Abstract
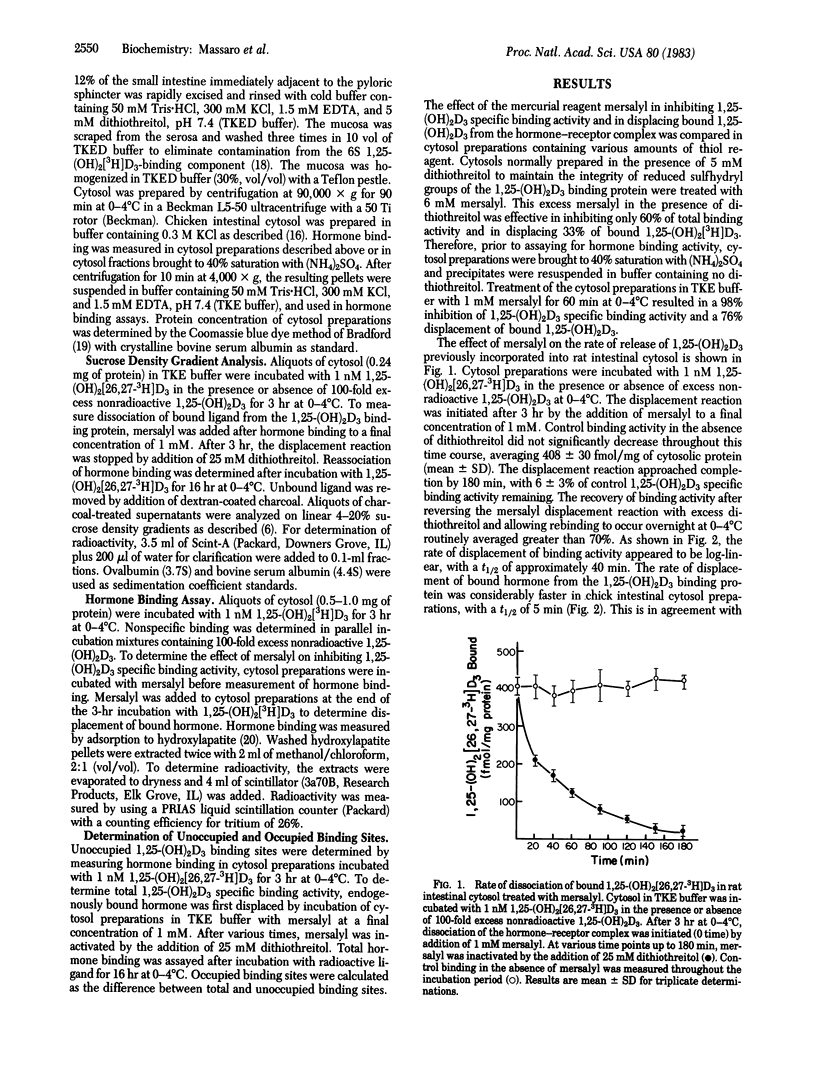
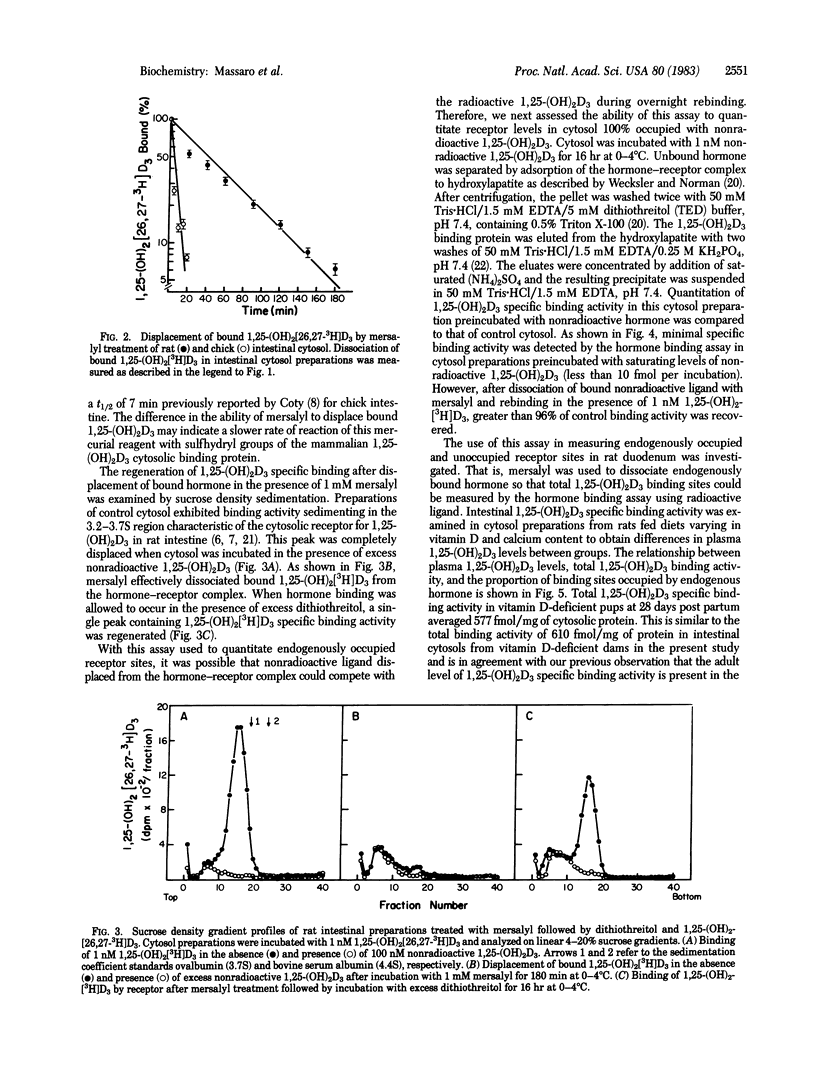
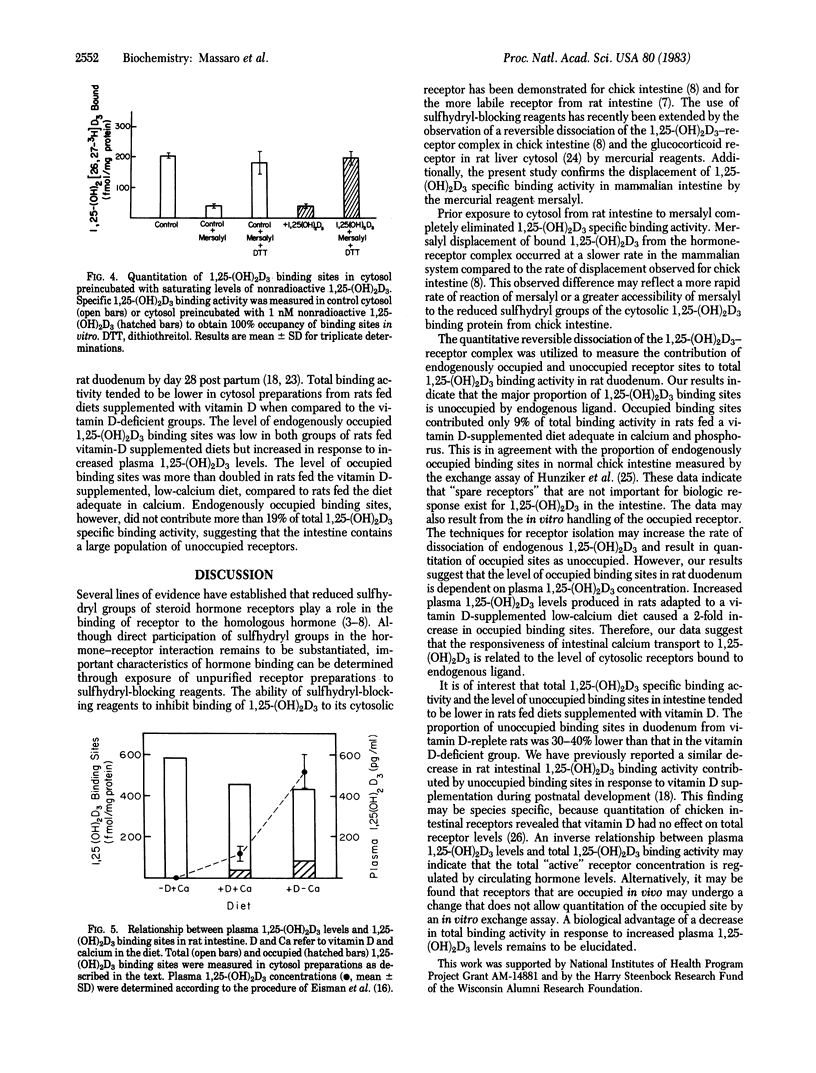
The quantitative reversible dissociation of the 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3]-receptor complex by the mercurial reagent mersalyl was used to develop an assay for endogenously occupied and unoccupied 1,25-(OH)2D3 binding sites. Incubation of intestinal cytosol preparations in buffer containing 50 mM Tris . HCl, 300 mM KCl, and 1.5 mM EDTA, pH 7.4, with 1 mM mersalyl for 60 min was effective in inhibiting 98% of 1,25-(OH)2D3 specific binding activity. Dissociation of bound 1,25-(OH)2[26,27-3H]D3 from the hormone-receptor complex approached completion by 180 min. In cytosol incubated with saturating levels of nonradioactive hormone, 96% of total binding activity was measurable with the hormone binding assay after displacement of bound nonradioactive ligand with 1 mM mersalyl. Endogenously occupied 1,25-(OH)2D3 binding sites contributed 0, 9, and 19% of total binding activity in rats with plasma 1,25-(OH)2D3 levels averaging 2, 121 +/- 36 and 516 +/- 92 pg/ml, respectively. Therefore, the major fraction of cytosolic 1,25-(OH)2D3 specific binding activity is unoccupied in rat intestine. The results suggest that only a small proportion of the measurable receptors are in the bound form to provide maximal 1,25-(OH)2D3-induced calcium transport.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji A., Kalimi M. Development of an [3H] glucocorticoid exchange assay in rat liver cytosol. Steroids. 1981 Apr;37(4):409–421. doi: 10.1016/0039-128x(81)90043-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Cone C. M., Morey-Holton E., Feldman D. Glucocorticoid regulation of 1,25(OH)2-vitamin D3 receptors in cultured mouse bone cells. J Biol Chem. 1982 Nov 25;257(22):13564–13569. [PubMed] [Google Scholar]
- Coty W. A. Reversible dissociation of steroid hormone x receptor complexes by mercurial reagents. J Biol Chem. 1980 Sep 10;255(17):8035–8037. [PubMed] [Google Scholar]
- DeLuca H. F., Franceschi R. T., Halloran B. P., Massaro E. R. Molecular events involved in 1,25-dihydroxyvitamin D3 stimulation of intestinal calcium transport. Fed Proc. 1982 Jan;41(1):66–71. [PubMed] [Google Scholar]
- Eisman J. A., Hamstra A. J., Kream B. E., DeLuca H. F. A sensitive, precise, and convenient method for determination of 1,25-dihydroxyvitamin D in human plasma. Arch Biochem Biophys. 1976 Sep;176(1):235–243. doi: 10.1016/0003-9861(76)90161-2. [DOI] [PubMed] [Google Scholar]
- Feldman D., McCain T. A., Hirst M. A., Chen T. L., Colston K. W. Characterization of a cytoplasmic receptor-like binder for 1 alpha, 25-dihydroxycholecalciferol in rat intestinal mucosa. J Biol Chem. 1979 Oct 25;254(20):10378–10384. [PubMed] [Google Scholar]
- Franceschi R. T., Simpson R. U., DeLuca H. F. Binding proteins for vitamin D metabolites: serum carriers and intracellular receptors. Arch Biochem Biophys. 1981 Aug;210(1):1–13. doi: 10.1016/0003-9861(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Halloran B. P., DeLuca H. F. Appearance of the intestinal cytosolic receptor for 1,25-dihydroxyvitamin D3 during neonatal development in the rat. J Biol Chem. 1981 Jul 25;256(14):7338–7342. [PubMed] [Google Scholar]
- Herman T. S., Fimognari G. M., Edelman I. S. Studies on renal aldosterone-binding proteins. J Biol Chem. 1968 Jul 25;243(14):3849–3856. [PubMed] [Google Scholar]
- Hunziker W., Walters M. R., Bishop J. E., Norman A. W. Effect of vitamin D status on the equilibrium between occupied and unoccupied 1,25-dihydroxyvitamin D intestinal receptors in the chick. J Clin Invest. 1982 Apr;69(4):826–833. doi: 10.1172/JCI110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Walters M. R., Norman A. W. 1,25-dihydroxyvitamin D3 receptors. Differential quantitation of endogenously occupied and unoccupied sites. J Biol Chem. 1980 Oct 25;255(20):9534–9537. [PubMed] [Google Scholar]
- Jensen E. V., Hurst D. J., DeSombre E. R., Jungblut P. W. Sulfhydryl groups and estradiol-receptor interaction. Science. 1967 Oct 20;158(3799):385–387. doi: 10.1126/science.158.3799.385. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S. Dynamics of steroid hormone receptor action. Annu Rev Physiol. 1980;42:17–35. doi: 10.1146/annurev.ph.42.030180.000313. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Yamada S., Schnoes H. K., DeLuca H. F. Specific cytosol-binding protein for 1,25-dihydroxyvitamin D3 in rat intestine. J Biol Chem. 1977 Jul 10;252(13):4501–4505. [PubMed] [Google Scholar]
- Massaro E. R., Simpson R. U., DeLuca H. F. Stimulation of specific 1,25-dihydroxyvitamin D3 binding protein in cultured postnatal rat intestine by hydrocortisone. J Biol Chem. 1982 Nov 25;257(22):13736–13739. [PubMed] [Google Scholar]
- Napoli J. L., Mellon W. S., Fivizzani M. A., Schnoes H. K., DeLuca H. F. Direct chemical synthesis of 1 alpha,25-dihydroxy[26,27-3H]vitamin D3 with high specific activity: its use in receptor studies. Biochemistry. 1980 May 27;19(11):2515–2521. doi: 10.1021/bi00552a033. [DOI] [PubMed] [Google Scholar]
- Omdahl J., Holick M., Suda T., Tanaka Y., DeLuca H. F. Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry. 1971 Jul 20;10(15):2935–2940. doi: 10.1021/bi00791a022. [DOI] [PubMed] [Google Scholar]
- Pike J. W. Evidence for a reactive sulfhydryl in the DNA binding domain of the 1,25-dihydroxyvitamin D3 receptor. Biochem Biophys Res Commun. 1981 Jun;100(4):1713–1719. doi: 10.1016/0006-291x(81)90716-6. [DOI] [PubMed] [Google Scholar]
- Sherman M. R., Corvol P. L., O'Malley B. W. Progesterone-binding components of chick oviduct. I. Preliminary characterization of cytoplasmic components. J Biol Chem. 1970 Nov 25;245(22):6085–6096. [PubMed] [Google Scholar]
- Simpson R. U., DeLuca H. F. Purification of chicken intestinal receptor for 1 alpha, 25-dihydroxyvitamin D3 to apparent homogeneity. Proc Natl Acad Sci U S A. 1982 Jan;79(1):16–20. doi: 10.1073/pnas.79.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr. 1970 Sep;100(9):1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Norman A. W. An hydroxylapatite batch assay for the quantitation of 1alpha,25-dihydroxyvitamin D3-receptor complexes. Anal Biochem. 1979 Jan 15;92(2):314–323. doi: 10.1016/0003-2697(79)90664-x. [DOI] [PubMed] [Google Scholar]
- Wecksler W. R., Ross F. P., Norman A. W. Characterization of the 1 alpha,25-dihydroxyvitamin D3 receptor from rat intestinal cytosol. J Biol Chem. 1979 Oct 10;254(19):9488–9491. [PubMed] [Google Scholar]


