvate, which were purchased from Roche Applied Science. All enzymes were purchased from Roche Applied Science, except aldolase (from rabbit muscle), citrate synthase (from porcine heart), glycerokinase (from Escherichia coli), phosphoglycerokinase (from Saccharomyces cerevisiae), phosphoglucomutase (from rabbit muscle), and triose phosphate isomerase (from rabbit muscle), which were purchased from Sigma-Aldrich Ltd. Bradford reagent was purchased from Bio-Rad.
Enzyme Activity Measurements
Aliquots of about 20 mg fresh weight were extracted by vigorous shaking with 500 µL extraction buffer composed of 20% (v/v) glycerol, 0.25% (w/v) bovine serum albumine, 1% (v/v) Triton-X100, 50 mm HEPES-KOH (pH 7.5), 10 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm ε-aminocaproic acid, 1 mm benzamidine, 10 mm leupeptin, 0.5 mm dithiothreitol, and 1 mm phenylmethylsulfonylfluoride, which was added just before extraction. Enzyme activities were assayed using a robotized platform as described in Gibon et al., 2004; Studart-Guimarães et al., 2005; Gibon et al., 2006; Gibon et al., 2009; and Steinhauser et al., 2010.
UGPase activity was assayed using a protocol adapted from Appeldoorn et al., 1997. The assay consisted of 5 µL extract in 100 mm Tricine-KOH (pH 8.0), 10 mm MgCl2, 2 mm EDTA, 1.2 mm NADP+, 1 mm UDP-Glc, 1 unit mL−1 Glc-6-P dehydrogenase, 1 unit mL−1 phosphoglucomutase, and 0.05% (v/v) Triton-X100. The reaction was started by the addition of inorganic pyrophosphate to a final concentration of 2.5 mm and a final volume of 100 µL.
Triose phosphate isomerase was assayed in the direction of dihydroxyacetone phosphate formation (Pichersky and Gottlieb, 1984). The assay consisted of 10 µL desalted extract in 100 mm HEPES-NaOH (pH 8.0), 0.2 mm NADH, 5 mm EDTA, and 1 unit mL−1 glycerol 3-phosphate dehydrogenase. The reaction was started by the addition of glyceraldehyde 3-phosphate to a final concentration of 1.5 mm.
Enolase was assayed in the direction of phosphoenolpyruvate production as described by Burrell et al., 1994. The assay consisted of 10 µL desalted extract in 100 mm HEPES-NaOH (pH 7.5), 10 mm MgCl2, 0.2 mm NADH, 2.7 mm ADP, 5 unit mL−1 PK, and 6 unit mL−1 lactate dehydrogenase. The reaction was started by the addition of 2-phosphoglycerate to a final concentration of 0.5 mm.
NAD-ME and NADP-ME activities were assayed using a protocol adapted from Wheeler et al., 2005. The assay consisted in 2 µL extract in 100 mm HEPES-KOH (pH 7.5), 10 mm MgCl2, 1 mm NADP+ or 6 mm NAD+, and 0.05% (v/v) Triton-X100. The reaction was started by the addition of malate to a final concentration of 10 mm and a final volume of 20 µL. After 40 min of incubation at 25°C, the reaction was stopped by the addition of 20 µL 0.5 m HCl and 0.1 m Tricine-KOH (pH 9). After mixing and waiting for 10 min, the acid was neutralized by the addition of 20 µL 0.5 m NaOH. The quantification of phosphoenolpyruvate accumulated during the incubation was then performed by the addition of 100 mm HEPES-KOH (pH 7.5), 10 mm MgCl2, 0.05% (v/v) Triton-X100, and 1 mm NADH for a final volume of 110 µL. The A340 was read until stabilized, and then, 2 µL lactate dehydrogenase (100 unit mL−1) was added to start the determination.
Metabolite Measurements
Aliquots of about 20 mg fresh weight were fractionated as by Hendriks et al., 2003. Suc, Glc, and Fru (Jelitto et al., 1992), malate (Nunes-Nesi et al., 2007), and citrate (Tompkins and Toffaletti, 1982) as well as Glc-6-P, Fru-6-P, and Glc-1-P (Gibon et al., 2002) were determined in the ethanolic supernatant. Starch (Hendriks et al., 2003) and protein (Bradford, 1976) contents were determined on the pellet resuspended in 100 mm NaOH. Assays were prepared in 96-well microplates using Starlet pipetting robots (Hamilton), and absorbance was read at 340, 570, or 595 nm in MP96 microplate readers (SAFAS).
Individual amino acids analysis was carried out using the AQC-tag method. Derivatization was performed using the AccQ-Fluor reagent kit (Waters) according to the manufacturer’s instructions. Amino acid derivatives were separated on an Acquity BEH C18 column (2.1 × 100 mm, 1.7 µm; Waters). Solvents were AccQ-tag Ultra eluent A (Waters) and acetonitrile/water (25 v:75 v). Detection was performed at 473 nm after excitation at 266 nm. Quantities of individual amino acids were calculated with external calibration curves prepared from commercial amino acid standards.
Statistical Analysis
All ANOVA, PCA, and Tukey’s test (P < 0.05) analyses were performed using R Software (http://www.r-project.org/) and the package FactomineR (Lê et al., 2008). Hierarchical clustering and heat maps were performed on mean-centered data scaled to unit variance using MEV software v4.8.1. (Saeed et al., 2003) with Pearson’s correlations and complete linkage.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Principal component analysis of metabolite profiles.
Supplemental Figure S2. Principal component analysis of enzyme (mg protein basis) and metabolite (fruit basis) profiles.
Supplemental Figure S3. Principal component analysis of enzyme (mg protein basis) and metabolite (g fresh weight basis) profiles.
Supplemental Figure S4. Principal component analysis of enzyme (mg protein basis) and metabolite (mg protein basis) profiles.
Supplemental Figure S5. Principal component analysis of enzyme (fruit and day basis) metabolite (fruit and day basis) profiles.
Supplemental Table S1. Enzyme data (control trusses).
Supplemental Table S2. Enzyme data: Tukey's test and coefficient of variation (control trusses).
Supplemental Table S3. Enzyme data (treatments).
Supplemental Table S4. Enzyme data: Tukey's test and coefficient of variation (treatments).
Supplemental Table S5. Enzyme data (growth locations).
Supplemental Table S6. Enzyme data: Tukey's test and coefficient of variation (growth locations).
Supplemental Table S7. Metabolite data (control trusses).
Supplemental Table S8. Metabolite data: Tukey's test and coefficient of variation (control trusses).
Supplemental Table S9. Individual amino acids (control truss 7).
Supplemental Table S10. Metabolite data (treatments).
Supplemental Table S11. Metabolite data: Tukey's test and coefficient of variation (treatments).
Supplemental Table S12. Metabolite data (growth locations).
Supplemental Table S13. Metabolite data: Tukey's test and coefficient of variation (growth locations).
Acknowledgments
We thank Dr. Alisdair Fernie and Dr. Sonia Osorio for providing the seeds used in the study, Pierre Gaillard, Jacques Longuesserre, Emilie Labadie and Benoît Collu for technical help, and our colleagues for their friendly contribution during harvests and sample preparation.
Glossary
- EC
European Community
- IDH
isocitrate dehydrogenase
- MDH
malate dehydrogenase
- ME
malic enzyme
- PEPC
phosphoenolpyruvate carboxylase
Footnotes
This work has been funded by the Eranet Erasysbio+ Fruit Integrative Modelling project and Institut National de la Recherche Agronomique.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ. (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17: 2954–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amemiya T, Kanayama Y, Yamaki S, Yamada K, Shiratake K. (2006) Fruit-specific V-ATPase suppression in antisense-transgenic tomato reduces fruit growth and seed formation. Planta 223: 1272–1280 [DOI] [PubMed] [Google Scholar]
- Appeldoorn NJG, de Bruijn SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, van der Plas LHW. (1997) Developmental changes of enzymes involved in conversion of sucrose to hexose-phosphate during early tuberisation of potato. Planta 202: 220–226 [Google Scholar]
- Balibrea ME, Martinez-Andujar C, Cuartero J, Bolarin MC, Perez-Alfocea F. (2006) The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Funct Plant Biol 33: 279–288 [DOI] [PubMed] [Google Scholar]
- Beckles DM. (2012) Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol Technol 63: 129–140 [Google Scholar]
- Beckles DM, Hong N, Stamova L, Luengwilai K. (2012) Biochemical factors contributing to tomato fruit sugar content: a review. Fruits 67: 49–64 [Google Scholar]
- Bénard C, Gautier H, Bourgaud F, Grasselly D, Navez B, Caris-Veyrat C, Weiss M, Génard M. (2009) Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J Agric Food Chem 57: 4112–4123 [DOI] [PubMed] [Google Scholar]
- Bolarin MC, Estañ MT, Caro M, Romero-Aranda R, Cuartero J. (2001) Relationship between tomato fruit growth and fruit osmotic potential under salinity. Plant Sci 160: 1153–1159 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brady CJ, Palmer JK, O'Connell PBH, Smillie RM. (1970) An increase in protein synthesis during ripening of the banana fruit. Phytochemistry 9: 1037–1047 [Google Scholar]
- Burrell MM, Mooney PJ, Bundy M, Carter D, Wilson F, Green J, Blandy KS, Rees TA. (1994) Genetic manipulation of 6-phosphofructokinase in potato tubers. Planta 194: 95–101 [Google Scholar]
- Carrari F, Asis R, Fernie AR. (2007) The metabolic shifts underlying tomato fruit development. Plant Biotechnol J 24: 45–55 [Google Scholar]
- Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor MI, Nunes-Nesi A, Nikiforova V, Centero D, Ratzka A, Pauly M, et al. (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol 142: 1380–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Fernie AR. (2006) Metabolic regulation underlying tomato fruit development. J Exp Bot 57: 1883–1897 [DOI] [PubMed] [Google Scholar]
- Carrari F, Nunes-Nesi A, Gibon Y, Lytovchenko A, Loureiro ME, Fernie AR. (2003) Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol 133: 1322–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockshull KE, Graves CJ, Cave CRJ. (1992) The influence of shading on yield of glasshouse tomatoes. J Hortic Sci 67: 11–24 [Google Scholar]
- Cuartero J, Fernández-Muñoz R. (1999) Tomato and salinity. Sci Hortic (Amsterdam) 78: 83–125 [Google Scholar]
- Demnitz-King A, Ho LC, Baker DA. (1997) Activity of sucrose hydrolysing enzymes and sugar accumulation during tomato fruit development. Plant Growth Regul 22: 193–201 [Google Scholar]
- Dias DCFS, Ribeiro FP, Dias LAS, Silva DJH, Vidigal DS. (2006) Tomato seed quality harvested from different trusses. Seed Sci Technol 34: 681–689 [Google Scholar]
- Do PT, Prudent M, Sulpice R, Causse M, Fernie AR. (2010) The influence of fruit load on the tomato pericarp metabolome in a Solanum chmielewskii introgression line population. Plant Physiol 154: 1128–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drincovich MF, Casati P, Andreo CS. (2001) NADP-malic enzyme from plants: a ubiquitous enzyme involved in different metabolic pathways. FEBS Lett 490: 1–6 [DOI] [PubMed] [Google Scholar]
- Eshed Y, Zamir D. (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141: 1147–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanasca S, Martino A, Heuvelink E, Stanghellini C. (2007) Effect of electrical conductivity, fruit pruning, and truss position on quality in greenhouse tomato fruit. J Hortic Sci Biotechnol 82: 488–494 [Google Scholar]
- Faurobert M, Mihr C, Bertin N, Pawlowski T, Negroni L, Sommerer N, Causse M. (2007) Major proteome variations associated with cherry tomato pericarp development and ripening. Plant Physiol 143: 1327–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D. (2004) Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305: 1786–1789 [DOI] [PubMed] [Google Scholar]
- Gautier H, Diakou-Verdin V, Bénard C, Reich M, Buret M, Bourgaud F, Poëssel JL, Caris-Veyrat C, Génard M. (2008) How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J Agric Food Chem 56: 1241–1250 [DOI] [PubMed] [Google Scholar]
- Gautier H, Rocci A, Buret M, Grasselly D, Causse M. (2005) Fruit load or fruit position alters response to temperature and subsequently cherry tomato quality. J Sci Food Agric 85: 1009–1016 [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks JH, Palacios N, Cross J, Selbig J, Stitt M. (2004) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Pyl ET, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. (2009) Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Usadel B, Blaesing OE, Kamlage B, Hoehne M, Trethewey R, Stitt M. (2006) Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol 7: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M. (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221–235 [DOI] [PubMed] [Google Scholar]
- Giovannoni J. (2001) Molecular Biology Of Fruit Maturation And Ripening. Annu Rev Plant Physiol Plant Mol Biol 52: 725–749 [DOI] [PubMed] [Google Scholar]
- Guan HP, Janes HW. (1991) Light regulation of sink metabolism in tomato fruit. I. Growth and sugar accumulation. Plant Physiol 96: 916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Just D, Bénard N, Destrac-Irvine A, Baldet P, Hernould M, Causse M, Raymond P, Rothan C. (2002) A fruit-specific phospho enolpyruvate carboxylase is related to rapid growth of tomato fruit. Planta 214: 717–726 [DOI] [PubMed] [Google Scholar]
- Hendriks JH, Kolbe A, Gibon Y, Stitt M, Geigenberger P. (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, ap Rees T. (1994) Fluxes of carbohydrate metabolism in ripening bananas. Planta 192: 52–60 [Google Scholar]
- Holtzapffel RC, Finnegan PM, Millar AH, Badger MR, Day DA. (2002) Mitochondrial protein expression in tomato fruit during on-vine ripening and cold storage. Funct Plant Biol 29: 827–834 [DOI] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M. (1992) Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188: 238–244 [DOI] [PubMed] [Google Scholar]
- Kisaka H, Kida T. (2003) Transgenic tomato plant carrying a gene for NADP-dependent glutamate dehydrogenase (gdhA) from Aspergillus nidulans. Plant Sci 164: 35–42 [Google Scholar]
- Klee HJ, Giovannoni JJ. (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Hayford MB, Kretzmer KA, Barry GF, Kishore GM. (1991) Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 3: 1187–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labboun S, Tercé-Laforgue T, Roscher A, Bedu M, Restivo FM, Velanis CN, Skopelitis DS, Moschou PN, Roubelakis-Angelakis KA, Suzuki A, et al. (2009) Resolving the role of plant glutamate dehydrogenase. I. In vivo real time nuclear magnetic resonance spectroscopy experiments. Plant Cell Physiol 50: 1761–1773; erratum Labboun S, Tercé-Laforgue T, Roscher A, Bedu M, Restivo FM, Velanis CN, Skopelitis DS, Moschou PN, Roubelakis-Angelakis KA, Suzuki A, et al. (2009) Plant Cell Physiol 50: 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê S, Josse J, Husson F. (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25: 1–18 [Google Scholar]
- Lemaire-Chamley M, Petit J, Garcia V, Just D, Baldet P, Germain V, Fagard M, Mouassite M, Cheniclet C, Rothan C. (2005) Changes in transcriptional profiles are associated with early fruit tissue specialization in tomato. Plant Physiol 139: 750–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HF, Génard M, Guichard S, Bertin N. (2007) Model-assisted analysis of tomato fruit growth in relation to carbon and water fluxes. J Exp Bot 58: 3567–3580 [DOI] [PubMed] [Google Scholar]
- Luengwilai K, Beckles DM. (2009) Starch granules in tomato fruit show a complex pattern of degradation. J Agric Food Chem 57: 8480–8487 [DOI] [PubMed] [Google Scholar]
- Luengwilai K, Fiehn OE, Beckles DM. (2010) Comparison of leaf and fruit metabolism in two tomato (Solanum lycopersicum L.) genotypes varying in total soluble solids. J Agric Food Chem 58: 11790–11800 [DOI] [PubMed] [Google Scholar]
- Miron D, Schaffer AA. (1991) Sucrose phosphate synthase, sucrose synthase, and invertase activities in developing fruit of lycopersicon esculentum mill. and the sucrose accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol 95: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Shennan C, Grattan SR, May DM. (1991) Tomato fruit yields and quality under water deficit and salinity. J Am Soc Hortic Sci 116: 215–221 [Google Scholar]
- Miyashita Y, Good AG. (2008) NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J Exp Bot 59: 667–680 [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Osorio S, Gehl B, Baxter CJ, Kruger NJ, Ratcliffe RG, Fernie AR, Sweetlove LJ. (2013) Metabolic engineering of tomato fruit organic acid content guided by biochemical analysis of an introgression line. Plant Physiol 161: 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounet F, Moing A, Garcia V, Petit J, Maucourt M, Deborde C, Bernillon S, Le Gall G, Colquhoun I, Defernez M, et al. (2009) Gene and metabolite regulatory network analysis of early developing fruit tissues highlights new candidate genes for the control of tomato fruit composition and development. Plant Physiol 149: 1505–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques M, Gibon Y. (2011) Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J Exp Bot 62: 1715–1729 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- N'tchobo H, Dali N, Nguyen-Quoc B, Foyer CH, Yelle S. (1999) Starch synthesis in tomato remains constant throughout fruit development and is dependent on sucrose supply and sucrose synthase activity. J Exp Bot 50: 1457–1463 [Google Scholar]
- Nafati M, Cheniclet C, Hernould M, Do PT, Fernie AR, Chevalier C, Gévaudant F. (2011) The specific overexpression of a cyclin-dependent kinase inhibitor in tomato fruit mesocarp cells uncouples endoreduplication and cell growth. Plant J 65: 543–556 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR. (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50: 1093–1106 [DOI] [PubMed] [Google Scholar]
- O’Leary B, Park J, Plaxton WC. (2011) The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436: 15–34 [DOI] [PubMed] [Google Scholar]
- Osorio S, Alba R, Damasceno CMB, Lopez-Casado G, Lohse M, Zanor MI, Tohge T, Usadel B, Rose JKC, Fei Z, et al. (2011) Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol 157: 405–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gottlieb LD. (1984) Plant triose phosphate isomerase isozymes: purification, immunological and structural characterization, and partial amino acid sequences. Plant Physiol 74: 340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piques M, Schulze WX, Hohne M, Usadel B, Gibon Y, Rohwer J, Stitt M. (2009) Ribosome and transcript copy numbers, polysome occupancy and enzyme dynamics in Arabidopsis. Mol Syst Biol 5: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudent M, Bertin N, Génard M, Muños S, Rolland S, Garcia V, Petit J, Baldet P, Rothan C, Causse M. (2010) Genotype-dependent response to carbon availability in growing tomato fruit. Plant Cell Environ 33: 1186–1204 [DOI] [PubMed] [Google Scholar]
- Rocco M, D’Ambrosio C, Arena S, Faurobert M, Scaloni A, Marra M. (2006) Proteomic analysis of tomato fruits from two ecotypes during ripening. Proteomics 6: 3781–3791 [DOI] [PubMed] [Google Scholar]
- Roessner-Tunali U, Hegemann B, Lytovchenko A, Carrari F, Bruedigam C, Granot D, Fernie AR. (2003) Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol 133: 84–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Gibon Y. (2009) Enzyme kinetics: theory and practice. In J Schwender, ed, Plant Metabolic Networks. Springer, Berlin, pp 71–103 [Google Scholar]
- Rosales MA, Rubio-Wilhelmi MM, Castellano R, Castilla N, Ruiz JM, Romero L. (2007) Sucrolyticactivities in cherrytomatofruits in relation to temperature and solarradiation. Sci Hortic (Amsterdam) 113: 244–249 [Google Scholar]
- Ruan YL, Patrick JW, Bouzayen M, Osorio S, Fernie AR. (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17: 656–665 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Saito T, Ariizumi T, Okabe Y, Asamizu E, Hiwasa-Tanase K, Fukuda N, Mizoguchi T, Yamazaki Y, Aoki K, Ezura H. (2011) TOMATOMA: a novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol 52: 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Levin I, Oguz I, Petreikov M, Cincarevsky F, Yeselson Y, Shen S, Gilboa N, Bar M. (2000) ADPglucose pyrophosphorylase activity and starch accumulation in immature tomato fruit: the effect of a Lycopersicon hirsutum-derived introgression encoding for the large subunit. Plant Sci 152: 135–144 [Google Scholar]
- Schaffer AA, Petreikov M. (1997) Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N, Semel Y, Balbo I, Steinfath M, Repsilber D, Selbig J, Pleban T, Zamir D, Fernie AR. (2008) Mode of inheritance of primary metabolic traits in tomato. Plant Cell 20: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N, Semel Y, Roessner U, Gur A, Balbo I, Carrari F, Pleban T, Perez-Melis A, Bruedigam C, Kopka J, et al. (2006) Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat Biotechnol 24: 447–454 [DOI] [PubMed] [Google Scholar]
- Sinesio F, Cammareri M, Moneta E, Navez B, Peparaio M, Causse M, Grandillo S. (2010) Sensory quality of fresh French and Dutch market tomatoes: a preference mapping study with Italian consumers. J Food Sci 75: S55–S67 [DOI] [PubMed] [Google Scholar]
- Smith CJS, Watson CF, Ray J, Bird CR, Morris PC, Schuch W, Grierson D. (1988) Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature 334: 724–726 [Google Scholar]
- Steinhauser MC, Steinhauser D, Koehl K, Carrari F, Gibon Y, Fernie AR, Stitt M. (2010) Enzyme activity profiles during fruit development in tomato cultivars and Solanum pennellii. Plant Physiol 153: 80–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studart-Guimarães C, Gibon Y, Frankel N, Wood CC, Zanor MI, Fernie AR, Carrari F. (2005) Identification and characterisation of the α and β subunits of succinyl CoA ligase of tomato. Plant Mol Biol 59: 781–791 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Sienkiewicz-Porzucek A, Osorio S, Krahnert I, Stitt M, Fernie AR, Nunes-Nesi A. (2010) Mild reductions in cytosolic NADP-dependent isocitrate dehydrogenase activity result in lower amino acid contents and pigmentation without impacting growth. Amino Acids 39: 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Saika H, Matsumura H, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M. (2011) Cell division and cell elongation in the coleoptile of rice alcohol dehydrogenase 1-deficient mutant are reduced under complete submergence. Ann Bot (Lond) 108: 253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley SD, Grandillo S, Fulton TM, Zamir D, Eshed Y, Petiard V, Lopez J, Beck-Bunn T. (1996) Advanced backcross QTL analysis in a cross between an elite processing line of tomato and its wild relative L. pimpinellifolium. Theor Appl Genet 92: 213–224 [DOI] [PubMed] [Google Scholar]
- Teusink B, Walsh MC, van Dam K, Westerhoff HV. (1998) The danger of metabolic pathways with turbo design. Trends Biochem Sci 23: 162–169 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JH, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P. (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D, Toffaletti J. (1982) Enzymic determination of citrate in serum and urine, with use of the Worthington “ultrafree” device. Clin Chem 28: 192–195 [PubMed] [Google Scholar]
- Wang F, Sanz A, Brenner ML, Smith A. (1993) Sucrose synthase, starch accumulation, and tomato fruit sink strength. Plant Physiol 101: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding RT. (1989) Malic enzymes of higher plants: characteristics, regulation, and physiological function. Plant Physiol 90: 367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MCG, Tronconi MA, Drincovich MF, Andreo CS, Flügge UI, Maurino VG. (2005) A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol 139: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor GW. (1979) Some factors affecting the quality and composition of tomatoes. Acta Hortic 93: 335–346 [Google Scholar]
- Winter H, Huber JL, Huber SC. (1997) Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett 420: 151–155 [DOI] [PubMed] [Google Scholar]
- Yelle S, Chetelat RT, Dorais M, Deverna JW, Bennett AB. (1991) Sink metabolism in tomato fruit. IV. Genetic and biochemical analysis of sucrose accumulation. Plant Physiol 95: 1026–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. (2007) The diurnal metabolism of leaf starch. Biochem J 401: 13–28 [DOI] [PubMed] [Google Scholar]