Light quality coordinates a CO2-concentrating mechanism during C4 photosynthesis.
Abstract
Unequal absorption of photons between photosystems I and II, and between bundle-sheath and mesophyll cells, are likely to affect the efficiency of the CO2-concentrating mechanism in C4 plants. Under steady-state conditions, it is expected that the biochemical distribution of energy (ATP and NADPH) and photosynthetic metabolite concentrations will adjust to maintain the efficiency of C4 photosynthesis through the coordination of the C3 (Calvin-Benson-Bassham) and C4 (CO2 pump) cycles. However, under transient conditions, changes in light quality will likely alter the coordination of the C3 and C4 cycles, influencing rates of CO2 assimilation and decreasing the efficiency of the CO2-concentrating mechanism. To test these hypotheses, we measured leaf gas exchange, leaf discrimination, chlorophyll fluorescence, electrochromatic shift, photosynthetic metabolite pools, and chloroplast movement in maize (Zea mays) and Miscanthus × giganteus following transitional changes in light quality. In both species, the rate of net CO2 assimilation responded quickly to changes in light treatments, with lower rates of net CO2 assimilation under blue light compared with red, green, and blue light, red light, and green light. Under steady state, the efficiency of CO2-concentrating mechanisms was similar; however, transient changes affected the coordination of C3 and C4 cycles in M. giganteus but to a lesser extent in maize. The species differences in the ability to coordinate the activities of C3 and C4 cycles appear to be related to differences in the response of cyclic electron flux around photosystem I and potentially chloroplast rearrangement in response to changes in light quality.
The CO2-concentrating mechanism in C4 plants reduces the carbon lost through the photorespiratory pathway by limiting the oxygenation of ribulose-1,5-bisphosphate (RuBP) by the enzyme Rubisco (Brown and Smith, 1972; Sage, 1999). Through the compartmentalization of the C4 cycle in the mesophyll cells and the C3 cycle in the bundle-sheath cells (Hatch and Slack, 1966), C4 plants suppress RuBP oxygenation by generating a high CO2 partial pressure around Rubisco (Furbank and Hatch, 1987). To maintain high photosynthetic rates and efficient light energy utilization, the metabolic flux through the C3 and C4 cycles must be coordinated. However, coordination of the C3 and C4 cycles is likely disrupted due to rapid changes in environmental conditions, particularly changes in light availability (Evans et al., 2007; Tazoe et al., 2008).
Spatial and temporal variations in light environments, including both light quantity and quality, are expected to alter the coordination of the C3 and C4 cycles. For example, it has been suggested that the coordination of C3 and C4 cycles is altered by changes in light intensity (Henderson et al., 1992; Cousins et al., 2006; Tazoe et al., 2006, 2008; Kromdijk et al., 2008, 2010; Pengelly et al., 2010). However, more recent publications indicate that some of the proposed light sensitivity of the CO2-concentrating mechanisms in C4 plants can be attributed to oversimplifications of leaf models of carbon isotope discrimination (Δ13C), in particular, errors in estimates of bundle-sheath CO2 partial pressure and omissions of respiratory fractionation (Ubierna et al., 2011, 2013). Alternatively, there is little information on the effects of light quality on the coordination of C3 and C4 cycle activities and the subsequent impact on net rate of CO2 assimilation (Anet).
In C3 plants, Anet is reduced under blue light compared with red or green light (Evans and Vogelmann, 2003; Loreto et al., 2009). This was attributed to differences in absorbance and wavelength-dependent differences in light penetration into leaves, where red and green light penetrate farther into leaves compared with blue light (Vogelmann and Evans, 2002; Evans and Vogelmann, 2003). Differences in light quality penetration into a leaf are likely to have profound impacts on C4 photosynthesis, because the C4 photosynthetic pathway requires the metabolic coordination of the mesophyll C4 cycle and the bundle-sheath C3 cycle. Indeed, Evans et al. (2007) observed a 50% reduction in the rate of CO2 assimilation in Flaveria bidentis under blue light relative to white light at a light intensity of 350 µmol quanta m−2 s−1. This was attributed to poor penetration of blue light into the bundle-sheath cells and subsequent insufficient production of ATP in the bundle-sheath cells to match the rates of mesophyll cell CO2 pumping (Evans et al., 2007). Recently, Sun et al. (2012) observed similar low rates of steady-state CO2 assimilation under blue light relative to red, green, and blue light (RGB), red light, and green light at a constant light intensity of 900 µmol quanta m−2 s−1.
Because the light penetration into a leaf depends on light quality, with blue light penetrating the least, this potentially results in changes in the energy available for carboxylation reactions in the bundle-sheath (C3 cycle) and mesophyll (C4 cycle) cells. Changes in the balance of energy driving the C3 and C4 cycles can alter the efficiency of the CO2-concentrating mechanisms, often represented by leakiness (ϕ), the fraction of CO2 that is pumped into the bundle-sheath cells that subsequently leaks back out (Evans et al., 2007). Unfortunately, ϕ cannot be measured directly, but it can be estimated through the combined measured and modeled values of Δ13C (Farquhar, 1983). Using measurements of Δ13C, it has been demonstrated that under steady-state conditions, changes in light quality do not affect ϕ (Sun et al., 2012); however, it remains unknown if ϕ is also constant during the transitions between different light qualities. In fact, sudden changes of light quality could temporally alter the coordination of the C3 and C4 cycles.
To understand the effects of light quality on C4 photosynthesis and the coordination of the activities of C3 and C4 cycles, we measured transitional changes in leaf gas exchange and Δ13C under RGB and broad-spectrum red, green, and blue light in the NADP-malic enzyme C4 plants maize (Zea mays) and Miscanthus × giganteus. Leaf gas exchange and Δ13C measurements were used to estimate ϕ using the complete model of C4 leaf Δ13C (Farquhar, 1983; Farquhar and Cernusak, 2012). Additionally, we measured photosynthetic metabolite pools, Rubisco activation state, chloroplast movement, and rates of linear versus cyclic electron flow during rapid transitions from red to blue light and blue to red light. We hypothesized that the limited penetration of blue light into the leaf would result in insufficient production of ATP in the bundle-sheath cells to match the rate of mesophyll cell CO2 pumping. We predicted that rapid changes in light quality would affect the coordination of the C3 and C4 cycles and cause an increase in ϕ, but this would equilibrate as leaf metabolism reached a new steady-state condition.
RESULTS
Gas Exchange and Photosynthetic Discrimination
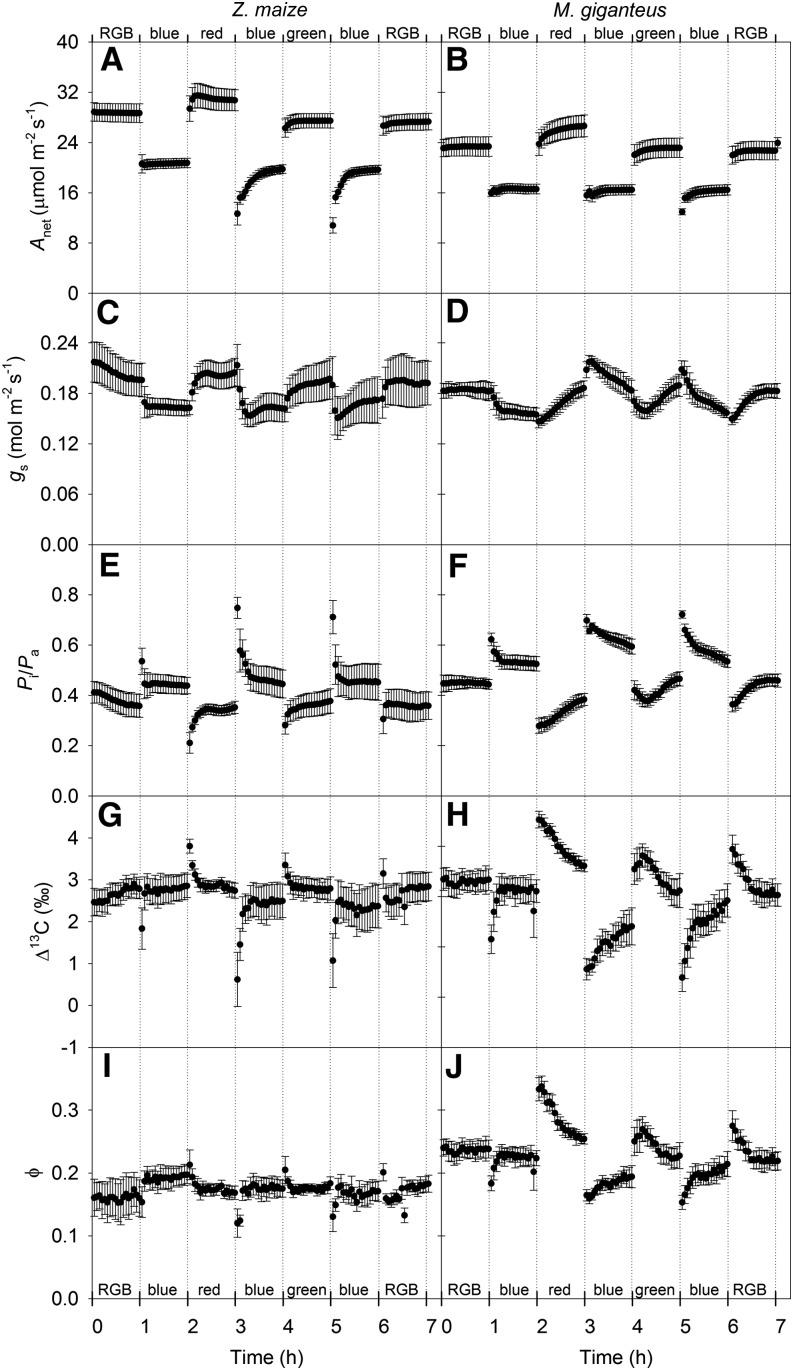
The Anet was significantly affected by changes in light treatment in both maize (Fig. 1A) and M. giganteus (Fig. 1B), with lower Anet under blue light compared with RGB, red, and green light at 900 µmol quanta m−2 s−1. In maize, it took 20 to 30 min for Anet to stabilize when transitioned from either red or green light to blue light (Fig. 1A). However, in M. giganteus, Anet stabilized within minutes under these light transitions (Fig. 1B). Both stomatal conductance (gs) and the ratio of intercellular to ambient CO2 partial pressure (Pi/Pa) responded within minutes to changes in light quality in maize; however, gs was lower and Pi/Pa was higher under blue light relative to other light treatments (Fig. 1, C and E). For M. giganteus, changing light treatment from RGB, red, and green to blue resulted in a gradual decrease in gs, whereas switching light from blue to red, green, and RGB caused a gradual increase in gs (Fig. 1D). Overall, the responses of gs to variation in light treatment in M. giganteus were not as rapid as in maize, and Pi/Pa was higher under blue light relative to other light treatments in M. giganteus (Fig. 1F).
Figure 1.
Transitional variation in Anet (µmol m−2 s−1; A and B), gs (mol m−2 s−1; C and D), Pi/Pa (unitless; E and F), Δ13C (‰; G and H), and ϕ (I and J) in maize and M. giganteus with changes in light treatment in the light sequence RGB, blue, red, blue, green, blue, and RGB. The light intensity was set to 900 µmol quanta m−2 s−1 for all light treatments. The CO2 partial pressure in the leaf chamber was maintained at 38.4 Pa, leaf temperature was 25°C, and relative humidity was between 50% and 70% for all light treatments. Data are reported as means ± se (n = 4).
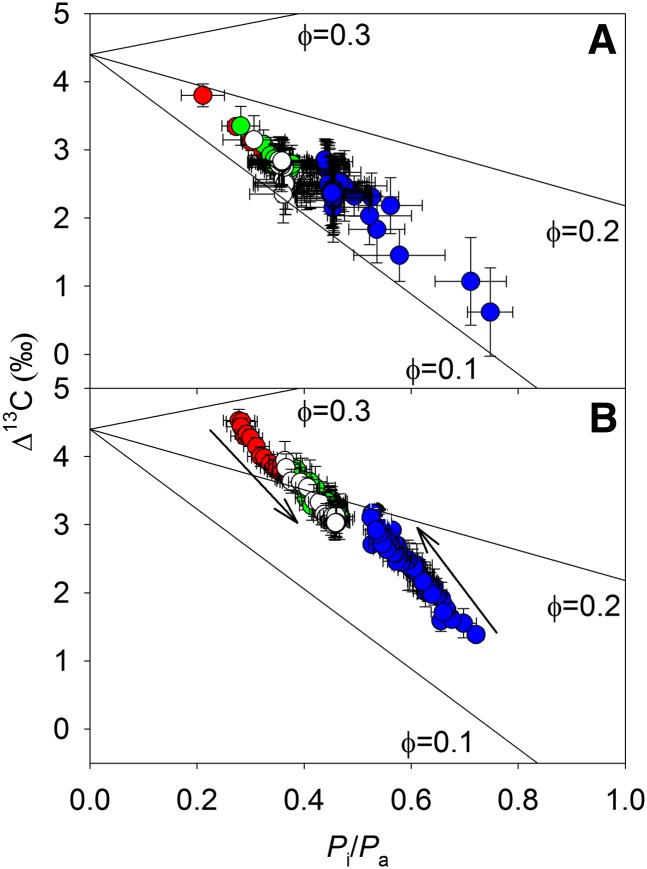
Δ13C and ϕ in maize (Fig. 1, G and I) were less sensitive to changes in light treatments than in M. giganteus (Fig. 1, H and J). Values of Δ13C and ϕ increased in M. giganteus during light transitions from blue to red, green, or RGB and decreased when switched from red or green to blue (Fig. 1, H and J). The Δ13C in maize was linearly related to Pi/Pa under all light qualities, consistent with a constant ϕ (Fig. 2A); however, the response in M. giganteus indicated a shift in ϕ with light quality (Fig. 2B). In both species, Δ13C and ϕ tended to converge to a constant value after 1 h of illumination in all light treatments (Figs. 1 and 2). Variation of Δ13C in maize was mainly related to changes in Pi/Pa, whereas in M. giganteus, shifts of Δ13C were associated with changes in both Pi/Pa and ϕ (Fig. 2).
Figure 2.
Δ13C (‰) as a function of Pi/Pa (unitless) for RGB (white circles), red (red circles), green (green circles), and blue (blue circles) light in maize (A) and M. giganteus (B). Solid lines were modeled (Eq. 7) with ϕ of 0.1, 0.2, and 0.3 from bottom to top. Arrows indicate the convergence of ϕ to a constant value in M. giganteus after switching from one light species to another. Data are reported as means ± se (n = 4).
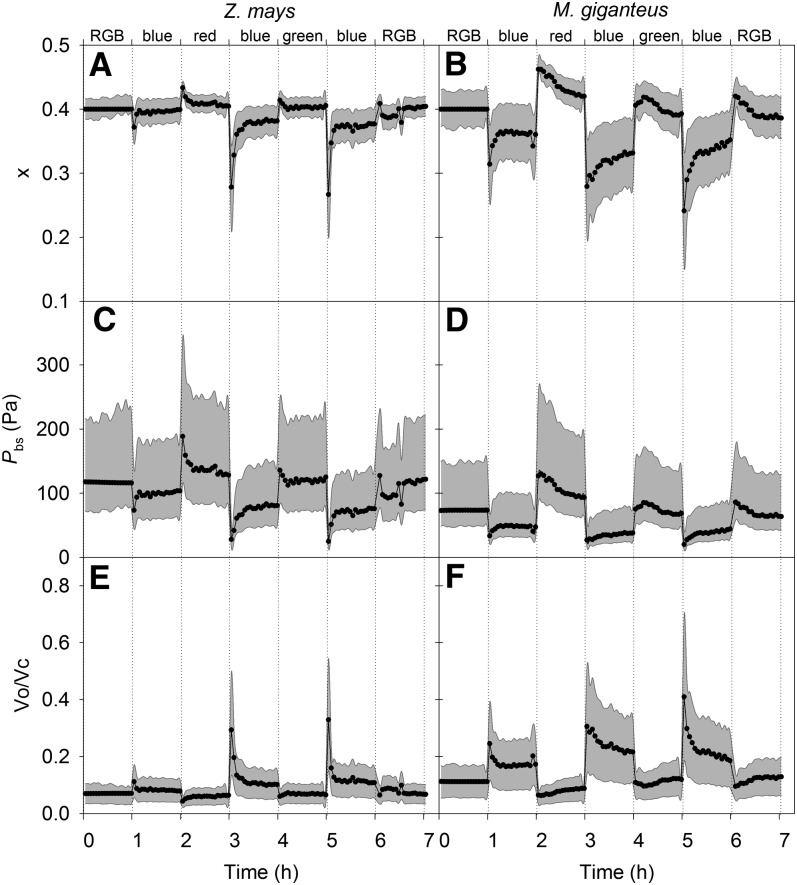
The allocation of absorbed excitation energy between the C3 and C4 cycles (x) was modeled by solving for the value of x that minimizes the difference between the modeled (see Eq. 3) and measured values of Δ13C, assuming that bundle-sheath conductance did not change with light quality and was equal to values estimated under RGB. To account for the measured 3.5‰ increase in Δ13C in M. giganteus, the x value had to increase 20%, with lower values under blue light compared with the other light treatments (Fig. 3). In maize, the required change in x needed to explain Δ13C was small compared with that in M. giganteus. The modeled bundle-sheath CO2 partial pressure (Pbs) was lower and the Rubisco oxygenation-carboxylation ratio (vo/vc) was higher under blue light compared with other light treatments in both maize and M. giganteus (Fig. 3, C–F).
Figure 3.
Modeled transitional variation in x (A and B), Pbs (Pa; C and D), and vo/vc (µmol m−2 s−1; E and F) in maize and M. giganteus with changes in light quality in the order RGB, blue, red, blue, green, blue, and RGB at an irradiance of 900 µmol quanta m−2 s−1. gbs was estimated (0.0046 μmol m−2 s−1 Pa−1 for maize and 0.01 μmol m−2 s−1 Pa−1 for M. giganteus) using data obtained from the first 1 h of RGB measurements assuming x = 0.4. For the other light treatments, x was solved, assuming gbs was constant, to minimize the least-square difference between modeled and measured Δ13C. The gray areas show the sensitivity of the modeled parameters assuming different values of gbs (between 0.0069 and 0.0023 μmol m−2 s−1 Pa−1 for maize and between 0.015 and 0.005 μmol m−2 s−1 Pa−1 for M. giganteus).
Linear Electron Flux and Proton Motive Force
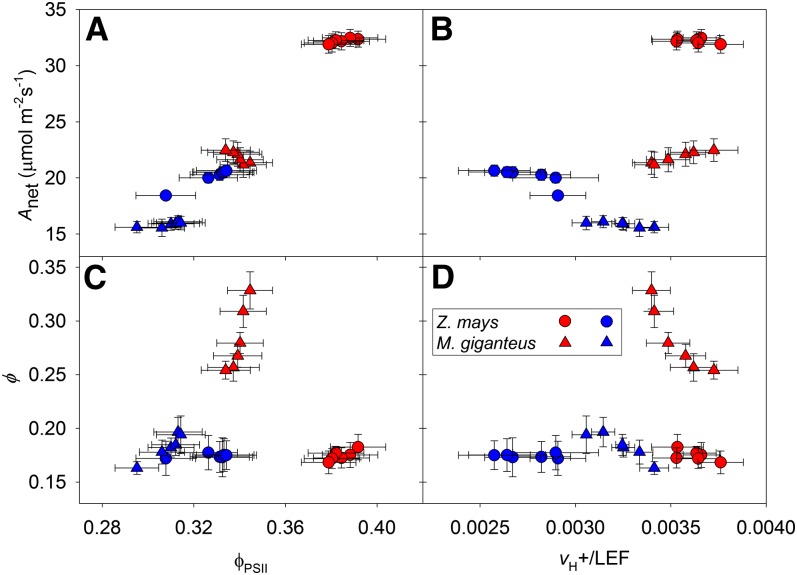
Measurements of Anet, gs, and Pi/Pa made with the LI-COR 6400 coupled to the electrochromatic shift (ECS) system were similar to those made with the LI-COR 6400 coupled to the tunable diode laser absorption spectroscope (TDLAS; compare Fig. 1 and Supplemental Fig. S1). The efficiency of photosystem II (ϕPSII) was higher under red light compared with blue light for both maize and M. giganteus (Supplemental Fig. S2D). However, the total ECS was lower under blue light compared with red light, and this difference was larger in maize compared with M. giganteus (Supplemental Fig. S2E). Rates of proton flux across the thylakoid membrane (vH+) relative to rates of linear electron flux (LEF), or vH+/LEF, were lower under blue light compared with red light for both species. However, the shift in vH+/LEF was greater in maize compared with M. giganteus (Supplemental Fig. S2F). In both species, Anet was positively correlated with both ϕPSII (Fig. 4A) and vH+/LEF (Fig. 4B). In M. giganteus, ϕ was positively related to ϕPSII, and under a given light treatment, there was a negative relationship of ϕ and vH+/LEF (Fig. 4, C and D). In maize, ϕ did not change with shifts in ϕPSII and vH+/LEF in response to red or blue light (Fig. 4, C and D). It should be noted that vH+/LEF and ϕ were measured on different leaves in separate gas-exchange systems; however, for comparison, measurements of Anet, gs and Pi/Pa from both setups are presented in Supplemental Figure S1.
Figure 4.
Anet (A and B) and ϕ (C and D) in response to variation in ϕPSII and vH+/LEF under red light (red symbols) and blue light (blue symbols) at 900 µmol quanta m−2 s−1. Data are reported as means ± se (n = 4–8).
Pools of Photosynthetic Intermediates and Rubisco Activation State
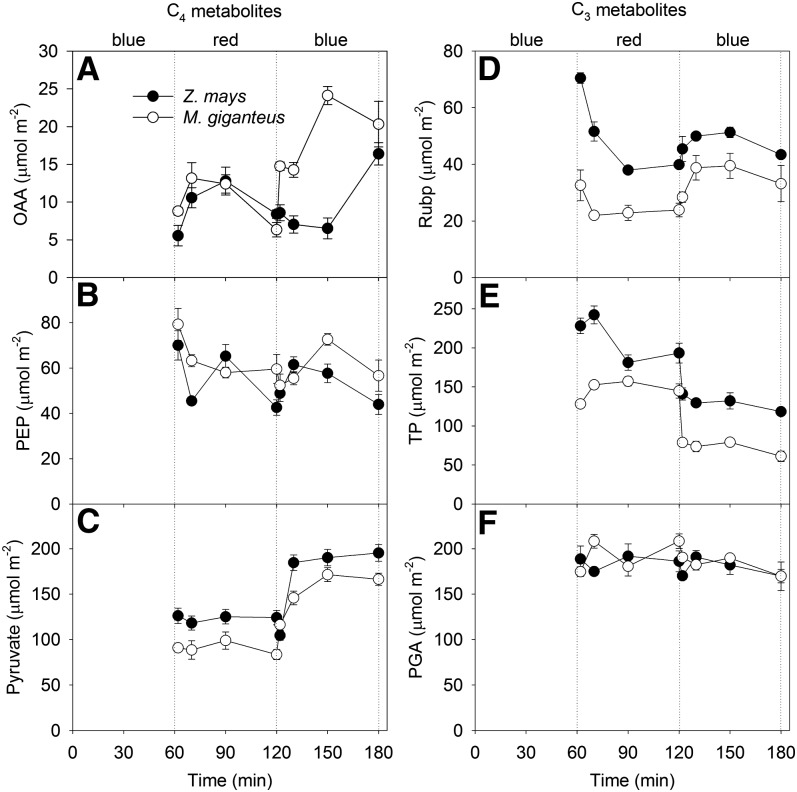
There was more oxaloacetic acid (OAA) under blue light for both species (Supplemental Table S1); however, the change in concentration was slower in maize than in M. giganteus (Fig. 5A; Supplemental Table S2). Pyruvate was constant under red light, but there was a significant change in content with time after the transition to blue light for both species (Supplemental Table S2), which took 30 min to reach a steady state in M. giganteus and only 10 min in maize (Fig. 5C). It took 30 min for RuBP to reach steady state under red light in maize but only 10 min in M. giganteus (Fig. 5D). Under blue light transitions, RuBP increased more in M. giganteus than in maize (Fig. 5D). The amount of triose phosphate (TP) was significantly lower under blue light compared with red light, whereas pyruvate was higher under blue light compared with red light for both maize and M. giganteus (Fig. 5E; Supplemental Tables S1 and S2). Phosphoenolpyruvate (PEP) and phosphoglycerate (PGA) were not significantly different between light treatments (Fig. 5F; Supplemental Table S2).
Figure 5.
Transitional variation in pools of the C4 metabolites OAA (µmol m−2; A), pyruvate (µmol m−2; B), and PEP (µmol m−2; C) and the C3 metabolites TP (µmol m−2; D), RuBP (µmol m−2; E), and PGA (µmol m−2; F) in maize and M. giganteus when light was switched in the sequence blue, red, and blue at a light intensity of 900 µmol quanta m−2 s−1. Data are reported as means ± se (n = 4).
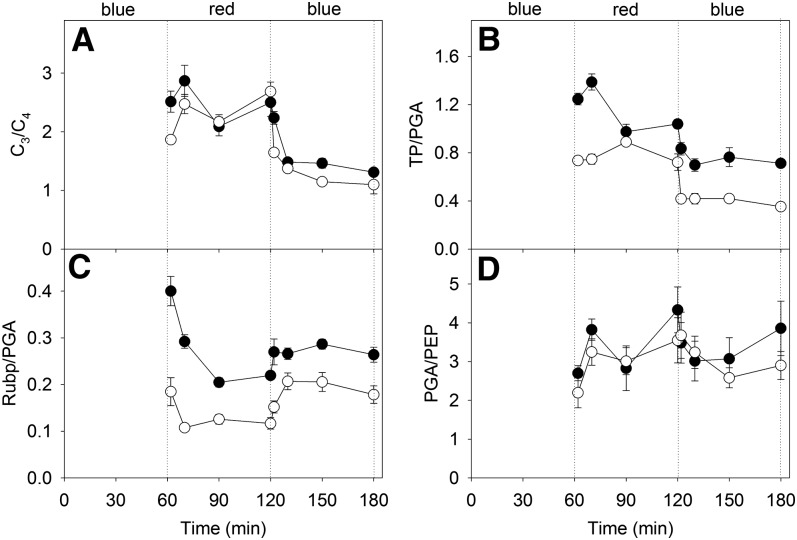
The ratios of C3 metabolites (TP + RuBP + PGA) over C4 metabolites (OAA + PEP + pyruvate) were significantly greater under red light compared with blue light treatment for both maize and M. giganteus (Fig. 6A; Supplemental Table S2). There was no statistically significant difference in PGA/PEP, but TP/PGA was lower under blue light compared with red light for both maize and M. giganteus (Fig. 6, B and D; Supplemental Table S2). The ratio of RuBP to PGA reached steady state within 10 min in M. giganteus but took 30 min in maize (Fig. 6C). After the transition to blue light, RuBP/PGA increased within 2 min for maize but took 10 min in M. giganteus (Fig. 6C). The activation state of Rubisco was constant across light treatments for both species (Supplemental Table S2).
Figure 6.
Transitional variation in the ratios of C3 metabolites (TP + RuBP + PGA) over C4 metabolites (OAA + PEP + pyruvate; A), TP over PGA (B), RuBP over PGA (C), and PEP over PGA (D) in maize (black circles) and M. giganteus (white circles) when light quality was switched in the sequence blue, red, and blue at a light intensity of 900 µmol quanta m−2 s−1. Data are reported as means ± se (n = 4).
Chloroplast Movement
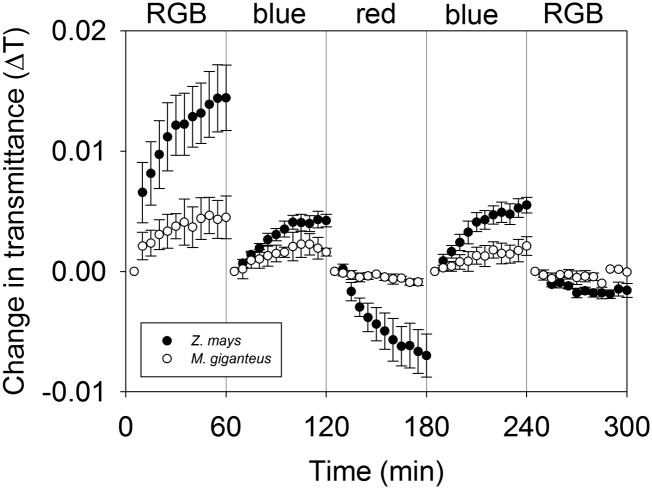
Light quality-driven movement and positioning of chloroplast can be monitored through changes in leaf light transmittance (Inoue and Shibata, 1973; Trojan and Gabrys, 1996; DeBlasio et al., 2003). In leaves of maize and M. giganteus, chloroplast movement was monitored through changes in leaf transmittance (ΔT) during 1-h exposures to RGB, blue, and red light under similar conditions used for the gas exchange and online discrimination measurements. In M. giganteus, there was little ΔT over the 60-min time period regardless of light treatment (Fig. 7). However, in maize, there was a relatively large response of ΔT under all light treatments compared with M. giganteus (Fig. 7).
Figure 7.
Transitional variation in ΔT in maize (black circles) and M. giganteus (white circles) when light treatment was switched in the sequence of RGB, blue, red, blue, and RGB. ΔT is defined as the difference between the initial averaged 5 min of transmittance of a given light quality and the transmittance at a given time point within the 60-min exposure. Data are reported as means ± se (n = 4 for maize; n = 3 for M. giganteus).
DISCUSSION
Light Quality and Anet
After a 1-h acclimation, the steady-state Anet was lower under blue light when compared with RGB, red light, and green light in both maize and M. giganteus (Fig. 1, A and B). The lower photosynthetic rate under blue light is consistent with previous findings for both C3 (Evans and Vogelmann, 2003; Loreto et al., 2009) and C4 (Evans et al., 2007; Sun et al., 2012) species. Low Anet under steady-state blue light has been discussed previously by Sun et al. (2012); however, in this study, there were changes in ϕ unrelated to changes in Anet that can provide insight into the mechanisms controlling the coordination of C4 photosynthesis. For example, transitions from blue light to any other light quality lead to a relatively fast recovery of Anet in both species; however, ϕ increased sharply in M. giganteus, but there was little change in maize (Fig. 1). Additionally, in maize, Anet decreased significantly when shifted to blue light but without a change in ϕ (Fig. 1), suggesting that ϕ can be maintained regardless of changes in Anet. It has been demonstrated that Anet is typically determined by total available energy, whereas ϕ is determined by x (Siebke et al., 1997; von Caemmerer, 2000; Ubierna et al., 2011; Pengelly et al., 2012). Over time, the coordination of both cycles was achieved under all light qualities; however, the rate at which this balance was achieved differed between maize and M. giganteus.
Blue light reduction in rates of C4 photosynthesis has been described before by Sun et al. (2012); therefore, this article focuses on the dynamic coordination between the C3 and C4 cycles during the transition from one light quality to another. In brief, the decrease in photosynthesis under blue light is the result of reduced energy availability to drive ATP and NADPH production. This is demonstrated by the measured changes in metabolite pools and the decrease in ϕPSII under blue light. First, the analysis of photosynthetic metabolite concentrations demonstrates lower ATP and NADPH availability under blue light when Anet is lower in both species. For example, pyruvate content under blue light was greater than under red light (Fig. 5C; Supplemental Table S2), which implies insufficient energy supply under blue light to convert pyruvate to PEP in mesophyll cells (Leegood and von Caemmerer, 1988, 1989; Sun et al., 2012). Moreover, TP content was lower under blue light relative to red light (Fig. 5E; Supplemental Table S2), whereas there were no differences in PGA content between red and blue light (Fig. 5F; Supplemental Table S2). These results suggest a lack of reducing equivalent for the reduction of PGA to TP.
There was also a strong positive relationship between Anet and ϕPSII (Fig. 4A) in response to light treatments, with lower ϕPSII under blue light (Supplemental Fig. S2). During C4 photosynthesis, there is typically a linear dependence of ϕPSII and rates of CO2 assimilation because the assimilation of CO2 is the primary sink for NADPH generated by electron flux through PSII (Edwards and Baker, 1993). The lower ϕPSII under blue light observed in both species may result from (1) an imbalance in absorption between PSI and PSII within the mesophyll chloroplast, leading to an overreduction of the chloroplast electron transport chain (Evans, 1986); (2) a change in the distribution of light energy absorption between the PSII-enriched mesophyll and the PSII-depleted bundle-sheath chloroplast; (3) an increase in nonphotochemical quenching; and/or (4) an overall decrease in the absorption of energy by the chlorophyll under blue light due to strong absorption of blue light by carotenoids and nonphotosynthetic structures within the leaf. As discussed below, some of these explanations will not only affect the total available energy but also x and, therefore, the overall efficiency of the CO2-concentrating mechanism.
Coordination of the C3 and C4 Cycles in Response to Transient Changes in Light Quality
Measurements of Δ13C indicated transitory changes in the coordination of the CO2-concentrating mechanism in response to changes in light quality for both maize (Fig. 1I) and M. giganteus (Fig. 1J). This response lasted about 1 h in M. giganteus but only a few minutes in maize (Figs. 1 and 2). As discussed above, and in more detail below, these differences between species may be associated with the ability to coordinate the production of ATP and NADPH under changing light environments. Transition from any light treatment to blue light caused a reduction in Δ13C, while the reverse transition from blue light to any other light quality resulted in increased Δ13C (Figs. 1 and 2).
A comparison of leaf-level models of Δ13C and C4 photosynthesis was used to investigate the variation of ϕ in response to rapid changes in light quality. This consisted of fitting measurements of Δ13C and gas exchange into theoretical models of C4 photosynthesis and leaf CO2 discrimination (Ubierna et al., 2013) and solving for several parameters of interest. These included x, Pbs, and the rates of Rubisco oxygenation (vo) versus carboxylation (vc; Fig. 3). The C4 photosynthesis model assumes steady-state conditions, which may be violated during the transition in light quality. However, there were no conditions where modeled parameters were outside physiologically feasible values. Additionally, this modeling assumed that the conductance of CO2 between the bundle-sheath and mesophyll cells was constant. This may be an oversimplification; however, there is currently no evidence that bundle-sheath conductance (gbs) changes in response to short-term manipulations in measurement conditions. Given these assumptions, these data illustrated that the transition from blue light to other light qualities resulted in a larger fraction of available energy allocated to the C4 cycle, resulting in an increase in Pbs, a decrease in vo/vc, and an increase in ϕ (Fig. 3, A and B). The change in ϕ was larger in M. giganteus compared with maize. This suggests a faster coordination of the C3 and C4 cycles in maize compared with M. giganteus. Unfortunately, this modeling does not specifically identify the mechanism driving the change in energy allocation, but it does demonstrate that changes in light quality cause variations in the production of energy and reducing equivalents between the mesophyll and bundle-sheath cells.
Additionally, under blue light, there was a lower ratio of vH+/LEF (Fig. 4B). A lower vH+/LEF could be interpreted as lower activity of bundle-sheath cyclic electron flux; however, the ECS measurements integrate across the leaf cross section, and it is impossible to distinguish between the contributions of mesophyll and bundle-sheath cells. Nevertheless, it is reasonable that blue light would decrease cyclic electron flux preferentially in the bundle-sheath cells because of the limited penetration of blue light into the bundle-sheath cells (Evans and Vogelmann, 2003; Evans et al., 2007) and the insufficient blue light absorption by bundle-sheath PSI (Evans, 1986). This would limit the Calvin-Benson-Bassham cycle, because approximately 50% of the ATP requirement for carbon assimilation is thought to be provided by bundle-sheath cyclic electron flux (Kanai and Edwards, 1999). In M. giganteus, there was a positive relationship of ϕ and vH+/LEF, with higher ϕ and higher vH+/LEF under red light compared with blue light. However, over time after the initial change in light quality, there was a relatively large decrease in ϕ and only a slight increase in vH+/LEF for M. giganteus. Alternatively, in maize, there was no change in ϕ between light treatments, but vH+/LEF increased from blue to red light (Fig. 4D). This indicates that changes in vH+/LEF and corresponding changes in ATP/NADPH production in maize may help maintain a balance in metabolic flux between the C3 and C4 cycles and low ϕ. For example, in maize, increased cyclic electron flux under red light could drive additional phosphoenolpyruvate carboxylase (PEPC) refixation of leaked CO2 from the bundle-sheath cells, maintaining a constant Δ13C and ϕ. In M. giganteus, there was only a small shift in vH+/LEF between blue and red light compared with maize, suggesting that change in cyclic electron flux in M. giganteus was not the only mechanism that ultimately balances the energy production and coordination between the C3 and C4 cycles. These findings in M. giganteus are similar to those found in F. bidentis, where the ratio of PSI to PSII quantum yields did not change in plants with higher ϕ due to genetically reduced levels of Rubisco, suggesting that both linear and cyclic electron flux contribute to the production of ATP with increased ϕ (Siebke et al., 1997)
Chloroplast movement could also influence the metabolic and energy coordination between the C3 and C4 cycles. It has long been reported that blue light induces chloroplast movement to optimize photosynthetic activity and/or to prevent photodamage under strong light in both C3 and C4 species (Senn, 1908; Inoue and Shibata, 1974; Kagawa et al., 2001; Wada et al., 2003; Yamada et al., 2009; Luesse et al., 2010; Maai et al., 2011). In C4 plants, blue light induced chloroplast movement in mesophyll but not in bundle-sheath cells (Yamada et al., 2009). This differential response could result in changes to the energy allocation between the C3 (bundle-sheath) and C4 (mesophyll) cycles, different rates of linear versus cyclic electron flux, and changes in the metabolite flux between both compartments, all of which could affect the coordination and efficiency of C4 photosynthesis. There was more chloroplast movement, estimated as ΔT, in response to the transition in light quality in maize compared with M. giganteus (Fig. 7). This corresponded to the transitional variation in ϕ and x in response to light quality in M. giganteus but not in maize. Although the apparent chloroplast movement was relatively small, it does suggest that, in maize, it may help balance light energy distribution between the mesophyll and bundle-sheath cells, leading to a coordination of C3 and C4 cycle activities. In contrast, the lack of chloroplast movement in M. giganteus might be related to the longer time to coordinate the activities of C3 and C4 cycles in this species.
Although ϕ responded differently to light transitions in maize and M. giganteus, there were no significant differences in the rapid response of the contents and ratios of metabolites between species (Figs. 5 and 6; Supplemental Tables S1 and S2). However, as demonstrated previously by Sun et al. (2012), there were strong correlations between Anet and various metabolite concentrations (Figs. 5E and 6; Supplemental Tables S1 and S2). However, the lack of detectible transitional variation in photosynthetic metabolites may partially be attributed to the inability to measure metabolite fluxes between the mesophyll and bundle-sheath cells as well as an inability to determine mesophyll- or bundle-sheath-specific changes in metabolite concentrations.
Finally, the observed transitional variation in Anet and ϕ could be caused by light quality-associated differences in stomatal heterogeneity. Stomatal heterogeneity or patchiness has been observed in many species, particularly in response to environmental stress conditions (Terashima et al., 1988; Mott and Buckley, 1998). Stomatal heterogeneity could alter the estimation of Δ13C and Pi/Pa by affecting the relationship between assimilation rate and stomatal conductance (Lloyd et al., 1992). However, Lloyd et al. (1992) demonstrated that the effects of stomatal heterogeneity on the estimation of Δ13C are significant only under low light and low rates of stomatal conductance. We measured transitional variation in CO2 assimilation rate and Δ13C at a constant photon density of 900 µmol m−2 s−1 for all light treatments. Furthermore, the rates of stomatal conductance were relatively high under all light treatments (Fig. 1, C and D). Therefore, the effects of stomatal heterogeneity on the variation in Anet and Δ13C are likely minimal in both species.
CONCLUSION
The in vivo coordination of the C3 and C4 cycles is complex and difficult to evaluate, requiring in many cases the interpretation of indirect evidence. We used several tools, including measurements of gas exchange, Δ13C, metabolite pools, ECS, and chloroplast movement, to characterize C4 photosynthesis in response to changes in light quality. In both maize and M. giganteus, there was a lower efficiency of PSII under blue light, suggesting that the reduction in Anet under this condition was caused by insufficient production of NADPH through LEF. This was further supported by measurement of the pools of C3 and C4 metabolites. In maize, x was quickly optimized to minimize ϕ; however, it took almost 1 h for M. giganteus to achieve steady-state values of ϕ. Changes in linear versus cyclic electron flux in M. giganteus were slower to respond to light quality compared with those in maize. This may have led to an imbalance in the coordination of the C3 and C4 pathways. Additionally, the rapid rearrangement of chloroplasts under blue light in maize likely optimized light energy production between the mesophyll and bundle-sheath cells. Together, these responses in maize may have helped coordinate the C4 photosynthetic pathway and potentially increased PEPC refixation of CO2 leaked from the bundle-sheath cells. In M. giganteus, there was a quick change in ϕPSII, but cyclic electron flux and chloroplast movement were not as responsive to changes in light quality. This potentially resulted in an imbalance of energy, leading to the overpumping of CO2 and increased ϕ. However, in both species, given enough time, the activities of C3 and C4 cycles appear to balance and maintain the efficiency of the C4-concentrating mechanism.
MATERIALS AND METHODS
Plants
Maize (Zea mays var. Trucker’s Favorite; Victory Seed Company) seeds and Miscanthus × giganteus rhizomes were planted in 6-L pots. The plants were grown in a greenhouse with day temperature of 25°C to 28°C, night temperature of 20°C to 25°C, and daylength of 14 h. Illumination in the greenhouse was a combination of sunlight and supplementary light provided by 400-W high-pressure sodium lamps. Plants were watered daily and fertilized weekly with Peters 20-20-20 (J.R. Peters) and a slow-release fertilizer (17-3-6 monopotassium phosphate). Four-week-old maize and 3-month-old M. giganteus were used for the measurements.
Gas Exchange and Online Leaf Δ13C
Leaf gas exchange and online Δ13C were measured on the uppermost fully expanded maize and M. giganteus leaves using the LI-COR 6400xt gas-exchange analyzer with the opaque conifer chamber and the RGB light source (LI-COR 6400-18; LI-COR Biosciences) coupled to a TDLAS (TGA 100A; Campbell Scientific). The RGB light source generates a broad-spectrum red, green, and blue light with peak wavelengths of 635, 522, and 460 nm and bandwidths of 16, 35, and 24 nm, respectively. The 12CO2 and 13CO2 partial pressures in the LI-COR reference and sample cells were measured by the TDLAS concurrently with a CO2-free tank and two standard tanks (Liquid Technology) with known 12CO2 and 13CO2 partial pressures. The TDLAS was sampled from each of the five sites at a flow rate of 150 mL min−1 and a frequency of 40 s per site. Only the last 10 s at each site was used for the calculation of 12CO2 and 13CO2 partial pressures. The partial pressures of 12CO2 and 13CO2 in the reference and sample lines were calibrated using a gain and offset calculated from the two calibration tanks (Bowling et al., 2003; Ubierna et al., 2011; Sun et al., 2012). The leaves were acclimated to 900 µmol m−2 s−1 RGB for 1 h in the opaque conifer chamber. The light treatment was then shifted in the order RGB, blue, red, blue, green, blue, and RGB, with an interval of 1 h per light treatment. The irradiance of 900 µmol m−2 s−1 was the maximum light intensity all light treatments could achieve with the LI-COR 6400-18 RGB light source. The CO2 partial pressure in the leaf chamber was maintained at 38.4 Pa, a leaf temperature of 25°C, and a relative humidity between 50% and 70% for all gas-exchange measurements. The simultaneous gas exchange and Δ13C measurements were conducted on four individual plants of maize and M. giganteus.
Δ13C was calculated as (Evans et al., 1986):
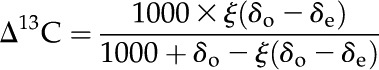 |
(1) |
where δe and δo represent the isotopic composition of CO2 entering and leaving the leaf chamber, respectively; ξ is calculated as:
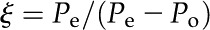 |
(2) |
where Pe and Po are the partial pressures of CO2 in the dry air entering and leaving the leaf chamber, respectively.
Leakiness
Following the recommendation of Ubierna et al. (2013), CO2 ϕ was estimated by rearranging the equation proposed by Farquhar (1983) and Farquhar and Cernusak (2012):
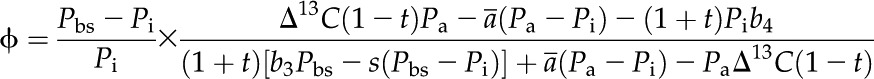 |
(3) |
where Pa, Pi, and Pbs are CO2 partial pressures in the atmosphere, the intercellular air spaces, and the bundle-sheath cells, respectively. The term 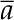 is the weighted fractionation across the boundary layer and stomata:
is the weighted fractionation across the boundary layer and stomata:
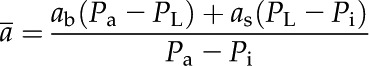 |
(4) |
where ab (2.9‰) and as (4.4‰) are the fractionation across the boundary layer and stomata, respectively. PL is CO2 partial pressure at the leaf surface. The terms b3 and b4 are defined as (Farquhar, 1983):
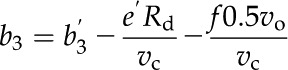 |
(5) |
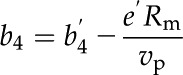 |
(6) |
where Rd is the nonphotorespiratory CO2 release in the light (assumed to equal measured rates of dark respiration, 1.80 and 1.27 µmol m−2 s−1 for maize and M. giganteus, respectively); Rm is the mesophyll cell dark respiration rate, which is assumed to be half of the Rd (von Caemmerer, 2000); vc and vo are as already defined and vp is the PEP carboxylation rate (for details on the modeling of vc, vo, vp, and Pbs, see Ubierna et al. [2011]); b′3 is fractionation by Rubisco, 30‰ (Roeske and O’Leary, 1984); b′4 represents the net effect of CO2 dissolution, hydration, and PEPC activity, which at 25°C has a value of −5.7‰ (Farquhar, 1983; Henderson et al., 1992); f is fractionation during photorespiration, 11.6‰ (Lanigan et al., 2008); and e′ is fractionation associated with dark respiration, which is calculated as (Wingate et al., 2007):
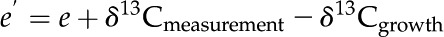 |
(7) |
where e is fractionation during decarboxylation, assumed to equal −6‰ (Ghashghaie et al., 2001; Sun et al., 2010); δ13Cmeasurement (−31‰) is the carbon isotope signature of CO2 used for the online discrimination measurement, which was measured by the TDLAS; and δ13Cgrowth is the carbon isotope composition of CO2 of the growth conditions, which is assumed to be −8‰.
The ternary effects term t is defined as (Farquhar and Cernusak, 2012):
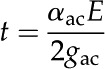 |
(8) |
where E is the transpiration rate, gac is the conductance to diffusion of air in CO2, and 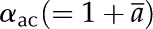 is the fractionation factor for the isotopologs of CO2 diffusing in air.
is the fractionation factor for the isotopologs of CO2 diffusing in air.
gbs was estimated from the least-square difference between predicted and observed leaf discrimination using data obtained from the first 1-h RGB measurements (Kromdijk et al., 2010; Ubierna et al., 2011). This resulted in gbs values of 0.0046 μmol m−2 s−1 Pa−1 for maize and 0.01 μmol m−2 s−1 Pa−1 for M. giganteus, which are within the range of values previously suggested for this parameter of 0.005 to 0.02 μmol m−2 s−1 Pa−1 (He and Edwards, 1996; von Caemmerer and Furbank, 2003).
Modeling Energy Partitioning between the C3 and C4 Cycles (x)
The light-limited C4 photosynthesis model (von Caemmerer, 2000), which describes the relationships between total electron flux (Jt) and Anet, was used to model how x could explain the changes in Δ13C observed in Figure 1. Jt can be partitioned between the C4 cycle (Jm) and the C3 cycle (Js) by x, which is the portion of ATP required for Jm, and 1 − x is the requirement for Js. The C4 photosynthetic pathway has a theoretical minimum energetic cost of five ATPs, three for the C3 cycle and two required to regenerate PEP from pyruvate in the mesophyll cells, so it is generally assumed that x = 0.4 (von Caemmerer, 2000). Therefore, during the first 1 h of RGB, x was assumed to be 0.4; however, for other light treatments, x was solved to minimize the least-square difference between modeled and measured Δ13C. Currently, there is no evidence that gbs changes in response to variation in measurement conditions. Therefore, during this modeling, it was assumed that gbs (0.0046 μmol m−2 s−1 Pa−1 for maize and 0.01 μmol m−2 s−1 Pa−1 for M. giganteus) and dark-type respiration (1.80 and 1.27 µmol m−2 s−1 for maize and M. giganteus, respectively) were constant for all light treatments and mesophyll conductance to CO2 was infinite. Additionally, the modeled relationship between light, Anet, and Jt was used to calculate Pbs and vo/vc (Fig. 3), as described (Ubierna et al., 2011; Supplemental Equations S1). To demonstrate the sensitivity of x, Pbs, and vo/vc to gbs, these parameters were modeled with gbs of 0.0069 and 0.0023 μmol m−2 s−1 Pa−1 for maize and 0.015 and 0.005 μmol m−2 s−1 Pa−1 for M. giganteus (Fig. 3, shaded areas).
Chlorophyll a Fluorescence and ECS
Measurements were performed with a LI-COR 6400 gas-exchange analyzer coupled to a nonfocusing optics spectrophotometer/chlorophyll fluorometer (Avenson et al., 2004, 2005; Kiirats et al., 2010). In brief, 0.79 cm2 of leaf was clamped into a custom-built leaf chamber that allowed simultaneous measurements of net CO2 exchange, chlorophyll fluorescence, and leaf optical properties. The leaf chamber was maintained at 38.4 Pa of CO2 and 900 µmol photon m−2 s−1. Leaves were acclimated for 1 h under blue actinic light (460 nm), and then the actinic light was switched to red (637 nm) for 1 h and then to blue for 1 h. Measurements of chlorophyll a fluorescence and 520-nm absorbance in the dark were taken every 10 min per light treatment. The measuring and actinic light were the same wavelength for the measurements of chlorophyll a fluorescence.
The ϕPSII derived from fluorescence measurements (Genty et al., 1989) was used to estimate the LEF as ϕPSII × Absleaf × fractionPSII (Donahue et al., 1997; Avenson et al., 2005), where Absleaf and fractionPSII are leaf absorbance and the fraction of light energy capture by PSII as compared with total photosystems, respectively. Leaf absorbance was determined from measurements of reflectance and transmittance as described previously by Sun et al. (2012), and the fractionPSII was assumed to be 0.5. Absorbance changes at 520 nm from rapid light-to-dark transitions were used to estimate ECS. The total proton motive force was defined as the amplitude of ECS decay from steady state to a quasistable minimum after a 300-ms dark period (Avenson et al., 2004, 2005). The relative rate of vH+ was estimated by ECS decay kinetics (Avenson et al., 2004, 2005; Livingston et al., 2010).
Rapid Freeze Clamping of Leaves and Photosynthetic Metabolite Measurements
Leaves similar in age and position to those used for the transitional gas exchange and Δ13C measurements were rapidly freeze clamped in situ using a custom-built rapid kill system attached to a LI-COR 6400 gas-exchange analyzer (Badger et al., 1984; Hendrickson et al., 2008). Leaves were acclimated to either red or blue light for 1 h at a light intensity of 900 µmol quanta m−2 s−1, a sample CO2 partial pressure of 38.4 Pa, and a leaf temperature of 25°C. Leaves were then rapidly freeze clamped between two liquid nitrogen-cooled copper rods at 2, 10, 30, and 60 min after light was switched from red to blue or blue to red. The frozen leaf discs were stored in a −80°C deep freezer prior to extraction for photosynthetic metabolites and Rubisco activation state assays (Sun et al., 2012). Leaf discs (approximately 4.5 cm2) were ground to a fine powder in liquid nitrogen in a precooled pestle and mortar and extracted with 1 m HClO4 (Leegood and von Caemmerer, 1988). OAA, pyruvate, and PEP were measured consecutively in an enzymatic assay by monitoring changes in absorbance at 340 nm (Leegood and Furbank, 1984). TP, PGA, and RuBP were also assayed spectrophotometrically by coupled enzyme as described previously (He et al., 1997) using Rubisco purified from tobacco (Nicotiana tabacum).
Rubisco Activation State
Rubisco activity was determined by the incorporation of 14CO2 into acid-stable products at 25°C (Salvucci and Anderson, 1987). The initial activity was determined by adding 20 µL of the leaf extract to the reaction mixture (100 mm Tricine-NaOH, pH 8.0, 10 mm MgCl2, 10 mm NaH14CO3 [0.2 µCi µmol−1], and 0.4 mm RuBP) and quenching the reaction after 30 s with 100 µL of acid (1 n HCl/4 n formic acid). Total activity was measured after incubating 20 µL of the same leaf extract in the reaction mixture for 3 min at 25°C minus RuBP to carbamylate all Rubisco catalytic sites. Subsequently, the reaction was initiated with RuBP and quenched after 30 s. Rubisco activation state was determined by the ratio of initial to total Rubisco activity.
Chloroplast Movement
Chloroplast movement was determined from the ΔT during exposure to different wavelengths of light (DeBlasio et al., 2003). Leaves were clamped to an integrating sphere (Labsphere) in a custom-built apparatus that included a multispectral light-emitting diode light source (Cree) that could be toggled between preset light qualities. Red, RGB, and blue light intensity was adjusted so that incident light had a similar intensity to that used during gas-exchange measurements. A photometer (LI-COR Biosciences) was attached to the integrating sphere, and light intensity was averaged over 5-min intervals using a data logger (Campbell Scientific). Leaves were exposed for 1 h each to the following light regime: RGB, blue, red, blue, and RGB. Reference traces were measured under the same regime without a leaf and used to calculate ΔT according to:
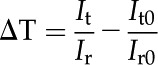 |
(9) |
where It and Ir are the light intensities measured under a given light treatment and time point for the leaf and reference runs, respectively. It0 and Ir0 are the averaged light intensities from the leaf and reference runs measured during the initial 5 min of each light quality treatment. Therefore, ΔT is defined as the difference between the initial averaged 5 min of transmittance of a given light quality and the transmittance at a given time point within the 60-min exposure.
Statistical Analyses
Three-way ANOVA was conducted to assess the effects of species (maize and M. giganteus), time (2, 10, 30, and 60 min), and light treatment (red and blue) on the contents of OAA, pyruvate, PEP, RuBP, TP, and PGA, the ratios of C3 to C4 metabolites (TP to PGA, RuBP to PGA, and PGA to PEP), and Rubisco activation state. Statistical analyses were carried out using SAS version 9.0 (SAS Institute). Average values are reported as arithmetic means ± se.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Comparison in gas exchange measurements between gas analyzers coupled to a tunable diode laser and an electrochromatic shift spectrophotometer.
Supplemental Figure S2. Transitional variation in gas exchange parameters, chlorophyll a fluorescence, and electrochromatic shift.
Supplemental Table S1. Metabolite profiles.
Supplemental Table S2. Statistical analysis.
Supplemental Equations S1. Calculation of total electron transport rate and CO2 partial pressure inside the bundle-sheath cells.
Acknowledgments
We thank Chuck Cody for plant growth management, Dr. Steve Long for M. giganteus plant material, and Gordon Stack, Sarah Waldo, and George Mount for their assistance and technical guidance in measuring leaf transmittance.
Glossary
- RuBP
ribulose-1,5-bisphosphate
- Δ13C
carbon isotope discrimination
- RGB
red, green, and blue light
- ϕ
leakiness
- Anet
net rate of CO2 assimilation
- gs
stomatal conductance
- Pi/Pa
ratio of intercellular to ambient CO2 partial pressure
- Pbs
bundle-sheath CO2 partial pressure
- vo/vc
Rubisco oxygenation-carboxylation ratio
- ECS
electrochromatic shift
- TDLAS
tunable diode laser absorption spectroscope
- ϕPSII
efficiency of photosystem II
- vH+
proton flux across the thylakoid membrane
- LEF
linear electron flux
- OAA
oxaloacetic acid
- TP
triose phosphate
- PEP
phosphoenolpyruvate
- PGA
phosphoglycerate
- ΔT
change in leaf transmittance
- vo
rate of Rubisco oxygenation
- vc
rate of Rubisco carboxylation
- gbs
bundle-sheath conductance
- PEPC
phosphoenolpyruvate carboxylase
- Jt
total electron flux
- x
energy partitioning between the C3 and C4 cycles
Footnotes
This work was supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, Photosynthetic Systems, Department of Energy (grant nos. DE–FG02–09ER16062 and DE–FG02–04ERl5559), by the National Science Foundation (grant no. 0923562 to A.B.C.), and by the National Natural Science Foundation of China (grant no. 31270445 to W.S.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Avenson TJ, Cruz JA, Kanazawa A, Kramer DM. (2005) Regulating the proton budget of higher plant photosynthesis. Proc Natl Acad Sci USA 102: 9709–9713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson TJ, Cruz JA, Kramer DM. (2004) Modulation of energy-dependent quenching of excitons in antennae of higher plants. Proc Natl Acad Sci USA 101: 5530–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Sharkey TD, von Caemmerer S. (1984) The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta 160: 305–313 [DOI] [PubMed] [Google Scholar]
- Bowling DR, Sargent SD, Tanner BD, Ehleringer JR. (2003) Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem-atmosphere CO2 exchange. Agric For Meteorol 118: 1–19 [Google Scholar]
- Brown WV, Smith BN. (1972) Grass evolution, the Kranz syndrome, 13C/12C ratios, and continental drift. Nature 239: 345–346 [DOI] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S. (2006) Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis: insights from antisense RNA in Flaveria bidentis. Plant Physiol 141: 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasio SL, Mullen JL, Luesse DR, Hangarter RP. (2003) Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Physiol 133: 1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RA, Poulson ME, Edwards GE. (1997) A method for measuring whole plant photosynthesis in Arabidopsis thaliana. Photosynth Res 52: 263–269 [Google Scholar]
- Edwards GE, Baker NR. (1993) Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res 37: 89–102 [DOI] [PubMed] [Google Scholar]
- Evans JR. (1986) A quantitative analysis of light distribution between the two photosystems, considering variation in both the relative amounts of the chlorophyll-protein complexes and the spectral quality of light. Photobiochemistry and Photobiophysics 10: 135–147 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Evans JR, Vogelmann TC. (2003) Profiles of 14C fixation through spinach leaves in relation to light absorption and photosynthetic capacity. Plant Cell Environ 26: 547–560 [Google Scholar]
- Evans JR, Vogelmann TC, von Caemmerer S. (2007) Balancing light capture with distributed metabolic demand during C4 photosynthesis. In JE Sheehy, PL Mitchell, B Hardy, eds, Charting New Pathways to C4 Rice. World Scientific Publishing, Singapore, pp 127–143 [Google Scholar]
- Farquhar GD. (1983) On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol 10: 205–226 [Google Scholar]
- Farquhar GD, Cernusak LA. (2012) Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant Cell Environ 35: 1221–1231 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Hatch MD. (1987) Mechanism of C4 photosynthesis: the size and composition of the inorganic carbon pool in bundle sheath cells. Plant Physiol 85: 958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Ghashghaie J, Duranceau M, Badeck FW. (2001) δ13C of CO2 respired in the dark in relation to δ13C of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ 24: 505–515 [Google Scholar]
- Hatch MD, Slack CR. (1966) Photosynthesis by sugar-cane leaves: a new carboxylation reaction and the pathway of sugar formation. Biochem J 101: 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DX, Edwards GE. (1996) Estimation of diffusive resistance of bundle sheath cells to CO2 from modeling of C4 photosynthesis. Photosynth Res 49: 195–208 [DOI] [PubMed] [Google Scholar]
- He ZL, von Caemmerer S, Hudson GS, Price GD, Badger MR, Andrews TJ. (1997) Ribulose-1,5-bisphosphate carboxylase/oxygenase activase deficiency delays senescence of ribulose-1,5-bisphosphate carboxylase/oxygenase but progressively impairs its catalysis during tobacco leaf development. Plant Physiol 115: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD. (1992) Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19: 263–285 [Google Scholar]
- Hendrickson L, Sharwood R, Ludwig M, Whitney SM, Badger MR, von Caemmerer S. (2008) The effects of Rubisco activase on C4 photosynthesis and metabolism at high temperature. J Exp Bot 59: 1789–1798 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Shibata K. (1973) Light-induced chloroplast rearrangements and their action spectra as measured by absorption spectrophotometry. Planta 114: 341–358 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Shibata K. (1974) Comparative examination of terrestrial plant leaves in terms of light-induced absorption changes due to chloroplast rearrangements. Plant Cell Physiol 15: 717–721 [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kanai R, Edwards GE. (1999) The biochemistry of C4 photosynthesis. In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 49–87 [Google Scholar]
- Kiirats O, Kramer DM, Edwards GE. (2010) Co-regulation of dark and light reactions in three biochemical subtypes of C4 species. Photosynth Res 105: 89–99 [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Griffiths H, Schepers HE. (2010) Can the progressive increase of C4 bundle sheath leakiness at low PFD be explained by incomplete suppression of photorespiration? Plant Cell Environ 33: 1935–1948 [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Schepers HE, Albanito F, Fitton N, Carroll F, Jones MB, Finnan J, Lanigan GJ, Griffiths H. (2008) Bundle sheath leakiness and light limitation during C4 leaf and canopy CO2 uptake. Plant Physiol 148: 2144–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanigan GJ, Betson N, Griffiths H, Seibt U. (2008) Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiol 148: 2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC, Furbank RT. (1984) Carbon metabolism and gas exchange in leaves of Zea mays L.: changes in CO2 fixation, chlorophyll a fluorescence and metabolite levels during photosynthetic induction. Planta 162: 450–456 [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S. (1988) The relationship between contents of photosynthetic metabolites and the rate of photosynthetic carbon assimilation in leaves of Amaranthus edulis L. Planta 174: 253–262 [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S. (1989) Some relationships between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Zea mays L. Planta 178: 258–266 [DOI] [PubMed] [Google Scholar]
- Livingston AK, Kanazawa A, Cruz JA, Kramer DM. (2010) Regulation of cyclic electron flow in C3 plants: differential effects of limiting photosynthesis at ribulose-1,5-bisphosphate carboxylase/oxygenase and glyceraldehyde-3-phosphate dehydrogenase. Plant Cell Environ 33: 1779–1788 [DOI] [PubMed] [Google Scholar]
- Lloyd J, Syvertsen JP, Kriedemann PE, Farquhar GD. (1992) Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ 15: 873–899 [Google Scholar]
- Loreto F, Tsonev T, Centritto M. (2009) The impact of blue light on leaf mesophyll conductance. J Exp Bot 60: 2283–2290 [DOI] [PubMed] [Google Scholar]
- Luesse DR, DeBlasio SL, Hangarter RP. (2010) Integration of Phot1, Phot2, and PhyB signalling in light-induced chloroplast movements. J Exp Bot 61: 4387–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maai E, Shimada S, Yamada M, Sugiyama T, Miyake H, Taniguchi M. (2011) The avoidance and aggregative movements of mesophyll chloroplasts in C4 monocots in response to blue light and abscisic acid. J Exp Bot 62: 3213–3221 [DOI] [PubMed] [Google Scholar]
- Mott KA, Buckley TN. (1998) Stomatal heterogeneity. J Exp Bot 49: 407–417 [Google Scholar]
- Pengelly JJL, Sirault XRR, Tazoe Y, Evans JR, Furbank RT, von Caemmerer S. (2010) Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. J Exp Bot 61: 4109–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly JJL, Tan J, Furbank RT, von Caemmerer S. (2012) Antisense reduction of NADP-malic enzyme in Flaveria bidentis reduces flow of CO2 through the C4 cycle. Plant Physiol 160: 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeske CA, O’Leary MH. (1984) Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23: 6275–6284 [DOI] [PubMed] [Google Scholar]
- Sage RF. (1999) Why C4 photosynthesis? In RF Sage, RK Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 3–14 [Google Scholar]
- Salvucci ME, Anderson JC. (1987) Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol 85: 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn G. (1908) Die Gestalts-und Lageveranderung der Pflanzench-romatophoren. W Engelmann, Leipzig, Germany [Google Scholar]
- Siebke K, Von Caemmerer S, Badger M, Furbank RT. (1997) Expressing an RbcS antisense gene in transgenic Flaveria bidentis leads to an increased quantum requirement for CO2 fixed in photosystems I and II. Plant Physiol 115: 1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Resco V, Williams DG. (2010) Nocturnal and seasonal patterns of carbon isotope composition of leaf dark-respired carbon dioxide differ among dominant species in a semiarid savanna. Oecologia 164: 297–310 [DOI] [PubMed] [Google Scholar]
- Sun W, Ubierna N, Ma JY, Cousins AB. (2012) The influence of light quality on C4 photosynthesis under steady-state conditions in Zea mays and Miscanthus × giganteus: changes in rates of photosynthesis but not the efficiency of the CO2 concentrating mechanism. Plant Cell Environ 35: 982–993 [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Hanba YT, Furumoto T, Noguchi K, Terashima I. (2008) Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant Cell Physiol 49: 19–29 [DOI] [PubMed] [Google Scholar]
- Tazoe Y, Noguchi K, Terashima I. (2006) Effects of growth light and nitrogen nutrition on the organization of the photosynthetic apparatus in leaves of a C4 plant, Amaranthus cruentus. Plant Cell Environ 29: 691–700 [DOI] [PubMed] [Google Scholar]
- Terashima I, Wong SC, Osmond CB, Farquhar GD. (1988) Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant Cell Physiol 29: 385–394 [Google Scholar]
- Trojan A, Gabrys H. (1996) Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol 111: 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Cousins AB. (2011) The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. J Exp Bot 62: 3119–3134 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Kramer DM, Cousins AB. (2013) The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus × giganteus and Flaveria bidentis. Plant Cell Environ 36: 365–381 [DOI] [PubMed] [Google Scholar]
- Vogelmann TC, Evans JR. (2002) Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence. Plant Cell Environ 25: 1313–1323 [Google Scholar]
- von Caemmerer S. (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia [Google Scholar]
- von Caemmerer S, Furbank RT. (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77: 191–207 [DOI] [PubMed] [Google Scholar]
- Wada M, Kagawa T, Sato Y. (2003) Chloroplast movement. Annu Rev Plant Biol 54: 455–468 [DOI] [PubMed] [Google Scholar]
- Wingate L, Seibt U, Moncrieff JB, Jarvis PG, Lloyd J. (2007) Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant Cell Environ 30: 600–616 [DOI] [PubMed] [Google Scholar]
- Yamada M, Kawasaki M, Sugiyama T, Miyake H, Taniguchi M. (2009) Differential positioning of C4 mesophyll and bundle sheath chloroplasts: aggregative movement of C4 mesophyll chloroplasts in response to environmental stresses. Plant Cell Physiol 50: 1736–1749 [DOI] [PubMed] [Google Scholar]