Expression of a bacterial effector alters plant responses to phosphorus nutrient and a bacterial pathogen.
Abstract
Phytoplasmas have the smallest genome among bacteria and lack many essential genes required for biosynthetic and metabolic functions, making them unculturable, phloem-limited plant pathogens. In this study, we observed that transgenic Arabidopsis (Arabidopsis thaliana) expressing the secreted Aster Yellows phytoplasma strain Witches’ Broom protein11 shows an altered root architecture, similarly to the disease symptoms of phytoplasma-infected plants, by forming hairy roots. This morphological change is paralleled by an accumulation of cellular phosphate (Pi) and an increase in the expression levels of Pi starvation-induced genes and microRNAs. In addition to the Pi starvation responses, we found that secreted Aster Yellows phytoplasma strain Witches’ Broom protein11 suppresses salicylic acid-mediated defense responses and enhances the growth of a bacterial pathogen. These results contribute to an improved understanding of the role of phytoplasma effector SAP11 and provide new insights for understanding the molecular basis of plant-pathogen interactions.
Nutrients are essential for the growth of both host cells and pathogens. Host cells obtain nutrients for their own metabolism, but they also provide a favorable environment for pathogens to exploit. To defend against the growth of pathogens, host cells can develop a strategy to sequester key nutrients from the pathogens (Radtke and O’Riordan, 2006; Hood and Skaar, 2012). However, nutritional deficiencies also impair immune responses and alter the host susceptibility to pathogens. In general, successful pathogens have evolved various ways of manipulating host nutrition by modulating the uptake and utilization of nutrients to satisfy their requirements. Global transcriptome analysis has shown that intracellular bacteria, such as Listeria monocytogenes, can up-regulate genes required for nutritional stresses in mammalian cells (Chatterjee et al., 2006). In the case of Xanthomonas oryzae, the pathogenicity factor of Xanthomonas oryzae1, a transcriptional activator-like type III effector, can activate the expression of ORYZA SATIVA SUGAR TRANSPORTER11 (OsSWEET11) in controlling cellular sugar efflux (Chen et al., 2010). Thus, it is not surprising that nutritional immunity is a complex process, and nutrition is an important factor in regulating the interaction between a host and a pathogen.
Recently, a study on huanglongbing disease through small RNA profiling analysis revealed that the infection of Candidatus Liberibacter asiaticus (Ca. L. asiaticus), an intracellular bacterial pathogen, specifically induced microRNA 399 (miR399) in citrus plants (Zhao et al., 2013). MiR399 is induced by phosphate (Pi) starvation and has the ability to regulate the homeostasis of Pi by repressing the expression of PHOSPHATE2 (PHO2; Aung et al., 2006; Chiou et al., 2006). PHO2 encodes a ubiquitin E2 enzyme required for the degradation of PHOSPHATE TRANSPORTER1 (PHT1) Pi transporters and PHO1 involved in Pi uptake and translocation Pi starvation signaling (Liu et al., 2012; Huang et al., 2013). The Pi deficiency symptom associated with huanglongbing disease implies that the availability of Pi is an important factor for the disease development caused by Ca. L. asiaticus. The application of Pi reduces the disease symptoms of huanglongbing (Zhao et al., 2013).
Pi is required for the synthesis of nucleic acids and phospholipids, and it also plays an important function in energy metabolism and signaling transduction (Shen et al., 2011). Although Pi is an essential nutrient for plant growth and development, the amount of available Pi in the soil is limited (Raghothama, 1999; Jain et al., 2012). As a result, plants have evolved a way to improve their Pi acquisition by forming mutualistic symbiosis with arbuscular mycorrhizal (AM) fungi (Bucher et al., 2009; Ohkama-Ohtsu and Wasaki, 2010). Colonization by AM fungi can modify the plants’ root architecture and enhance the uptake of mineral nutrients, in particular Pi (Gu et al., 2011; Volpe et al., 2013). Interestingly, several studies also reported that AM fungi reduce the disease symptoms caused by phytoplasmas (Lingua et al., 2002; Romanazzi et al., 2009; Kamińska et al., 2010; Sampò et al., 2012).
Similar to Ca. L. asiaticus, phytoplasmas are intracellular bacterial pathogens and depend on sap-feeding insects for transmission (Lee et al., 2000). Phytoplasmas cause various disease symptoms, including witches’ broom, phyllody, virescence, bunchy roots, and stunting and yellowing of plants (Hogenhout et al., 2008). Owing to the significant reduction in the genome of phytoplasmas, they cannot be cultured in vitro and are restricted to phloem sieve cells with a rich growth environment (Oshima et al., 2004; Bai et al., 2006). Although phytoplasmas depend on their plant host for survival, they are able to manipulate the plant’s physiology through their secreted effectors (Sugio et al., 2011a).
Recent studies have shown that the protein 11 (SAP11AYWB) secreted by the Aster Yellows phytoplasma strain Witches’ Broom contains a nuclear localization signal for nuclear targeting outside phloem cells (Bai et al., 2009). Arabidopsis (Arabidopsis thaliana) plants that overexpress SAP11AYWB display crinkled leaves that resemble transgenic plants overexpressing miR319 (Sugio et al., 2011b). MiR319 has been shown to target transcripts of TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR1 (TCP) genes, which are involved in the control of cell proliferation in leaf morphogenesis and the biosynthesis of jasmonate (JA; Ori et al., 2007; Schommer et al., 2008). Although SAP11AYWB has not been shown to interfere with microRNA (miRNA) expression, it has been reported that it can destabilize TCP transcription factors through direct interaction (Sugio et al., 2011b). Because TCPs can directly regulate the expression of LIPOXGENASE2 (LOX2), a key component in the biosynthesis of JA, destabilization of TCPs leads to a decrease in JA (Schommer et al., 2008). In agreement with the findings that JA plays an important role in defense responses against insect herbivores, impairment of JA biosynthesis in SAP11AYWB-overexpressing plants leads to an increase in the progeny of Macrosteles quadrilineatus (Kessler et al., 2004; Sugio et al., 2011b).
In this study, we have shown that the expression of phytoplasma effector SAP11AYWB in Arabidopsis triggers Pi starvation responses and suppresses plant defense responses against a bacterial pathogen. These findings advance our understanding of the molecular mechanism underlying the disease symptoms elicited by the secreted effectors and provide new insights into the interaction between host plants and phytoplasmas.
RESULTS
Expression of SAP11AYWB Alters miRNA Accumulation Involved in Pi Starvation and Auxin Responses in Arabidopsis
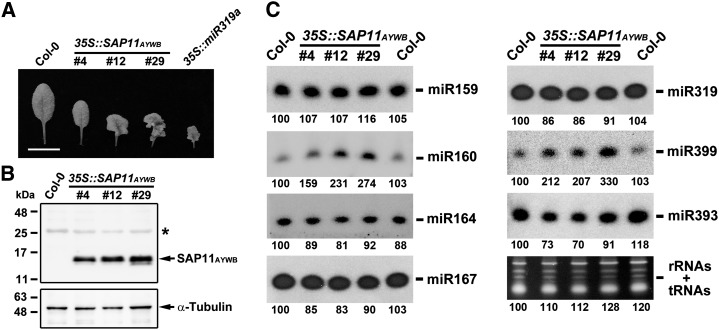
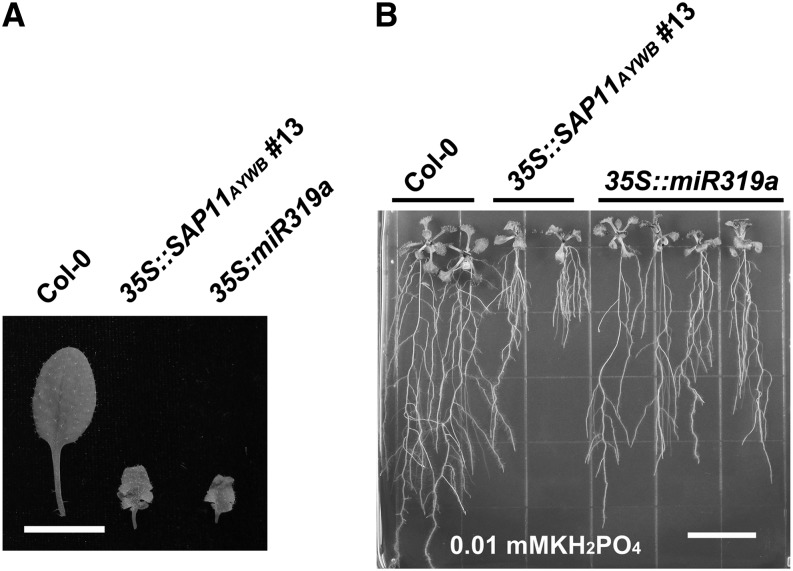
The phenotypical similarities in the leaf morphologies of SAP11AYWB-overexpressing plants (35S::SAP11AYWB) and miR319a-overexpressing (35S::miR319a) plants (Sugio et al., 2011b) prompted us to examine whether the expression of SAP11AYWB can regulate the accumulation of miRNAs in host cells (Fig. 1A). Expression of SAP11AYWB was confirmed by using the anti-SAP11AYWB antibody (Fig. 1B). Then, total RNA was extracted from plants grown on one-half-strength Murashige and Skoog (1/2× MS) medium for small RNA northern-blotting assays. Here, we compared the accumulation of miR159, miR160, miR164, miR167, miR319, miR399, and miR393 in Arabidopsis wild-type ecotype Columbia (Col-0) and 35S::SAP11AYWB transgenic lines. Interestingly, the accumulation of miR319 and miR159, miR164, and miR167 was not affected by the expression of SAP11AYWB (Fig. 1C). However, a decrease of miR393 and an induction of miR399 and miR160 were detected in 35S::SAP11AYWB transgenic lines (Fig. 1C). MiR399 plays a key role in regulating Pi starvation responses, whereas miR393 and miR160 are involved in auxin responses. In general, auxin is regulated upon Pi depletion to change root architecture with the aim of increasing Pi uptake (Péret et al., 2011). Thus, auxin has been shown to play an important role in stimulating root architecture during Pi starvation responses (Nacry et al., 2005; Jain et al., 2007; Pérez-Torres et al., 2008). Because miR393 and miR160 are involved in auxin responses, it is possible that those miRNAs have indirect effects on the root architecture alterations required for Pi uptake. Our results suggest that Pi starvation and auxin responses are altered in 35S::SAP11AYWB transgenic plants.
Figure 1.
Expression of SAP11AYWB regulates the accumulation of miRNAs involved in Pi- and auxin-signaling responses in Arabidopsis. A, Comparison of leaf morphologies between wild-type (Col-0) and transgenic plants carrying SAP11AYWB or miR319a driven by Cauliflower mosaic virus 35S promoter. Bar = 8 mm. B, Examination of the translated product of SAP11AYWB by western blotting using specific antibody against SAP11AYWB. Asterisk indicates the cross-reacting band appeared in all samples. Antitubulin was used for loading control. C, Comparisons of the expression levels of miRNAs in the wild type and 35S::SAP11AYWB transgenic lines by small RNA northern blotting. The RNAs staining bands were used as a loading control. The values of band intensities were measured using ImageJ.
Pi Starvation Responses and Pi Homeostasis Are Altered in SAP11 Transgenic Arabidopsis Plants
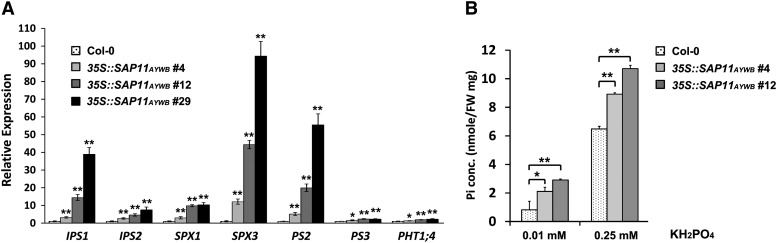
To investigate whether Pi starvation responses have been altered in 35S::SAP11AYWB transgenic plants, the expression levels of Pi starvation-induced genes were examined using quantitative reverse transcription (qRT)-PCR. RNA samples were extracted from seedlings grown in 1/2× MS (Pi-sufficient) medium, and typical Pi starvation-induced genes such as INDUCED BY PHOSPHATE STARVATION1 (IPS1), IPS2, SPX DOMAIN GENE1 (SPX1), SPX3, PHOSPHATE STARVATION-INDUCED GENE2 (PS2), PS3, and PHT1;4 were examined. IPS1, IPS2, SPX1, SPX3, PS2, PS3, and PHT1;4 were all elicited in 35S::SAP11AYWB transgenic plants, compared with wild-type plants (Fig. 2A). To examine the levels of cellular Pi, Arabidopsis seedlings grown in hydroponic solution were collected. A higher amount of Pi was detected in the aerial parts of 35S::SAP11AYWB transgenic plants grown in 0.01 mm KH2PO4 (Pi-deficient) and 0.25 mm KH2PO4 (Pi-sufficient) hydroponic solutions than in wild-type plants (Fig. 2B). These results indicate that Pi starvation responses and Pi homeostasis are altered in 35S::SAP11AYWB transgenic plants.
Figure 2.
SAP11AYWB elicits the expression of Pi starvation-induced genes and increases the accumulation of Pi in Arabidopsis. A, The mRNA levels of genes triggered by Pi starvation responses in wild-type (Col-0) plants and 35S::SAP11AYWB transgenic lines were examined by qRT-PCR and normalized to Actin2. The relative expression levels of each gene in wild-type plants were set at 1. B, The levels of Pi concentration in the aerial parts of the wild type and 35S::SAP11AYWB transgenic lines grown in hydroponic solutions with 0.01 or 0.25 mm KH2PO4 were measured. Statistically significant differences were determined using Student’s t test (*P < 0.05, **P < 0.005 for transgenic plants versus the wild type).
Expression of SAP11AYWB Reduces the Accumulation of Anthocyanin and Alters the Root Architecture in Arabidopsis
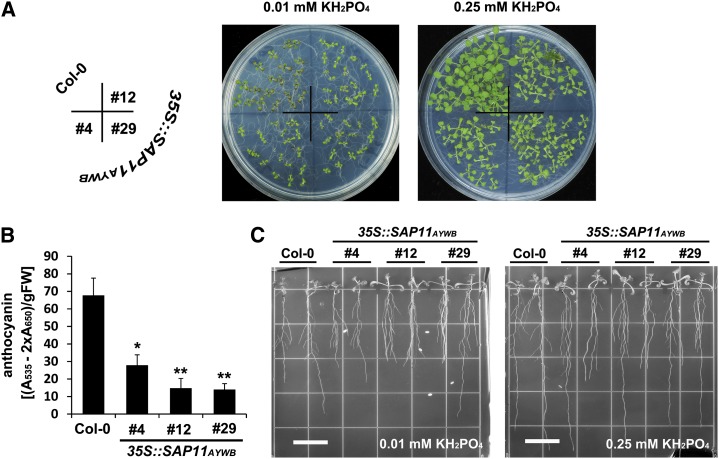
Pi is a major limiting factor for the growth and development of plants. Thus, plants have evolved a series of morphological and physiological modifications triggered by Pi deficiency, such us an increase in root/shoot ratio, proliferation of lateral roots, and accumulation of anthocyanin (Rouached et al., 2010). To examine the effects of Pi deficiency, variations in the development of 35S::SAP11AYWB transgenic lines grown on 0.01 and 0.25 mm KH2PO4 medium were compared. As a control, wild-type plants showed a significant reduction in plant size and displayed red/purple color in leaf when grown on 0.01 mm KH2PO4 medium (Fig. 3A). Compared with wild-type plants, 35S::SAP11AYWB transgenic plants showed a growth-inhibiting phenotype on both 0.01 and 0.25 mm KH2PO4 media (Fig. 3A). However, the accumulation of anthocyanin was strongly reduced in 35S::SAP11AYWB transgenic plants grown on 0.01 mm KH2PO4 medium (Fig. 3B). We further investigated the root architecture of 35S::SAP11AYWB transgenic plants. Compared with wild-type plants, 35S::SAP11AYWB transgenic plants grown on both 0.01 and 0.25 mm KH2PO4 media showed a clear inhibition of primary roots, a significant proliferation of adventitious roots, and an elongation of lateral roots (Fig. 3C). Taken together, the molecular alterations associated with the expression of SAP11AYWB were correlated with the morphological changes observed in the 35S::SAP11AYWB transgenic plants.
Figure 3.
Expression of SAP11AYWB in Arabidopsis alters the phenotypes in anthocyanin accumulation and root architecture. Comparisons of plant size (A), anthocyanin accumulation (B), and root morphology (C) between wild-type (Col-0) plants and 35S::SAP11AYWB transgenic lines grown on horizontal (A) or vertical (C) plates containing 0.01 or 0.25 mm KH2PO4. Statistically significant differences were determined using Student’s t test (*P < 0.05, **P < 0.005 for transgenic plants versus the wild type). FW, Fresh weight. Bars = 15 mm.
Genome-Wide Identification of Differentially Expressed mRNAs Altered by the Expression of SAP11AYWB in Arabidopsis
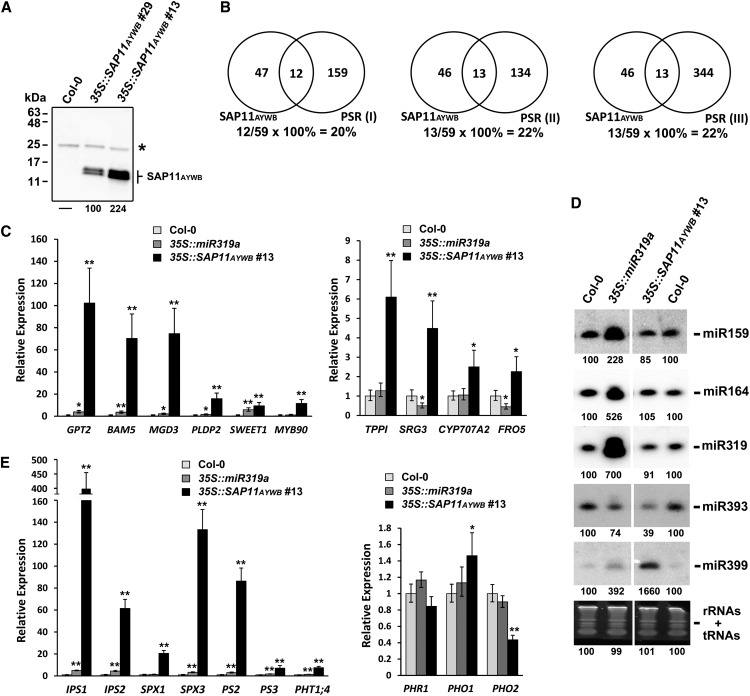
To examine the genome-wide expression profile of mRNAs in response to the expression of SAP11AYWB in Arabidopsis, the 35S::SAP11AYWB transgenic line number 13 with higher expression of SAP11AYWB was selected for a comparative transcriptome analysis (Fig. 4A). Here, total RNA from 35S::SAP11AYWB transgenic seedlings and wild-type seedlings grown on 1/2× MS medium were extracted for next-generation sequencing. In summary, a total of 26.2 million reads mapped to the Arabidopsis genome were generated after quality trimming from two complementary DNA libraries (Supplemental Table S1). To identify differentially expressed genes altered by SAP11AYWB, the expression levels of mapped genes from two samples were quantified and analyzed using DESeq, a differential gene expression data analysis program (Anders and Huber, 2010). A total of 395 differentially expressed genes (P < 0.01) were identified in the 35S::SAP11AYWB transgenic plants. To narrow down the differentially expressed genes, a criterion of P < 0.001 was selected, and 163 differentially expressed genes were identified in the 35S::SAP11AYWB transgenic plants (Supplemental Data S1). Among them, 59 genes were up-regulated, and 104 genes were down-regulated.
Figure 4.
Comparison of gene expression profiles. A, Comparison of the expression levels of SAP11AYWB in Arabidopsis 35S::SAP11AYWB transgenic lines by western blotting using specific antibodies against SAP11AYWB. Asterisk indicates the cross-reacting band appeared in all samples. B, Venn diagrams show the comparisons of SAP11AYWB-elicited genes (P < 0.001) with the Pi starvation response (PSR) genes (up-regulation, 2-fold higher). Pi starvation response (I) indicates the data set collected by Müller et al. (2007) from leaf samples in response to Pi starvation. Pi starvation responses (II) and (III) indicate the data set collected by Liu et al. (2011) from leaf and root samples, respectively, in response to Pi starvation. C, The mRNA levels of differentially expressed genes identified in the 35S::SAP11AYWB transgenic line number 13 were examined by qRT-PCR and normalized to Actin2. The relative expression levels of each gene in wild-type (Col-0) plants were set at 1. D, Comparisons of the expression levels of miRNAs by small RNA northern blotting. The RNAs staining bands were used as a loading control. E, The mRNA levels of genes involved in Pi starvation responses were examined by qRT-PCR and normalized to Actin2. The relative expression levels of each gene in wild-type (Col-0) plants were set at 1. Statistically significant differences were determined using Student’s t test (*P < 0.05, **P < 0.005 for transgenic plants versus the wild type). The values of band intensities were measured using ImageJ.
Functional annotations on the 59 up-regulated genes revealed that a total of 18 Pi starvation-induced genes, including MYB DOMAIN PROTEIN90 (MYB90), IPS1, GLC-6-PHOSPHATE/PHOSPHATE TRANSLOCATOR2 (GPT2), SULFATE TRANSPORTER1;3 (SULTR1;3), SPX3, MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE3 (MGD3), β-AMYLASE5 (BAM5), AT5G09570, PS2, PHOSPHOETHANOLAMINE/PHOSPHOCHOLINE PHOPHATASE1 (PEPC1), AT5G20790, AT1G23110, IPS2, SPX1, PHOSPHOLIPASE D P2 (PLDP2), PS3, SENESCENCE-RELATED GENE3 (SRG3), and PURPLE ACID PHOSPHATASE17 (PAP17), were highly elicited by SAP11AYWB (Table I). Notably, a total of seven Pi starvation-induced genes were found in the top 10 list of SAP11AYWB-elicited genes. Further comparison revealed that more than 20% of the SAP11AYWB-elicited genes (P < 0.001) overlapped with the differentially expressed genes (more than 2-fold up-regulation) identified by Müller et al. (2007) and Liu et al. (2011) under Pi starvation conditions (Fig. 4B). In addition to the Pi starvation responses, a small subset of genes involved in sugar metabolic processes and iron deficiency responses were also elicited by SAP11AYWB (Table I). To evaluate the quality of the RNA Sequencing (RNA-Seq) data, SAP11AYWB-elicited genes were randomly selected for validation by using the qRT-PCR method. The differentially expressed genes, including GPT2, BAM5, MGD3, PLDP2, SWEET1, MYB90, TREHALOSE-6-PHOSPHATE PHOSPHATASEI (TPPI), SRG3, CYTOCHROME P450 FAMILY 707 SUBFAMILY A POLYPEPTIDE2, and FERRIC REDUCTION OXIDASE5, were all elevated in the 35S::SAP11AYWB transgenic plants (Fig. 4C). Taken together, these results indicate that Pi starvation-induced genes are highly up-regulated by the expression of SAP11AYWB in Arabidopsis.
Table I. Classification and annotation of the differentially expressed genes (P < 0.001) elicited by SAP11AYWB.
| Category |
AGIb No. | Base Meana |
Fold Changec | Short Description | Referenced | ||
|---|---|---|---|---|---|---|---|
| Rank | Name | Wild Type | 35S::SAP11AYWB | ||||
| Pi starvation response | |||||||
| 1st | MYB90 | At1g66390 | 1.11 | 60.23 | 54.14 | Myb domain protein90 | B, D |
| 2nd | IPS1 | At3g09922 | 18.91 | 620.26 | 32.8 | Induced by phosphate starvation1 | |
| 3rd | GPT2 | At1g61800 | 7.79 | 175.29 | 22.51 | Glc-6-phosphate/phosphate translocator2 | B, D |
| 5th | SULTR1;3 | At1g22150 | 5.56 | 82.7 | 14.87 | Sulfate transporter1;3 | A, C |
| 6th | SPX3 | At2g45130 | 5.56 | 79.11 | 14.22 | SPX domain gene3 | B, C, D |
| 8th | MGDC | At2g11810 | 30.04 | 374.86 | 12.48 | Monogalactosyldiacylglycerol synthase type C | A, B, C, D |
| 10th | BAM5 | At4g15210 | 13.35 | 161.81 | 12.12 | β-Amylase5 | A, B, C |
| 11th | At5g09570 | 7.79 | 89.89 | 11.54 | Cytochrome-c oxidase19-like Coiled-coil Helix Coiled-coil Helix family protein | C | |
| 14th | PS2 | At1g73010 | 67.86 | 737.13 | 10.86 | Phosphate starvation-induced gene2 | A, B, C, D |
| 15th | PEPC1 | At1g17710 | 46.72 | 468.34 | 10.02 | Phosphoethanolamine/phosphocholine phophatase1 | A, B, C, D |
| 21th | At5g20790 | 84.55 | 747.01 | 8.84 | Unknown protein | A, B, C, D | |
| 24th | At1g23110 | 28.92 | 232.82 | 8.05 | Unknown protein | A | |
| 35th | IPS2 | At5g03545 | 199.13 | 1141.65 | 5.73 | Induced by phosphate starvation2 | |
| 36th | SPX1 | At5g20150 | 284.78 | 1629.77 | 5.72 | SPX domain gene1 | A, B, C, D |
| 45th | PLDP2 | At3g05630 | 73.42 | 317.32 | 4.32 | Phospholipase D P2 | A, B, C, D |
| 48th | PS3 | At3g47420 | 543.98 | 2238.34 | 4.11 | Phosphate starvation-induced gene3 | A, B, C, D |
| 52th | SRG3 | At3g02040 | 327.05 | 1253.11 | 3.83 | Senescence-related gene3 | A, B, C, D |
| 56th | PAP17 | At3g17790 | 263.65 | 944.78 | 3.58 | Purple acid phosphatase17 | A, B, C, D |
| Sugar metabolic processes | |||||||
| 3rd | GPT2 | At1g61800 | 7.79 | 175.29 | 22.51 | Glc-6-phosphate/phosphate translocator2 | |
| 10th | BAM5 | At4g15210 | 13.35 | 161.81 | 12.12 | β-Amylase5 | |
| 16th | TPPI | At5g10100 | 13.349 | 133.04 | 9.97 | Trehalose-6-phosphate phosphataseI | |
| 44th | SWEET1 | At1g21460 | 350.42 | 1552.46 | 4.43 | Medicago truncatula nodule3 family protein | |
| Iron deficiency responses | |||||||
| 20th | bHLH100 | At2g41240 | 20.02 | 191.47 | 9.56 | Basic helix-loop-helix protein100 | |
| 25th | bHLH38 | At3g56970 | 32.26 | 249.01 | 7.72 | Basic helix-loop-helix protein38 | |
| 29th | bHLH39 | At3g56980 | 35.6 | 251.7 | 7.07 | Basic helix-loop-helix protein39 | |
Base mean: the number of reads divided by the size factor (normalized constant) of sample. bAGI, Arabidopsis Genome Initiative. cFold change: 35S::SAP11AYWB base mean/wild-type base mean. dReference: Pi starvation-induced genes (>2-fold change) reported by Müller et al. (2007; A), Liu et al. (2011; B, shoot, C, root), and Morcuende et al. (2007; D).
Genome-Wide Identification of Differentially Expressed miRNAs Altered by the Expression of SAP11AYWB in Arabidopsis
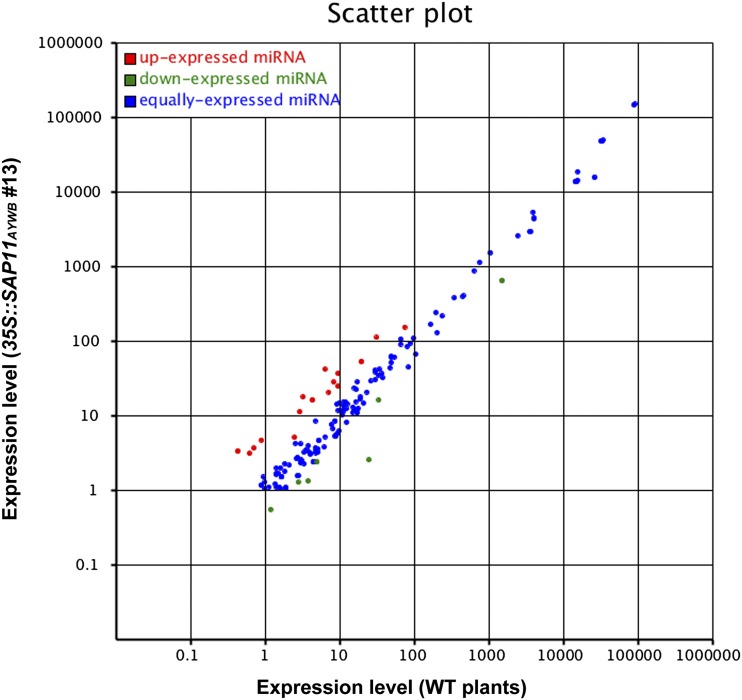
To investigate the genome-wide expression profile of miRNAs in response to the expression of SAP11AYWB in Arabidopsis, RNA samples were collected from seedlings grown on 1/2× MS medium. Two small RNA libraries from 35S::SAP11AYWB transgenic line number 13 and wild-type plants were constructed for deep sequencing. In summary, a total of 34.5 million reads mapped to the Arabidopsis genome were generated after quality trimming from two complementary DNA libraries (Supplemental Table S2). Among them, about 9.3 million reads were mapped to Arabidopsis miRNAs deposited in miRBase (Kozomara and Griffiths-Jones, 2011). The expression levels of known miRNAs in two samples were normalized, and the differentially expressed miRNAs with fold change greater than 2 or less than 0.5 were identified (Fig. 5; Supplemental Data S2). Compared with wild-type plants, 35S::SAP11AYWB transgenic plants showed significant up-regulation of miR160, miR2111, miR394, miR399, miR5639, miR5655, miR827, miR850, and miR863. By contrast, miR172, miR396, miR5020, miR5629, miR5645, and miR5648 showed significant down-regulation in the 35S::SAP11AYWB transgenic plants. Among them, miR2111, miR399, and miR827 have been shown to be up-regulated by Pi starvation (Hsieh et al., 2009; Pant et al., 2009), and miR399 and miR827 play important roles in regulating Pi homeostasis (Liu et al., 2012; Huang et al., 2013; Lin et al., 2013); miR160 and miR396 are required for regulating the auxin signaling response and cell proliferation during leaf and root development (Gutierrez et al., 2009; Pulido and Laufs, 2010; Debernardi et al., 2012; Bazin et al., 2013); miR172 has been shown to regulate the transition from the vegetative to reproductive phases (Aukerman and Sakai, 2003; Wu et al., 2009); and miR394 is required for the regulation of leaf morphology and shoot meristem stem-cell maintenance (Song et al., 2012; Knauer et al., 2013). However, miR5020b, miR5629, miR5639, miR5645d, miR5648, miR5655, and miR850 are newly identified miRNAs with unknown functions.
Figure 5.
Comparison of miRNA expression profiles. Scatter plot shows the related expression levels of known miRNAs in Arabidopsis wild-type (WT) plants (x axis) and 35S::SAP11AYWB transgenic line number 13 (y axis), in which the scales indicate the expression intensity of normalized miRNAs. Red points refer to miRNAs whose expression levels were 2-fold higher in 35S::SAP11AYWB transgenic line number 13 as compared with wild-type plants. Green points refer to miRNAs whose expression levels were 2-fold lower in 35S::SAP11AYWB transgenic line number 13 as compared with wild-type plants. Blue points refer to miRNAs whose expression levels were not higher or lower than 2-fold in the 35S::SAP11AYWB transgenic line number 13 as compared with wild-type plants.
We further evaluated the results of small RNA-Seq by using small RNA northern blotting. Here, several miRNAs were randomly selected for validation, and the results were consistent with the genome-wide miRNA expression profiling (Fig. 4D). Moreover, we investigated the expression level of PHO2 in 35S::SAP11AYWB transgenic line number 13, because miR399 has been shown to down-regulate PHO2 by directly targeting its transcripts (Aung et al., 2006; Chiou et al., 2006). Consistent with the up-regulation of miR399, a significant down-regulation of PHO2 was observed in 35S::SAP11AYWB transgenic line number 13 (Fig. 4E). Taken together, these results indicate that miRNAs involved in Pi starvation and auxin-signaling pathways are altered by the expression of SAP11AYWB in Arabidopsis.
Role of MiR319a in SAP11AYWB-Triggered Pi Starvation Responses
Although SAP11AYWB did not interfere with the accumulation of miR319 (Figs. 1C and 4D), the crinkled leaves that appeared in 35S::SAP11AYWB transgenic plants resembled those in the 35S::miR319a transgenic plants (Figs. 1A and 6A), which prompted us to examine whether miR319a has an effect on the SAP11AYWB-triggered Pi starvation responses. We investigated the accumulation of miR159, miR164, miR319, miR393, and miR399 in 35S::miR319a transgenic plants grown on 1/2× MS medium. An increase in the amount of miR159, miR164, and miR399 was observed with the overexpression of miR319 in Arabidopsis (Fig. 4D). However, the amount of increase for miR399 was much lower in 35S::miR319a transgenic plants than in 35S::SAP11AYWB transgenic line number 13. Consistently, the expression levels of Pi starvation-induced genes were much lower in 35S::miR319a transgenic plants, compared with 35S::SAP11AYWB transgenic line number 13 (Fig. 4, C and E). We further investigated whether miR319a has an effect on the root architecture. Compared with 35S::SAP11AYWB transgenic line number 13, 35S::miR319a transgenic plants grown on 0.01 mm KH2PO4 medium showed no proliferation of adventitious roots and strong inhibition of primary roots (Fig. 6B). The root architecture in 35S::miR319a transgenic plants was more similar to that in wild-type plants (Fig. 6B). Because SAP11AYWB does not affect miR319 expression levels and the root architecture of miR319 transgenic plants are more similar to the wild type, it is unlikely that miR319 is involved in the SAP11AYWB-triggered Pi starvation responses. However, the transcript levels of Pi starvation-induced genes and miR399 were lightly up-regulated in 35S::miR319a transgenic plants. Therefore, TCP transcripts targeted by miR319 may have minimal contribution to the Pi starvation responses.
Figure 6.
Phenotypic comparisons in leaf morphology and root architecture between 35S::miR319a and 35S::SAP11AYWB transgenic plants. A, Leaf morphologies of Arabidopsis seedlings grown on 1/2× MS medium. Bar = 8 mm. B, Root architectures of Arabidopsis seedlings grown on the vertical plate containing 0.01 mm KH2PO4. Bar = 15 mm.
SAP11AYWB-Triggered Pi Starvation Responses Are Mainly Dependent on PHR1
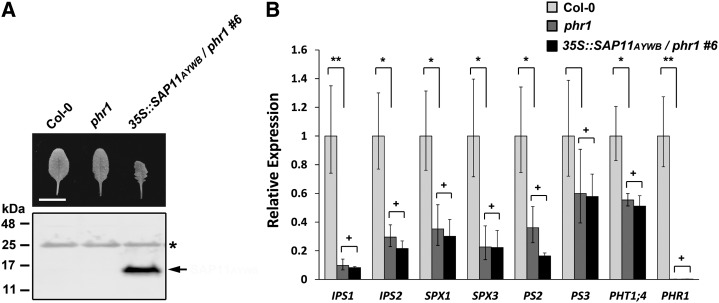
Molecular mechanisms underlying Pi starvation responses remain largely unknown (Rouached et al., 2010). Only a few transcription factors have been identified as important players in the Pi starvation signaling pathway. Among them, PHOSPHATE STARVATION RESPONSE1 (PHR1) that encodes a MYB transcription factor plays a key role in responding to the Pi deficiency in Arabidopsis (Rubio et al., 2001). However, there was no significant difference in the transcript level of PHR1 between wild-type and SAP11AYWB transgenic plants (Fig. 4E). When we analyzed the potential cis-regulatory elements on the Pi starvation-induced genes regulated by SAP11AYWB using PlantPAN, a navigator for plant promoter analysis (Chang et al., 2008), we found that 14 out of 18 Pi starvation-induced genes contain PHR1 binding sites in their promoter regions (Table II). Thus, to understand whether the PHR1-dependent pathway contributes to the SAP11AYWB-triggered Pi starvation responses, 35S::SAP11AYWB was introduced into a phr1 mutant. With the expression of SAP11AYWB, 35S::SAP11AYWB/phr1 transgenic plants showed a wavy-margin phenotype in the leaves (Fig. 7A). However, the Pi starvation-induced genes, including IPS1, IPS2, SPX1, SPX3, PS2, PS3, and PHT1;4, were all suppressed when SAP11AYWB was expressed under a phr1 mutant background (Fig. 7B). These results indicate that PHR1 is required for SAP11AYWB-triggered Pi starvation responses.
Table II. The potential sequence of PHR1-binding site found at the upstream region of Pi starvation-induced genes regulated by SAP11AYWB.
| Gene | AGIa No. | Sequence (Positionb) |
|---|---|---|
| IPS1 | At3G09922 | GCATATTC (–581); GCATATTC (–547) |
| GPT2 | At1G61800 | GTATATTC (–125) |
| SULTR1;3 | At1G22150 | GGATATTC (–439) |
| SPX3 | At2G45130 | GAATATGC (–212); GCATATCC (–89) |
| At5G09570 | GGATATAC (–1656); GAATATTC (–142) | |
| PS2 | At1G73010 | GGATATTC (–693); GAATATTC (–308) |
| PEPC1 | At1G17710 | GAATATTC (–765); GAATATTC (–370) |
| At5G20790 | GAATATGC (–1325) | |
| At1G23110 | GAATATGC (–346); GAATATGC (–171) | |
| IPS2 | At5G03545 | GTATATGC (–687); GCATATTC (–149) |
| SPX1 | At5G20150 | GAATATTC (–824); GGATATTC (–67); GAATATTC (–43) |
| PLDP2 | At3G05630 | GAATATTC (–698); GGATATTC (–664); GCATATAC (–635); GCATATAC (–244) |
| SRG3 | At3G02040 | GTATATGC (–334); GAATATTC (–137); GAATATCC (–73); GAATATTC (–73) |
| PAP17 | At3G17790 | GAATATCC (–131) |
AGI, Arabidopsis Genome Initiative. bPosition: the position in the 5′-upstream region is given with respect to the ATG strat codon.
Figure 7.
PHR1 is required for SAP11AYWB-triggered Pi starvation responses in Arabidopsis. A, Leaf morphologies of Arabidopsis transgenic plants overexpressing SAP11AYWB in the background of a phr1 mutant. The expression level of SAP11AYWB was detected by western blotting using specific antibodies against SAP11AYWB. Asterisk indicates the cross-reacting band appeared in all samples. Bar = 8 mm. B, The mRNA levels of genes involved in Pi starvation responses among wild-type (Col-0), phr1 mutant, and 35S::SAP11AYWB/phr1 transgenic plants were examined by qRT-PCR and normalized to Actin2. The relative expression levels of each gene in wild-type plants were set at 1. Statistically significant differences were determined using Student’s t test (*P < 0.05, **P < 0.005 for the wild type versus phr1; +P > 0.05 for phr1 versus 35S::SAP11AYWB/phr1).
SAP11AYWB Transgenic Plants Show Altered Hormone Synthesis and Signaling and Are More Susceptible to the Bacterial Pathogen Pseudomonas syringae
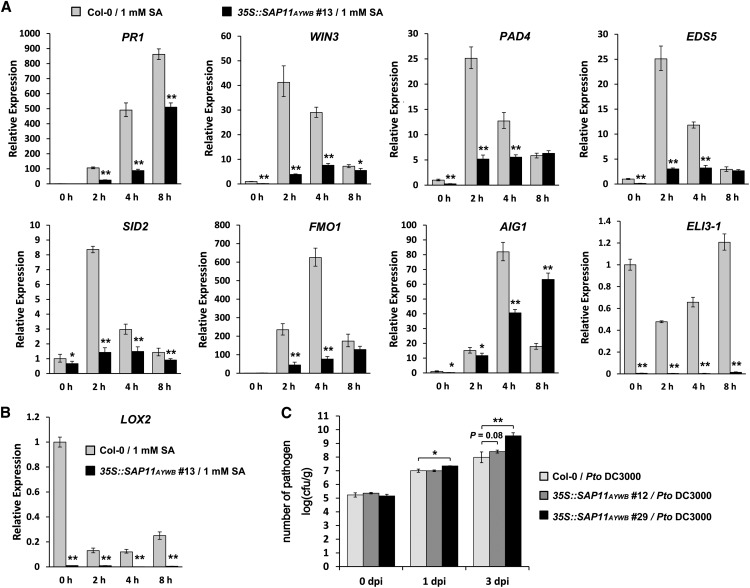
RNA-Seq analysis of the down-regulated genes in 35S::SAP11AYWB number 13 transgenic line revealed that a subset of genes, including ELICITOR-ACTIVATED GENE3-1 (ELI3-1), At3g05730 (defensin-like family protein), PATHOGENESIS-RELATED GENE1 (PR1), and LOX2, were highly suppressed (Table III). The RNA-Seq data were validated using the qRT-PCR method (Fig. 8, A and B). The down-regulation of LOX2 is consistent with the previous studies reported by Sugio et al. (2011b). Interestingly, LOX2 is involved in JA synthesis, whereas PR1 and ELI3-1 are salicylic acid (SA)-responsive genes (Kiedrowski et al., 1992; Spoel et al., 2003). These results suggest that SAP11AYWB not only interferes with JA synthesis but also suppresses SA-signaling responses. We found that SAP11AYWB repressed the expression of miR393, a bacterial flagellin peptide-induced miRNA that participates in basal resistance against bacterial pathogens (Navarro et al., 2006). Thus, repression of miR393 and PR1 in 35S::SAP11AYWB transgenic plants prompted us to investigate whether the expression of SAP11AYWB can suppress innate immunity against bacterial pathogens in Arabidopsis. To this end, we monitored the expression levels of genes involved in the SA-mediated defense-signaling network under the treatment of 1 mm SA. Genes involved in SA biosynthesis (ENHANCED DISEASE SUSCEPTIBILITY5 and SALICYLIC ACID INDUCTION DEFICIENT2) and SA accumulation (FLAVIN-DEPENDENT MONOOXYGENASE1, HOPW1-1-INTERACTING3, and PHYTOALEXIN DEFICIENT4), as well as the standard marker genes for SA-mediated response (PR1 and AVRRPT2-INDUCED GENE1), were all repressed in 35S::SAP11AYWB number 13 transgenic line (Fig. 8A). We further investigated the effect of SAP11AYWB on the multiplication of P. syringae pv tomato DC3000 (Pto DC3000) in Arabidopsis. In this study, we used the 35S::SAP11AYWB numbers 12 and 29 transgenic lines for bacterial inoculation assays because the leaves of 35S::SAP11AYWB number 13 were too crinkled and small for inoculation. Compared with wild-type plants, both 35S::SAP11AYWB numbers 12 and 29 transgenic lines showed an increase in bacterial growth (Fig. 8C). Taken together, these results indicate that the expression of SAP11AYWB suppresses the host’s innate immunity and makes the plants more susceptible to a bacterial infection.
Table III. Classification and annotation of the top 10 differentially expressed genes (P < 0.001) suppressed by SAP11AYWB.
| Category |
AGIb No. | Base Meana |
Fold Changec | Short Description | ||
|---|---|---|---|---|---|---|
| Rank | Name | Wild Type | 35S::SAP11AYWB | |||
| 1st | At3g16670 | 814.29 | 0.89 | 914.9 | Pollen Ole e 1 allergen and extensin family protein | |
| 2nd | ELI3-1 | At4g37980 | 1311.55 | 2.69 | 487.6 | Elicitor-activated gene3-1 |
| 3rd | At3g05730 | 2143.65 | 8.09 | 264.9 | Defensin-like family protein | |
| 4th | PR1 | At2g14610 | 150.17 | 0.89 | 168.7 | Pathogenesis-related gene1 |
| 5th | At2g29300 | 518.39 | 5.393 | 96.1 | NAD(P)-binding Rossmann-fold superfamily protein | |
| 6th | At3g19620 | 109.01 | 1.79 | 60.9 | Glycosyl hydrolase family protein | |
| 7th | At4g12900 | 58.95 | 1.79 | 32.9 | γ Interferon responsive lysosomal thiol reductase family protein | |
| 8th | LOX2 | At3g45140 | 2688.74 | 86.29 | 31.2 | Lipoxygenase2 |
| 9th | At2g29290 | 80.09 | 3.59 | 22.3 | NAD(P)-binding Rossmann-fold superfamily protein | |
| 10th | At2g32160 | 75.64 | 3.59 | 21.1 | S-Adenosyl-l-Met-dependent methyltransferases superfamily protein | |
Base mean: the number of reads divided by the size factor (normalized constant) of sample.bAGI, Arabidopsis Genome Initiative.cFold change, wild-type base mean/35S::SAP11AYWB base mean.
Figure 8.
SAP11AYWB suppresses the innate immunity against bacterial pathogens in Arabidopsis. RNA samples were extracted from SA-treated wild-type (Col-0) and 35S::SAP11AYWB transgenic plants harvested at indicated times. The mRNA levels of genes involved in SA-mediated defense signaling network (A) and JA-signaling responses (B) were examined by qRT-PCR and normalized to Actin2. The relative expression levels of each gene in wild-type plants without SA treatment were set at 1. C, Bacterial growth of Pto DC3000 in wild-type and 35S::SAP11AYWB transgenic plants were measured to examine the effects of SAP11AYWB on the resistance of Arabidopsis against bacterial pathogens. Hand-infiltrated leaves were collected at the indicated times for measuring the in planta growth of Pto DC3000. Statistically significant differences were determined using Student’s t test (*P < 0.05, **P < 0.005 for transgenic plants versus the wild type).
DISCUSSION
SAP11AYWB has been shown to mediate the destabilization of Arabidopsis TCPs, which leads to the down-regulation of LOX2, a key component in the biosynthesis of JA (Schommer et al., 2008; Sugio et al., 2011b). In this study, our RNA-Seq analysis also showed a significant decrease in LOX2 transcripts in 35S::SAP11AYWB transgenic plants (Table III). In addition, we showed that the expression of miR319 was not changed in the SAP11AYWB-overexpressing plants (Figs. 1C and 4D). These data are in agreement with previous published results showing that SAP11AYWB degrades TCPs (Sugio et al., 2011b) and suggest that the SAP11AYWB-mediated suppression of TCPs’ activities probably occurs only through posttranslational regulation. In contrast to miR319, a significant increase in the amount of miR399, miR2111, and miR827, which have been shown to be up-regulated by Pi starvation (Hsieh et al., 2009; Pant et al., 2009), was observed in 35S::SAP11AYWB transgenic plants (Fig. 1C; Supplemental Data S2). Among them, miR399 and miR827 play positive roles in regulating Pi uptake and translocation (Chiou et al., 2006; Liu et al., 2012; Huang et al., 2013; Lin et al., 2013). Consistent with this, 35S::SAP11AYWB transgenic plants accumulated a higher amount of Pi in cells and displayed reduced symptoms in the Pi-deficient condition, e.g. a lower level of anthocyanin accumulation (Figs. 2B and 3B). These phenomena were paralleled by an increase in the transcript levels of Pi starvation-induced genes, e.g. IPS1, IPS2, SPX1, SPX3, PS2, PS3, and PHT1;4 (Fig. 2A).
Although Pi homeostasis is altered in 35S::SAP11AYWB transgenic plants, the molecular mechanism of SAP11AYWB-triggered Pi starvation responses remains unclear and has yet to be clarified. In this study, we show that the Pi starvation responses triggered by SAP11AYWB are mainly dependent on the pathway regulated by PHR1 (Fig. 7B). PHR1, a MYB transcription factor, plays a crucial role as a central hub in the transcriptional activation of genes involved in Pi transport and translocation, root architecture remodeling, anthocyanin accumulation, and sugar metabolic processes (Bustos et al., 2010; Chiou and Lin, 2011). Thus, more than 75% of the Pi starvation-induced genes regulated by SAP11AYWB contain the potential cis-regulatory elements for PHR1 binding in the promoter region (Table II). These results might provide some information in elucidating the functional relationships between SAP11AYWB and Pi starvation responses. In Arabidopsis, there are 24 TCPs divided into two classes based on sequence similarities (Aguilar-Martínez et al., 2007). Recently, Sugio et al. (2011b) showed that class II CINCINNATA (CIN)-TCPs were selectively destabilized by SAP11AYWB. Class II CIN-TCPs contains eight members, but only five of them can be targeted by miR319 (Ori et al., 2007). In this study, we found that the transcript levels of Pi starvation-induced genes and miR399 were not changed significantly in 35S::miR319a transgenic plants (Fig. 4, C–E), suggesting that miR319-mediated regulation may have minimal contribution to the SAP11AYWB-triggered Pi starvation responses. Nevertheless, because only five transcripts out of eight CIN-TCPs are targeted by miR319, involvement of other class II CIN-TCP members in Pi starvation responses cannot be excluded. On the other hand, whether the class II CYCLOIDEA/TEOSINTE BRANCHED1-TCPs are also involved in Pi starvation responses of 35S::SAP11AYWB transgenic plants requires further investigation.
Phytoplasmas have the smallest genome among bacteria, and many essential genes required for standard metabolic functions are lost, including ATP synthase subunits (Oshima et al., 2004; Chung et al., 2013). Thus, phytoplasmas might uptake essential substances from the cytoplasm of surrounding host cells. Here, we showed that overexpression of SAP11AYWB could trigger Pi starvation responses and alter the Pi homeostasis in host cells (Fig. 2, A and B). Pi is an essential nutrient required for various basic biological functions, e.g. synthesis of nucleic acids and phospholipids, regulation of energy metabolism, and signal transduction cascades (Raghothama, 1999; Shen et al., 2011). Although the molecular mechanism of SAP11AYWB-triggered Pi starvation responses remains elucidated, it might alter the physiology of plant cells into a metabolically rich environment, which might be able to facilitate the growth of phytoplasmas.
Pi deficiency is typically accompanied with the accumulation of Fe and sugar (Jain et al., 2007; Ward et al., 2008; Rouached et al., 2010). Similarly, our RNA-Seq analysis uncovers transcriptomic variations not only in genes involved in Pi starvation responses, but also in genes related to sugar metabolic processes and Fe deficiency responses, e.g. GPT2, BAM5, TPPI, SWEET1, BASIC HELIX-LOOP-HELIX PROTEIN100 (bHLH100), bHLH38, and bHLH39 (Table I). These molecular changes might play a role in the cross regulation between Pi starvation, Suc signaling, and Fe responses. Because sugar and Fe are also essential nutrients for the growth and development of pathogens (Chen et al., 2010; Hood and Skaar, 2012), up-regulation of genes involved in sugar metabolic processes and Fe deficiency responses might provide a better environment for the growth of phytoplasmas in plant cells.
The importance of Pi in the development of disease symptoms could be revealed in huanglongbing disease, in which an increase in the expression levels of miR399 and Pi starvation-induced genes was observed in citrus plants infected by Ca. L. asiaticus. (Zhao et al., 2013). Noticeably, the disease symptoms of Ca. L. asiaticus-infected citrus were reduced after the application of Pi. In the case of phytoplasmas, although the correlation between Pi and the disease symptoms remains unclear, the disease symptoms in phytoplasma-infected plants could be reduced by the application of AM fungi (Lingua et al., 2002; Romanazzi et al., 2009; Kamińska et al., 2010; Sampò et al., 2012). AM fungi are symbiotic organisms that are able to enhance the uptake of Pi through up-regulation of Pi starvation responses in host plants (Branscheid et al., 2010; Gu et al., 2010; Volpe et al., 2013). Thus, it will be interesting to explore whether the application of Pi could reduce the phytoplasma-mediated disease symptoms in the future. Although it is reasonable to assume that the availability of Pi in host cells might affect the disease symptoms in phytoplasma-infected plants, other factors might alter the disease symptoms as well. For example, the plant responses elicited by AM fungi, such as SA and JA defense responses (Gutjahr and Paszkowski, 2009), might participate in counteracting the colonization of phytoplasmas, leading to the reduction of disease symptoms.
In addition to the regulation of Pi uptake, we found that the expression of SAP11AYWB is able to suppress host’s innate immunity by repressing and delaying the SA-mediated defense responses (Fig. 8A; Table III). As a result, SAP11AYWB-overexpressing plants become more susceptible to bacterial infections (Fig. 8C). Because miR393 plays an important role in modulating plant defense responses against bacterial pathogens through the repression of auxin signaling pathway (Navarro et al., 2006; Katiyar-Agarwal and Jin, 2010), the changes of host’s innate immunity in 35S::SAP11AYWB transgenic plants might correlate with the down-regulation of miR393. However, the role of class II TCPs in 35S::SAP11AYWB transgenic plants remains unclear. The suppression of host’s innate immunity might be possible to be an indirect consequence caused by the SAP11AYWB-mediated destabilization of TCPs.
Root architecture remodeling induced by Pi starvation requires the regulation of auxin signaling (Nacry et al., 2005; Jain et al., 2007; Pérez-Torres et al., 2008; Péret et al., 2011). In 35S::SAP11AYWB transgenic plants, the formation of adventitious roots, elongation of lateral roots, and reduction of primary roots was associated with the differential expression of miRNAs involved in auxin signaling (Figs. 3C and 6B; Supplemental Data S2). These miRNAs include miR160, miR393, and miR396, which target transcripts encoding the auxin response factors, auxin receptors, and growth-regulating factors, respectively (Mallory et al., 2005; Parry et al., 2009; Debernardi et al., 2012). Although the molecular changes in auxin signaling triggered by SAP11AYWB remain largely unknown, the phenotypic alterations in root architecture resembling the disease symptoms appeared in the roots of several phytoplasma-infected plants (Del Serrone et al., 2001; Lee et al., 2006).
Our findings provide useful information for the activities of SAP11AYWB in plant immune responses and suggest that the expression of SAP11AYWB not only suppresses the JA signaling responses against insect vectors but also down-regulates the SA-mediated defense responses against bacterial pathogens. These studies not only contribute to the understanding of molecular mechanisms underlying the interaction between phytoplasmas and plants, but also provide new insights into development of the strategy in controlling phytoplasma diseases.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) Col-0 was used for the generation of transgenic plants and bacterial inoculation. Nicotiana benthamiana was used for agroinfiltration. Plants were grown at 21°C in a semicontrolled walk-in chamber with a 16-h/8-h photoperiod for Agrobacterium tumefaciens transformation and Pi measurement; a 12-h/12-h photoperiod was used for SA treatment and bacterial inoculation.
Generation of Transgenic Arabidopsis Plants
A synthetic DNA template with the codon-optimized version of SAP11AYWB was used for PCR amplification. A DNA fragment encoding SAP11AYWB without the signal peptide was amplified using AccuPrime pfx DNA polymerase (Invitrogen) and subcloned into the pBA002 vector under the control of the Cauliflower mosaic virus 35S promoter. After verification by DNA sequencing, plasmid DNAs were introduced into A. tumefaciens strain ABI by using the freeze-thaw method and transformed into Arabidopsis by using the floral-dip method. To obtain homozygous transgenic lines expressing SAP11AYWB, seeds were screened on 1/2× MS medium containing Basta (25 μg mL–1) and carbenicillin (100 μg mL–1) and examined with western blotting using specific anti-SAP11 antibodies.
Root Architecture Investigation and Anthocyanin Measurement
Arabidopsis seeds were germinated on 1/2× MS medium with 1% (w/v) Suc and 1% (w/v) agar. After incubation for 3 d, seedlings were transferred to the one-half-strength modified Hoagland nutrient solution containing 1% (w/v) Suc and 1% (w/v) agar with the indicated concentration of KH2PO4. For root architecture investigation, seedlings were placed vertically to allow root growth along the surface of the agar, and the root architecture was observed at 14 d after germination. For anthocyanin measurement, seedlings were harvested at 13 d after germination, and the anthocyanin content was determined as described with modifications (Saijo et al., 2009). Briefly, seedlings were homogenized in the extraction buffer (propanol:HCl:water [18:1:81]) and immersed into boiling water for 1.5 min. After centrifugation, the supernatant was collected for measuring the absorbance at 535 and 650 nm. The relative anthocyanin amount was calculated by the following equation: (A535 – [2 × A650])/fresh weight (grams).
Pi Measurement
Pi contents were measured according to the method described by Chiou et al. (2006). Briefly, Arabidopsis seeds were germinated on agar plate with one-half-strength modified Hoagland medium containing 0.25 mm KH2PO4 for 11 d. The seedlings were then transferred to hydroponic culture. Before Pi starvation treatment, seedlings were grown on 0.25 mm KH2PO4. Fresh tissues were homogenized with extraction buffer (10 mm Tris-HCl, pH 8.0, 1 mm EDTA, 100 mm NaCl, 1 mm mercaptoethanol, and 1 mm phenylmethylsulfonyl fluoride) and then mixed with 1% (v/v) glacial acetic acid. After centrifugation at 13,000g for 5 min, Pi assay solution (0.35% [w/v] NH4MoO4, 0.86 NH2SO4, and 1.4% [v/v] ascorbic acid) was added to the supernatant and then incubated at 42°C for 30 min. Pi content was measured using Tecan Infinite 200 PRO at A820.
Small RNA Northern Blotting
For miRNA analysis, 12 μg of total RNA was fractionated on a 15% (w/v) polyacrylamide gel containing 8 m urea and then transferred to a Hybond-N+ membrane (GE Biosciences). Oligonucleotide probes were end labeled with [γ-32P]ATP by using T4 polynucleotide kinase (New England Biolabs). Hybridization was performed overnight at 42°C with the ULTRAHyb-Oligo hybridization buffer (Ambion). Signals were detected using autoradiography.
Antibody Production and Western Blotting
To generate recombinant proteins, a PCR product encoding SAP11AYWB without the signal peptide was subcloned into the SUMO-pET vector to generate an N-terminal His-SUMO fusion construct. After verification by DNA sequencing, plasmid DNA was introduced into Escherichia coli BL21 (DE3) cells, and His-SUMO-SAP11AYWB protein was purified using Ni2+-NTA resin (Qiagen), according to the manufacturer’s instruction. After being cleaved with ubiquitin-like-specific protease1 to remove the His-SUMO tag, recombinant SAP11AYWB protein was purified for antibody production in a rabbit. The polyclonal antibody against SAP11AYWB was obtained by affinity purification using a polyvinylidene difluoride membrane as a coupling matrix. To detect SAP11AYWB in Arabidopsis transgenic plants, total extracts were prepared by directly adding 2.5× SDS sample buffer into plant samples after grinding. Amersham ECL western-blotting reagents were used for the reactions, and the chemiluminescence signals were captured using an ImageQuant LAS 4000 Mini (GE Healthcare).
RNA-Seq Analysis
To perform the RNA-Seq analysis, 3-week-old 35S::SAP11AYWB transgenic plants grown in 1/2× MS medium were used, and next-generation sequencings were performed using HisEquation 2000 (Illumina) with total RNA samples extracted by the RNeasy Plant Mini Kit (Qiagen). For transcriptome analysis, sequence reads were aligned using CLC bio, and gene expression levels were normalized as reads per kilobase of exon model per million mapped reads. To identify differentially expressed genes in 35S::SAP11AYWB transgenic plants, DEseq (Anders and Huber, 2010) analysis was performed, and differentially expressed genes with P < 0.05 were selected for further analysis.
qRT-PCR
For qRT-PCR analysis, Arabidopsis total RNA was extracted using the Trizol reagent (Invitrogen), and complementary DNAs were synthesized using SuperScript III First-Strand Synthesis SuperMix (Invitrogen), according to the manufacturer’s instructions. The RNA expression analyses were performed using the KAPA SYBR Fast qPCR Kit (Kapa Biosystems) and Illumina Eco real-time PCR system. The expression levels of the selected genes were determined by normalizing to the reference gene Actin2. Experiments were repeated at least three times.
SA Treatments and Bacterial Inoculations
To examine the immune responses in 35S::SAP11AYWB transgenic plants, 4- to 5-week-old plants grown in the soil were treated with 1 mm SA with foliar sprays. After spraying, the aerial parts of the plants were harvested at indicated times and frozen immediately in liquid nitrogen. For bacterial inoculations, Pto DC3000 was cultured in nutrient broth (3 g beef extract, 5 g peptone, and 3 g yeast [Saccharomyces cerevisiae] extract in 1 L of water) at 28°C. The 4- to 5-week-old 35S::SAP11AYWB transgenic plants were inoculated with the bacterial suspension (5 × 105 colony forming units mL–1). The inoculated plants were maintained at 21°C, and bacterial populations were determined at indicated time intervals by using NY agar plates containing rifampicin (50 μg mL–1). Experiments were repeated at least three times.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Reads abundance and average length of RNA sequencing data.
Supplemental Table S2. Reads counting of small RNA sequencing data.
Supplemental Data S1. Differential expression analysis of RNA sequencing data.
Supplemental Data S2. Differential expression analysis of microRNA data.
Acknowledgments
We thank Dr. Yuval Eshed (Weizmann Institute of Science) for providing us with 35S::miR319a seeds and Dr. Nai-Chun Lin (National Taiwan University) for the Pto DC3000 strain.
Glossary
- Pi
phosphate
- AM
arbuscular mycorrhizal
- JA
jasmonate
- miRNA
microRNA
- 1/2× MS
one-half-strength Murashige and Skoog
- Col-0
ecotype Columbia
- qRT
quantitative reverse transcription
- RNA-Seq
RNA Sequencing
- SA
salicylic acid
- Pto DC3000
Pseudomonas syringae pv tomato DC3000
Footnotes
This work was supported by the National Science Council (NSC 100–2321–B–005–007–MY3, NSC 101–2911–I–005–301, and NSC 102–2911–I–005–301) and Ministry of Education (Aim for the Top University project), Taiwan.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P. (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H. (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141: 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Correa VR, Toruño TY, Ammar D, Kamoun S, Hogenhout SA. (2009) AY-WB phytoplasma secretes a protein that targets plant cell nuclei. Mol Plant Microbe Interact 22: 18–30 [DOI] [PubMed] [Google Scholar]
- Bai X, Zhang J, Ewing A, Miller SA, Jancso Radek A, Shevchenko DV, Tsukerman K, Walunas T, Lapidus A, Campbell JW, et al. (2006) Living with genome instability: the adaptation of phytoplasmas to diverse environments of their insect and plant hosts. J Bacteriol 188: 3682–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin J, Khan GA, Combier JP, Bustos-Sanmamed P, Debernardi JM, Rodriguez R, Sorin C, Palatnik J, Hartmann C, Crespi M, et al. (2013) miR396 affects mycorrhization and root meristem activity in the legume Medicago truncatula. Plant J 74: 920–934 [DOI] [PubMed] [Google Scholar]
- Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkrog A, Schauser L, Scheible WR, Krajinski F. (2010) Expression pattern suggests a role of MiR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Mol Plant Microbe Interact 23: 915–926 [DOI] [PubMed] [Google Scholar]
- Bucher M, Wegmüller S, Drissner D. (2009) Chasing the structures of small molecules in arbuscular mycorrhizal signaling. Curr Opin Plant Biol 12: 500–507 [DOI] [PubMed] [Google Scholar]
- Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Pérez-Pérez J, Solano R, Leyva A, Paz-Ares J. (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6: e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WC, Lee TY, Huang HD, Huang HY, Pan RL. (2008) PlantPAN: plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genomics 9: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Hossain H, Otten S, Kuenne C, Kuchmina K, Machata S, Domann E, Chakraborty T, Hain T. (2006) Intracellular gene expression profile of Listeria monocytogenes. Infect Immun 74: 1323–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, et al. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18: 412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI. (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62: 185–206 [DOI] [PubMed] [Google Scholar]
- Chung WC, Chen LL, Lo WS, Lin CP, Kuo CH. (2013) Comparative analysis of the peanut witches’ broom phytoplasma genome reveals horizontal transfer of potential mobile units and effectors. PLoS ONE 8: e62770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi JM, Rodriguez RE, Mecchia MA, Palatnik JF. (2012) Functional specialization of the plant miR396 regulatory network through distinct microRNA-target interactions. PLoS Genet 8: e1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Serrone P, Marzachì C, Bragaloni M, Galeffi P. (2001) Phytoplasma infection of tomato in central Italy. Phytopathol Mediterr 40: 137–142 [Google Scholar]
- Gu M, Chen A, Dai X, Liu W, Xu G. (2011) How does phosphate status influence the development of the arbuscular mycorrhizal symbiosis? Plant Signal Behav 6: 1300–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Xu K, Chen A, Zhu Y, Tang G, Xu G. (2010) Expression analysis suggests potential roles of microRNAs for phosphate and arbuscular mycorrhizal signaling in Solanum lycopersicum. Physiol Plant 138: 226–237 [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Bussell JD, Păcurar DI, Schwambach J, Păcurar M, Bellini C. (2009) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21: 3119–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Paszkowski U. (2009) Weights in the balance: JA and SA signaling in root-biotroph interactions. Mol Plant Microbe Interact 22: 763–772 [DOI] [PubMed] [Google Scholar]
- Hogenhout SA, Oshima K, Ammar D, Kakizawa S, Kingdom HN, Namba S. (2008) Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol 9: 403–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10: 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ. (2009) Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151: 2120–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TK, Han CL, Lin SI, Chen YJ, Tsai YC, Chen YR, Chen JW, Lin WY, Chen PM, Liu TY, et al. (2013) Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25: 4044–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Nagarajan VK, Raghothama KG. (2012) Transcriptional regulation of phosphate acquisition by higher plants. Cell Mol Life Sci 69: 3207–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG. (2007) Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol 144: 232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamińska M, Klamkowski K, Berniak H, Sowik I. (2010) Response of mycorrhizal periwinkle plants to aster yellows phytoplasma infection. Mycorrhiza 20: 161–166 [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Jin H. (2010) Role of small RNAs in host-microbe interactions. Annu Rev Phytopathol 48: 225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305: 665–668 [DOI] [PubMed] [Google Scholar]
- Kiedrowski S, Kawalleck P, Hahlbrock K, Somssich IE, Dangl JL. (1992) Rapid activation of a novel plant defense gene is strictly dependent on the Arabidopsis RPM1 disease resistance locus. EMBO J 11: 4677–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer S, Holt AL, Rubio-Somoza I, Tucker EJ, Hinze A, Pisch M, Javelle M, Timmermans MC, Tucker MR, Laux T. (2013) A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev Cell 24: 125–132 [DOI] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39: D152–D157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Bottner KD, Munyaneza JE, Davis RE, Crosslin JM, du Toit LJ, Crosby T. (2006) Carrot purple leaf: a new spiroplasmal disease associated with carrots in Washington state. Plant Dis 90: 989–993 [DOI] [PubMed] [Google Scholar]
- Lee IM, Davis RE, Gundersen-Rindal DE. (2000) Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol 54: 221–255 [DOI] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Chiou TJ. (2013) Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25: 4061–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingua G, D’Agostino G, Massa N, Antosiano M, Berta G. (2002) Mycorrhiza-induced differential response to a yellows disease in tomato. Mycorrhiza 12: 191–198 [DOI] [PubMed] [Google Scholar]
- Liu TY, Aung K, Tseng CY, Chang TY, Chen YS, Chiou TJ. (2011) Vacuolar Ca2+/H+ transport activity is required for systemic phosphate homeostasis involving shoot-to-root signaling in Arabidopsis. Plant Physiol 156: 1176–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Tseng CY, Lai YS, Lin SI, Lin WY, Chen JW, Chiou TJ. (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Bartel DP, Bartel B. (2005) MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17: 1360–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Bari R, Gibon Y, Zheng W, Pant BD, Blasing O, Usadel B, Czechowski T, Udvardi MK, Stitt M, et al. (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 30: 85–112 [DOI] [PubMed] [Google Scholar]
- Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH. (2007) Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol 143: 156–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P. (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138: 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG. (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312: 436–439 [DOI] [PubMed] [Google Scholar]
- Ohkama-Ohtsu N, Wasaki J. (2010) Recent progress in plant nutrition research: cross-talk between nutrients, plant physiology and soil microorganisms. Plant Cell Physiol 51: 1255–1264 [DOI] [PubMed] [Google Scholar]
- Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, Menda N, Amsellem Z, Efroni I, Pekker I, et al. (2007) Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet 39: 787–791 [DOI] [PubMed] [Google Scholar]
- Oshima K, Kakizawa S, Nishigawa H, Jung HY, Wei W, Suzuki S, Arashida R, Nakata D, Miyata SI, Ugaki M, et al. (2004) Reductive evolution suggested from the complete genome sequence of a plant-pathogenic phytoplasma. Nat Genet 36: 27–29 [DOI] [PubMed] [Google Scholar]
- Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR. (2009) Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol 150: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M. (2009) Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA 106: 22540–22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16: 442–450 [DOI] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. (2008) Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido A, Laufs P. (2010) Co-ordination of developmental processes by small RNAs during leaf development. J Exp Bot 61: 1277–1291 [DOI] [PubMed] [Google Scholar]
- Radtke AL, O’Riordan MXD. (2006) Intracellular innate resistance to bacterial pathogens. Cell Microbiol 8: 1720–1729 [DOI] [PubMed] [Google Scholar]
- Raghothama KG. (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50: 665–693 [DOI] [PubMed] [Google Scholar]
- Romanazzi G, Musetti R, Marzachì C, Casati P. (2009) Induction of resistance in the control of phytoplasma diseases. Petria 19: 113–129 [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. (2010) Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol Plant 3: 288–299 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J. (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15: 2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, Dong X, Robatzek S, Schulze-Lefert P. (2009) Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 28: 3439–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampò S, Massa N, D’Agostino U, Bosco D, Marzachì C, Berta G. (2012) Effects of two AM fungi on phytoplasma infection in the model plant Chrysanthemum carinatum. Agric Food Sci 21: 39-51 [Google Scholar]
- Schommer C, Palatnik JF, Aggarwal P, Chételat A, Cubas P, Farmer EE, Nath U, Weigel D. (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6: e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F. (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156: 997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JB, Huang SQ, Dalmay T, Yang ZM. (2012) Regulation of leaf morphology by microRNA394 and its target LEAF CURLING RESPONSIVENESS. Plant Cell Physiol 53: 1283–1294 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–77012615947 [Google Scholar]
- Sugio A, Kingdom HN, MacLean AM, Grieve VM, Hogenhout SA. (2011b) Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc Natl Acad Sci USA 108: E1254–E1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio A, MacLean AM, Kingdom HN, Grieve VM, Manimekalai R, Hogenhout SA. (2011a) Diverse targets of phytoplasma effectors: from plant development to defense against insects. Annu Rev Phytopathol 49: 175–195 [DOI] [PubMed] [Google Scholar]
- Volpe V, Dell’Aglio E, Giovannetti M, Ruberti C, Costa A, Genre A, Guether M, Bonfante P. (2013) An AM-induced, MYB-family gene of Lotus japonicus (LjMAMI) affects root growth in an AM-independent manner. Plant J 73: 442–455 [DOI] [PubMed] [Google Scholar]
- Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. (2008) The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol 147: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138: 750–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sun R, Albrecht U, Padmanabhan C, Wang A, Coffey MD, Girke T, Wang Z, Close TJ, Roose M, et al. (2013) Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for citrus huanglongbing disease. Mol Plant 6: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]