Abstract
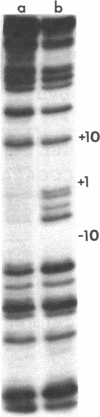
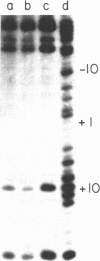
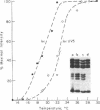
A method based on the differential rate of cytosine methylation in single- and double-stranded nucleic acids by dimethyl sulfate [Peattie, D.A. & Gilbert, W. (1980) Proc. Natl. Acad. Sci. USA 77, 4679-4682] has been developed for probing unpaired cytosines in DNA and DNA-protein complexes at the sequence level. Application of the method to the complexes between Escherichia coli RNA polymerase (EC 2.7.7.6) and three related promoters, lac UV5, trp, and a hybrid promoter tac resulting from the fusion of the two, reveals distinct differences in the way RNA polymerase unpairs DNA in these promoters. No single-stranded region is detectable in the complex with the trp promoter. For the lac UV5 promoter, the cytosines at positions -6, -4, -2, and -1 are in an unpaired region. The same cytosines in the tac promoter, which is homologous in sequence to lac UV5 in this region, are also found to be single stranded. For the pair of promoters lac UV5 and tac, the cytosine methylation reaction has also been used to demonstrate the steep temperature dependence of opening of base pairs by RNA polymerase. One striking feature is that the midpoint of this transition for the tac promoter is 3 degrees C lower than the corresponding value for lac UV5, even though the sequence of the unpaired region in the two promoters is identical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):113–137. doi: 10.1016/s0022-2836(78)80001-1. [DOI] [PubMed] [Google Scholar]
- Crick F. H., Wang J. C., Bauer W. R. Is DNA really a double helix? J Mol Biol. 1979 Apr 15;129(3):449–457. doi: 10.1016/0022-2836(79)90506-0. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K., Wang J. C. Mapping the topography of DNA wrapped around gyrase by nucleolytic and chemical probing of complexes of unique DNA sequences. Cell. 1981 Mar;23(3):721–729. doi: 10.1016/0092-8674(81)90435-9. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Effects of some chemical mutagens and carcinogens on nucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:89–131. doi: 10.1016/s0079-6603(08)60232-9. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melnikova A. F., Beabealashvilli R., Mirzabekov A. D. A study of unwinding of DNA and shielding of the DNA grooves by RNA polymerase by using methylation with dimethylsulphate. Eur J Biochem. 1978 Mar;84(1):301–309. doi: 10.1111/j.1432-1033.1978.tb12169.x. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Bennett G. N., Yanofsky C. Escherichia coli RNA polymerase and trp repressor interaction with the promoter-operator region of the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1980 Dec 5;144(2):133–142. doi: 10.1016/0022-2836(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Functional analysis of wild=type and altered tryptophan operon promoters of Salmonella typhimurium in Escherichia coli. J Mol Biol. 1980 Dec 5;144(2):143–161. doi: 10.1016/0022-2836(80)90030-3. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Herr W. Chemical probing of the tRNA--ribosome complex. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2273–2277. doi: 10.1073/pnas.78.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979 Jun 14;279(5714):651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Kubo M., Imamoto F. Promoter-specific inhibition of transcription by antibiotics which act on DNA gyrase. Nature. 1978 Oct 5;275(5679):420–423. doi: 10.1038/275420a0. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. D. Spacer mutations in the lac ps promoter. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1069–1072. doi: 10.1073/pnas.79.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strothkamp R. E., Oakley J. L., Coleman J. E. Promoter melting by T7 ribonucleic acid polymerase as detected by single-stranded endonuclease digestion. Biochemistry. 1980 Mar 18;19(6):1074–1080. doi: 10.1021/bi00547a005. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Heller K., Gellert M., Zubay G. Differential sensitivity of gene expression in vitro to inhibitors of DNA gyrase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3304–3308. doi: 10.1073/pnas.76.7.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]