An ethylene response factor transcription factor positively affects the interactions between stress responses to the pathogen Rhizoctonia cerealis and freezing in wheat.
Abstract
Sharp eyespot disease (primarily caused by the pathogen Rhizoctonia cerealis) and freezing stress are important yield limitations for the production of wheat (Triticum aestivum). Here, we report new insights into the function and underlying mechanisms of an ethylene response factor (ERF) in wheat, Pathogen-Induced ERF1 (TaPIE1), in host responses to R. cerealis and freezing stresses. TaPIE1-overexpressing transgenic wheat exhibited significantly enhanced resistance to both R. cerealis and freezing stresses, whereas TaPIE1-underexpressing wheat plants were more susceptible to both stresses relative to control plants. Following both stress treatments, electrolyte leakage and hydrogen peroxide content were significantly reduced, and both proline and soluble sugar contents were elevated in TaPIE1-overexpressing wheat, whereas these physiological traits in TaPIE1-underexpressing wheat exhibited the opposite trend. Microarray and quantitative reverse transcription-polymerase chain reaction analyses of TaPIE1-overexpressing and -underexpressing wheat plants indicated that TaPIE1 activated a subset of defense- and stress-related genes. Assays of DNA binding by electrophoretic mobility shift and transient expression in tobacco (Nicotiana tabacum) showed that the GCC boxes in the promoters of TaPIE1-activated genes were essential for transactivation by TaPIE1. The transactivation activity of TaPIE1 and the expression of TaPIE1-activated defense- and stress-related genes were significantly elevated following R. cerealis, freezing, and exogenous ethylene treatments. TaPIE1-mediated responses to R. cerealis and freezing were positively modulated by ethylene biosynthesis. These data suggest that TaPIE1 positively regulates the defense responses to R. cerealis and freezing stresses by activating defense- and stress-related genes downstream of the ethylene signaling pathway and by modulating related physiological traits in wheat.
Wheat (Triticum aestivum) is one of the most widely cultivated and important food crops, feeding more than 40% of the world’s population (Gill et al., 2004). Global demand for wheat is increasing with world population growth. Biotic and abiotic stresses often affect wheat production. For example, sharp eyespot, which is primarily caused by the necrotrophic fungus Rhizoctonia cerealis, is a devastating disease for wheat around the world. In China, the disease has recently become one of the most serious limiting factors in wheat production, with yield losses of 10% to 40% and losses even reaching greater than 50% in the most serious cases (Chen et al., 2008, 2009). Because of changes in global weather patterns, freezing stress often causes severe yield losses in winter and spring wheat because of severe winters and late freezes (Frederiks et al., 2012). To improve wheat’s tolerance to R. cerealis and freezing stresses, it is vital to identify the important regulatory genes associated with these stresses and to elucidate the mechanisms underlying these gene functions.
Plants have evolved a tuning adaptation network to cope with biotic and abiotic stresses. These stresses can induce the expression of both distinct and overlapping sets of genes, resulting in a series of physical and biochemical changes in plants (Mengiste et al., 2003; Seo et al., 2010). Genome-scale screening and molecular genetic studies, mostly in Arabidopsis (Arabidopsis thaliana), have identified a group of genes encoding various transcription factors (TFs) that are regulated by biotic and abiotic stresses (McGrath et al., 2005; Yamaguchi-Shinozaki and Shinozaki, 2006; Seo et al., 2010). Accumulating evidence indicates that cold/freezing signals are closely linked to pathogen resistance (Tsutsui et al., 2009; Seo et al., 2010; Yang et al., 2010). TFs may play a crucial role in the cross talk between cold tolerance and pathogen resistance. For example, the transcription repressor of a dehydration-responsive element-binding (DREB) gene called DEAR1 (for DREB and EAR motif protein1) plays a role in the balanced regulation of pathogen resistance and cold stress response in Arabidopsis (Tsutsui et al., 2009). In Arabidopsis, cold stimulates the proteolytic activation of a plasma membrane-tethered NAC (for NO APICAL MERISTEM/ARABIDOPSIS TRANSCRIPTION ACTIVATION FACTOR1/2/CUP-SHAPED COTYLEDON2) TF named NTL6, leading to an induced resistance response to the bacterial pathogen Pseudomonas syringae (Seo et al., 2010). The ectopic expression of a pepper (Capsicum annuum) ETHYLENE RESPONSE FACTOR (ERF) gene named Pathogen and Freezing Tolerance-Related Protein1 improved tolerance to a bacterial pathogen and freezing stress in transgenic Arabidopsis (Yi et al., 2004).
Ethylene is a very important phytohormone that participates in myriad developmental processes and fitness responses in plants, including seed germination, flower and leaf senescence, fruit ripening, leaf abscission, and responses to stress and pathogen attack (Johnson and Ecker, 1998). In Arabidopsis, ethylene can be captured by its receptors (Hua and Meyerowitz, 1998), which remove the block of CONSTITUTIVE TRIPLE RESPONSE1 on ETHYLENE INSENSITIVE2 (EIN2; Solano et al., 1998; Zhang et al., 2009). The release of EIN2 further activates the ethylene signal primary TF EIN3/EIN3-like1 (Chao et al., 1997), leading to the expression of secondary TFs including Arabidopsis ERF1. These secondary TFs regulate the expression of downstream defense- and stress-related genes, consequently enhancing the plant’s tolerance to stress (Solano et al., 1998; Berrocal-Lobo et al., 2002).
The ERF TFs containing an ERF DNA-binding domain were first identified as GCC box (with the core sequence GCCGCC)-binding proteins in Arabidopsis and tobacco (Nicotiana tabacum; Ohme-Takagi and Shinshi, 1995; Solano et al., 1998). An increasing number of studies have shown that various ERF proteins have diverse functions in ethylene-related pathogen resistance, plant development, and various environmental stress responses by interacting with multiple cis-acting elements in the promoters of regulated genes (Berrocal-Lobo et al., 2002; Yi et al., 2004; Zhang et al., 2004, 2007, 2012a; McGrath et al., 2005; Xu et al., 2006, 2007; Oh et al., 2009; Zhang and Huang, 2010; Cheng et al., 2013; Schmidt et al., 2013). Earlier studies showed that the ERF members of the B3 subgroup primarily participated in disease resistance (Berrocal-Lobo et al., 2002; Gutterson and Reuber, 2004; McGrath et al., 2005; Oñate-Sánchez et al., 2007). Recent investigations indicated that certain B3-type ERF proteins participated in both biotic and abiotic stress responses by regulating specific defense- and stress-related genes (Berrocal-Lobo et al., 2002; Zhang et al., 2007; Cheng et al., 2013). For example, Arabidopsis ERF1 up-regulates specific suites of genes in response to biotic and abiotic stresses by stress-specific binding to GCC box or dehydration-responsive element (DRE)/C-repeat motifs. ERF1 positively regulates Arabidopsis resistance to the pathogens Botrytis cinerea and Plectosphaerella cucumerina by binding GCC boxes in defense-related genes (Solano et al., 1998; Berrocal-Lobo et al., 2002). Conversely, in response to abiotic stress, Arabidopsis ERF1 binds to DRE motifs but not GCC boxes. Although there has been substantial progress in understanding the roles of ERFs in model plants, ERF TFs in wheat have not been well characterized because of the plant’s polyploidy and the complexity of the wheat genome (Dong et al., 2012).
Given that distinct ERF TFs may have different regulatory roles depending on the species, investigation of wheat-specific ERFs is very important for understanding their regulatory functions in wheat. An in silico analysis based on the presence of the conserved AP2/ERF domain amino acid sequence of Arabidopsis identified at least 47 ERF genes from ESTs of wheat (Zhuang et al., 2011). However, the functions of these wheat ERFs, especially in necrotrophic pathogen and freezing stress responses, and the underlying mechanisms of these functions in wheat remain poorly understood, primarily because it is difficult and time consuming to generate stably transgenic wheat lines and mutants. We previously isolated the pathogen-induced wheat ERF gene TaPIEP1/TaPIE1 (GenBank accession no. EF583940). It belongs to the B3 subgroup within the ERF subfamily, but its entire sequence shares a low identity with known B3-type ERFs. The TaPIE1 protein was demonstrated to be an ERF transcription activator with in vitro binding activity to the GCC box cis-element; moreover, TaPIE1 overexpression conferred significantly enhanced resistance to the fungal pathogen Bipolaris sorokiniana infection in transgenic wheat (Dong et al., 2010). We recently found that TaPIE1 expression was also induced following R. cerealis infection and freezing stress. These findings prompted us to explore whether TaPIE1 regulates freezing tolerance and R. cerealis resistance in wheat and to examine the physiological and molecular mechanisms underlying its functions.
In this study, TaPIE1-overexpressing and -underexpressing wheat plants were used to investigate the effects of TaPIE1 on R. cerealis, freezing, and ethylene responses. To explore the possible physiological mechanisms, we examined the electrolyte leakage and Pro, soluble sugar, and hydrogen peroxide (H2O2) contents in these wheat plants after R. cerealis and freezing stimuli. To investigate the molecular mechanism underlying these functions, microarrays, real-time quantitative reverse transcription (qRT)-PCR, transient expression analyses, and electrophoretic mobility shift assays (EMSAs) were used to characterize the defense- and stress-related genes up-regulated by TaPIE1. The results showed that TaPIE1 positively regulated wheat responses to both R. cerealis and freezing stresses and that TaPIE1 directly activated a range of defense- and stress-related genes by interacting with the GCC boxes in the promoters. The TaPIE1-enhanced resistance and the expression of these genes were correlated with ethylene biosynthesis.
RESULTS
Molecular Characterization of TaPIE1-Overexpressing and -Underexpressing Wheat
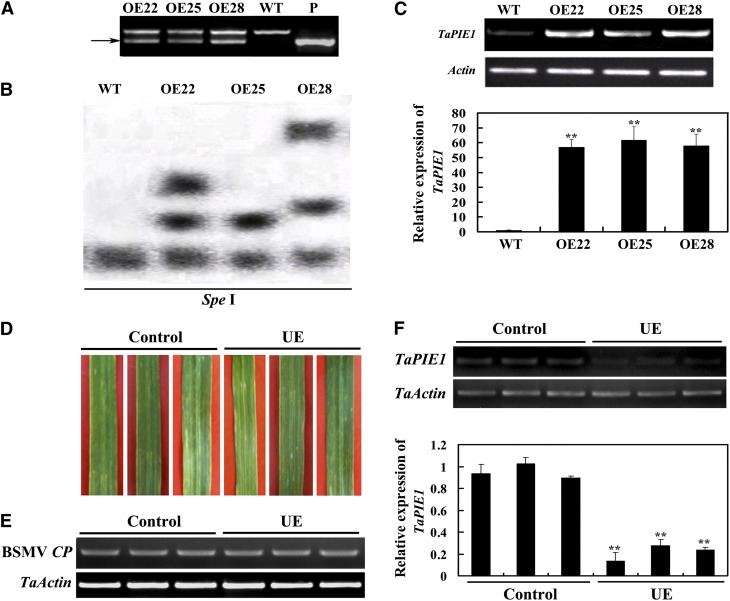
To dissect the function of TaPIE1 in wheat, we previously generated TaPIE1-overexpressing transgenic wheat lines (OE22, OE25, and OE28) by bombarding the expression vector pA25-TaPIE1 into the spring wheat cv Yangmai 12 (Dong et al., 2010). In these transgenic wheat lines, TaPIE1 was driven by the maize UBIQUITIN promoter, which is known to be induced by extreme stresses, including heat shock, drought, and B. sorokiniana treatments (Streatfield et al., 2004; Zhang et al., 2012b). The TaPIE1-overexpressing transgene was detected in all the T4 and T5 plants from the three TaPIE1-overexpressing transgenic lines (OE22, OE25, and OE28) by specific amplification (Fig. 1A), suggesting that the introduced transgene was inheritable. Southern-blot results showed that the TaPIE1-overexpressing transgene was integrated into the genomes of the three transgenic lines with one or two copies by different hybridization patterns (Fig. 1B), which were derived from independent transformation events. Reverse transcription (RT)-PCR and qRT-PCR assays showed that the transcript abundance of TaPIE1 was significantly elevated in TaPIE1-overexpressing lines when compared with wild-type cv Yangmai 12 (Fig. 1C), and they were markedly induced following R. cerealis infection or freezing treatment (Supplemental Fig. S1).
Figure 1.
Molecular characterization of TaPIE1-overexpressing transgenic wheat and TaPIE1-underexpressing wheat plants. A, PCR patterns of TaPIE1-overexpressing lines (OE; OE22, OE25, and OE28) and wild-type (WT) cv Yangmai 12 by using transgene-specific primers. P, Transformation vector pA25-TaPIE1 (positive control). The arrow indicates the transgene-specific amplification band (296 bp). B, Southern-blot assay of the OE and wild-type plants. Twenty micrograms of genomic DNA digested by the restriction enzyme SpeI was hybridized with the probe derived from the TaPIE1 transgene-specific fragment (296 bp). C, RT-PCR and qRT-PCR analyses of TaPIE1 transcription in the OE and wild-type plants. The relative transcript abundance of TaPIE1 in the three OE transgenic lines was compared with that in wild-type wheat (set to 1). D, Chlorotic mosaic symptoms on the fourth leaves of BSMV:00-infected (control) and BSMV:TaPIE1-infected (TaPIE1-underexpressing [UE]) wheat cv Yangmai 12 seedlings. The photographs were taken at 14 dpi. E and F, RT-PCR and qRT-PCR analyses of relative transcript levels of the BSMV COAT PROTEIN (CP) gene (E) and TaPIE1 (F) in the fourth leaves of the UE and control seedlings. The relative transcript abundance of TaPIE1 in the UE plants was related to that in a control plant (set to 1). The amplification of wheat ACTIN (TaActin) was used as an internal control to normalize all the data. Significant differences between the OE and wild-type plants or the UE and control plants were derived from the results of three independent replications (Student’s t test: **P < 0.01). Error bars indicate se. [See online article for color version of this figure.]
Virus-induced gene silencing as developed with Barley stripe mosaic virus (BSMV) has been demonstrated to be an effective reverse-genetics tool for investigating the function of target genes in barley (Hordeum vulgare) and wheat (Hein et al., 2005; Scofield et al., 2005). To obtain reverse-genetic evidence on the function of TaPIE1 in wheat, a recombinant BSMV:TaPIE1 virus was prepared (Supplemental Fig. S2) and used to suppress TaPIE1 expression in cv Yangmai 12 plants. The results showed that the TaPIE1 transcript was substantially decreased in cv Yangmai 12 plants from 10 d post inoculation (dpi) with the BSMV:TaPIE1 virus to wheat maturity. For example, at 14 dpi with BSMV:TaPIE1 virus or BSMV:00 virus, all the fourth leaves of the inoculated wheat plants exhibited BMSV infection symptoms (Fig. 1D), and the transcript of the BSMV COAT PROTEIN gene was readily detected (Fig. 1E). As expected, the TaPIE1 transcript was down-regulated in the plants infected with BSMV:TaPIE1 (Fig. 1F). Hereafter, these TaPIE1-underexpressing plants are referred to as TaPIE1 underexpressors, and BSMV:00-infected wheat plants were used as the control for the underexpressor experiments.
TaPIE1 Promotes Wheat Defense Responses to R. cerealis and Freezing Stresses
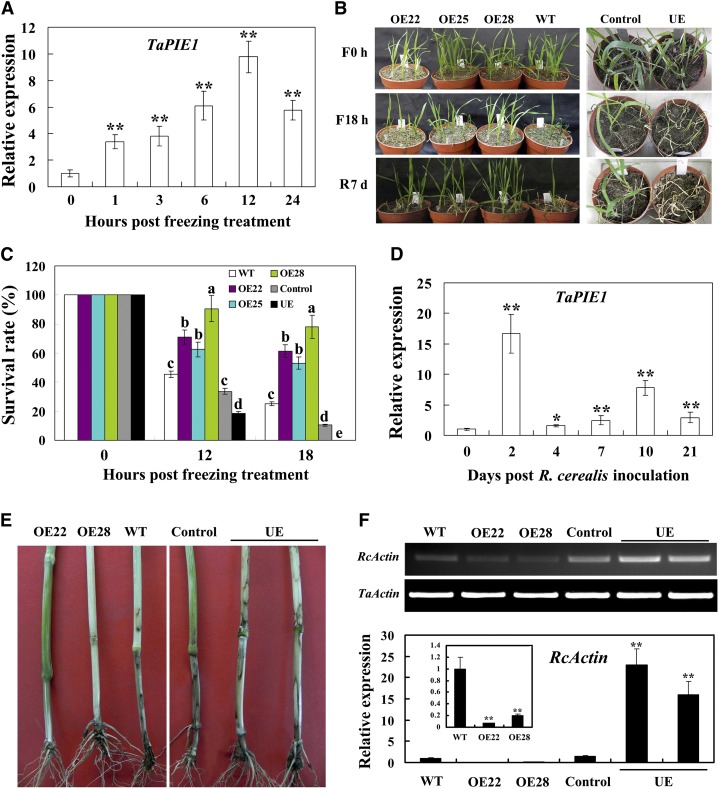
TaPIE1 transcript was markedly and rapidly induced by freezing stress (Fig. 2A), suggesting that TaPIE1 may participate in freezing stress responses in wheat. Following a freezing treatment at –9°C for 12 h, 61.5% to 90.62% of plants from three overexpressing lines (OE22, OE25, and OE28) survived, whereas only 45.45% of the wild-type plants survived (Fig. 2, B and C). After 18 h of –9°C treatment, the survival rates of the TaPIE1-overexpressing plants were 53.12% to 78.12% compared with only 25.15% of wild-type plants (Fig. 2, B and C), suggesting that TaPIE1 overexpression significantly enhanced freezing tolerance in wheat. Furthermore, 18.75% of TaPIE1 underexpressors survived following –5°C treatment for 12 h, whereas 33.75% of the control plants survived; when exposed to –5°C for 18 h, none of the TaPIE1-underexpressing plants survived (survival rate = 0%), whereas 10.48% of the control plants survived (Fig. 2, B and C), indicating that TaPIE1 underexpression in wheat markedly compromised freezing tolerance. These results demonstrated that TaPIE1 is required for freezing stress tolerance in wheat.
Figure 2.
TaPIE1 promotes wheat responses to freezing and R. cerealis stresses. A, qRT-PCR analysis of TaPIE1 induction by freezing stimulus. Total RNA was extracted from the leaves of cv Yangmai 12 plants after –5°C freezing treatment for the indicated times. B, Freezing-tolerant phenotypes of the wild-type (WT) cv Yangmai 12, TaPIE1-overexpressing (OE; OE22, OE25, and OE28) TaPIE1-underexpressing (UE), and control plants. F0 h and F18 h indicate growth under freezing conditions for 0 and 18 h, respectively; R7 d indicates recovery growing under normal conditions for 7 d after 18 h of freezing treatment. C, Survival rates of the OE, wild type, UE, and control cv Yangmai 12 seedlings at 12 and 18 h under freezing stresses. For A to C, seedlings at the four-leaf stage were grown at 4°C for 6 h and then kept at –9°C (for the OE and control plants) or –5°C (for the UE and control plants) for the indicated times. D, qRT-PCR analysis of TaPIE1 induction after R. cerealis inoculation. Total RNA was extracted from the leaf sheaths of cv Yangmai 12 plants for the indicated times. E, Typical infection phenotypes of the OE, UE, wild type, and control cv Yangmai 12 plants after R. cerealis inoculation for 55 d. F, qRT-PCR analysis of R. cerealis relative biomass based on the transcript level of the R. cerealis ACTIN gene (RcActin). For C to F, seedlings at the four-leaf stage were infected with R. cerealis. The amplification of the wheat ACTIN gene was used as an internal control to normalize all the data. Significant differences were analyzed based on the results of three replications (Student’s t test: *P < 0.05, **P < 0.01). Error bars indicate se. [See online article for color version of this figure.]
Upon R. cerealis infection, TaPIE1 expression was significantly induced (Fig. 2D), suggesting that TaPIE1 may be involved in the wheat defense response to the pathogen. Following inoculation with R. cerealis, three TaPIE1-overexpressing lines (OE22, OE25, and OE28) displayed significantly enhanced resistance when compared with the wild-type plants, whereas TaPIE1-underexpressing plants exhibited higher susceptibility than the control plants (Fig. 2, E and F). The average disease indexes of the TaPIE1-overexpressing lines were 22.88% to 24.61%, and that of wild-type cv Yangmai 12 was 50.79% (Supplemental Table S1); the disease index of TaPIE1-underexpressing plants was 67.91%, and that of the control plants was 53.18% (Supplemental Table S1). Moreover, we analyzed the relative biomass of R. cerealis in wheat stems based on the transcript level of the R. cerealis ACTIN gene according to Chacón et al. (2010). The results indicated that the R. cerealis biomass was lower in TaPIE1-overexpressing lines than in wild-type plants, and it was highest in the TaPIE1-underexpressing plants (Fig. 2F). These data suggest that TaPIE1 positively regulates the wheat defense response to R. cerealis infection.
TaPIE1 Regulates Physiological Traits for Freezing and Biotic Stresses
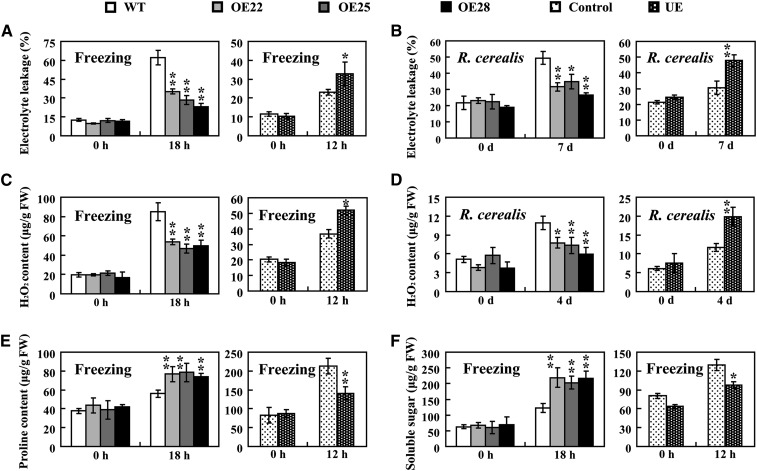
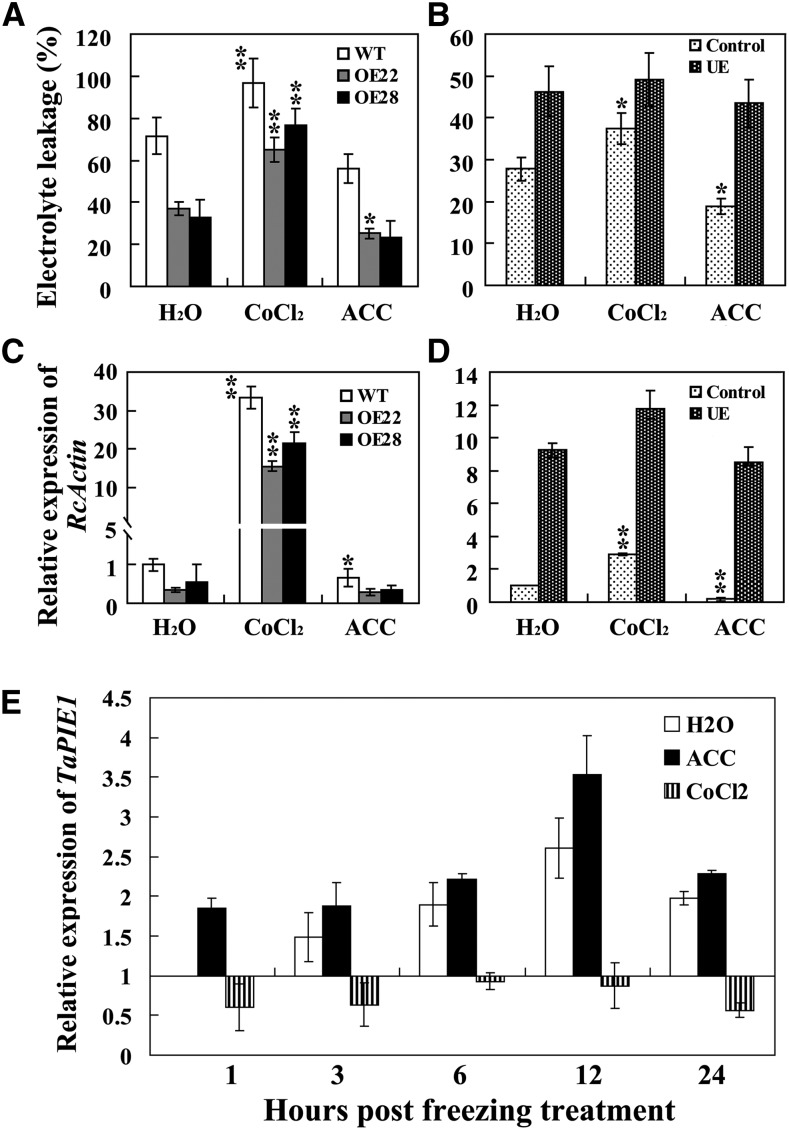
During freezing, the plasma membranes of cells are injured and ions leak from the cytoplasm (González-Aguilar et al., 2000). Ion leakage is often used as an indicator of damage to plasma membranes (Zhang and Huang, 2010). Under normal conditions, there were no significant differences in electrolyte leakage between wild-type and TaPIE1-overexpressing seedlings or between TaPIE1-underexpressing seedlings and control plants (Fig. 3, A and B). After treatment at –9°C for 18 h, the electrolyte leakage from the wild-type plants was significantly higher (60.10%) than those of the TaPIE1-overexpressing lines (OE22, 35.27%; OE25, 28.44%; and OE28, 23.06%); following 12 h of freezing treatment at –5°C, the leakage from TaPIE1 underexpressors (32.78%) was significantly higher than that of the control plants (22.96%; Fig. 3A). Interestingly, at 7 d after R. cerealis inoculation, the electrolyte leakage increased markedly, but it was still relatively lower in overexpressing plants than in wild-type plants, and the leakage in TaPIE1 underexpressors was highest (Fig. 3B). These data indicated that TaPIE1 overexpression reduced but TaPIE1 underexpression enhanced the plasma membrane damage, and the plasma membrane integrity might be important for wheat defense against freezing stress and R. cerealis infection.
Figure 3.
Physiological traits of wild-type (WT), TaPIE1-overexpressing (OE) and TaPIE1-underexpressing (UE), and control plants. A and B, Comparison of electrolyte leakage from wild-type, OE, UE, and control seedlings with freezing treatment (A) or R. cerealis infection (B) for the indicated times. C and D, H2O2 content in wild-type, OE, UE, and control wheat plants with freezing treatment (C) or R. cerealis infection (D) for the indicated times. E and F, Free Pro (E) and soluble sugar (F) contents of OE, wild-type, UE, and control plants with or without freezing treatment. FW, Fresh weight. For freezing treatment, seedlings at the four-leaf stage from the wild-type cv Yangmai 12 and OE lines were exposed to –9°C for 18 h; UE and control plants at the four-leaf stage were maintained at –5°C for 12 h. The leaves of plants at the end of the freezing treatment or the leaf sheaths of plants after R. cerealis treatment were harvested and used for the above assays. Significant differences between the OE and wild-type plants or the UE and control plants under the same conditions were analyzed based on three replications (Student’s t test: *P < 0.05, **P < 0.01). Error bars indicate se.
Freezing and necrotrophic pathogens often promote the generation of reactive oxygen species (ROS; Mengiste et al., 2003; Zhang et al., 2012a), the overproduction of which can cause oxidative stress and damage proteins, lipids, carbohydrates, and nucleotides in plant cells (Wu et al., 2008). Nitroblue tetrazolium and 3,3-diaminobenzidine staining assays showed that under normal conditions, there were few superoxide anion (O2−) and H2O2 formation spots on the leaves of the tested wheat plants (Supplemental Fig. S3, A, C, E, and G). Following the freezing stress treatment, the O2− and H2O2 formation spots were more numerous on the wild-type leaves than in TaPIE1-overexpressing plants (Supplemental Fig. S3, B and F), and the greatest number were those on the underexpressing plants (Supplemental Fig. S3, D and H). Quantitative assays consistently showed that after freezing treatment, the H2O2 content of TaPIE1-overexpressing plants was lowest and that TaPIE1-underexpressing plants had the highest H2O2 content (Fig. 3C). Moreover, at 4 d after R. cerealis inoculation, the H2O2 content was significantly higher in wild-type plants than in TaPIE1-overexpressing lines, and it was highest in TaPIE1-underexpressing plants (Fig. 3D). These results indicated that the ROS accumulation was affected by TaPIE1 expression.
When exposed to abiotic stresses, plants can increase their accumulation of osmotic substances (including free Pro and soluble sugar) to maintain high intracellular osmotic pressure and normal physiological functions of cells (Sharma et al., 2005). Therefore, we measured the contents of free Pro and soluble sugar in wild-type cv Yangmai 12, TaPIE1-overexpressing and -underexpressing plants, and control plants. There were no significant differences in the free Pro and soluble sugar contents between the TaPIE1 overexpressors and wild-type cv Yangmai 12 plants under normal conditions (Fig. 3, E and F). Following freezing treatment, the contents of free Pro and soluble sugars in TaPIE1-overexpressing lines increased more significantly than those of the wild type, but they were lowest in the TaPIE1-underexpressing plants (Fig. 3, E and F). These results revealed that the accumulation of free Pro and soluble sugars was associated with TaPIE1 expression.
TaPIE1 Activates the Expression of Stress- and Defense-Related Genes
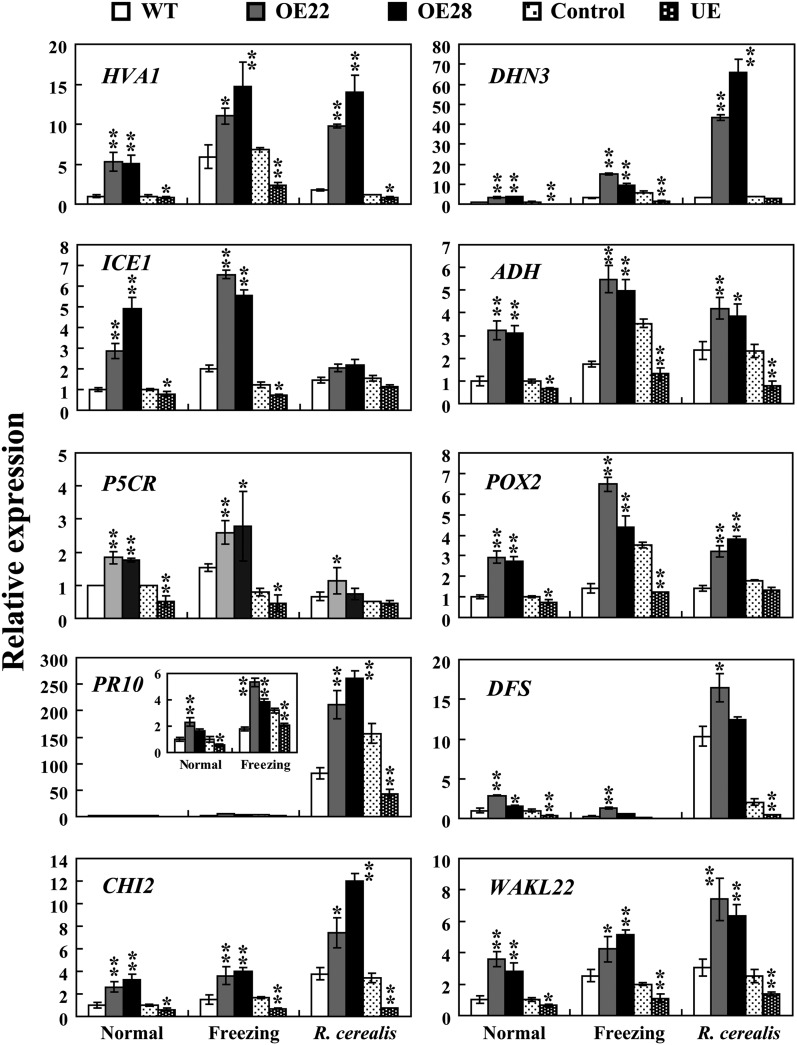
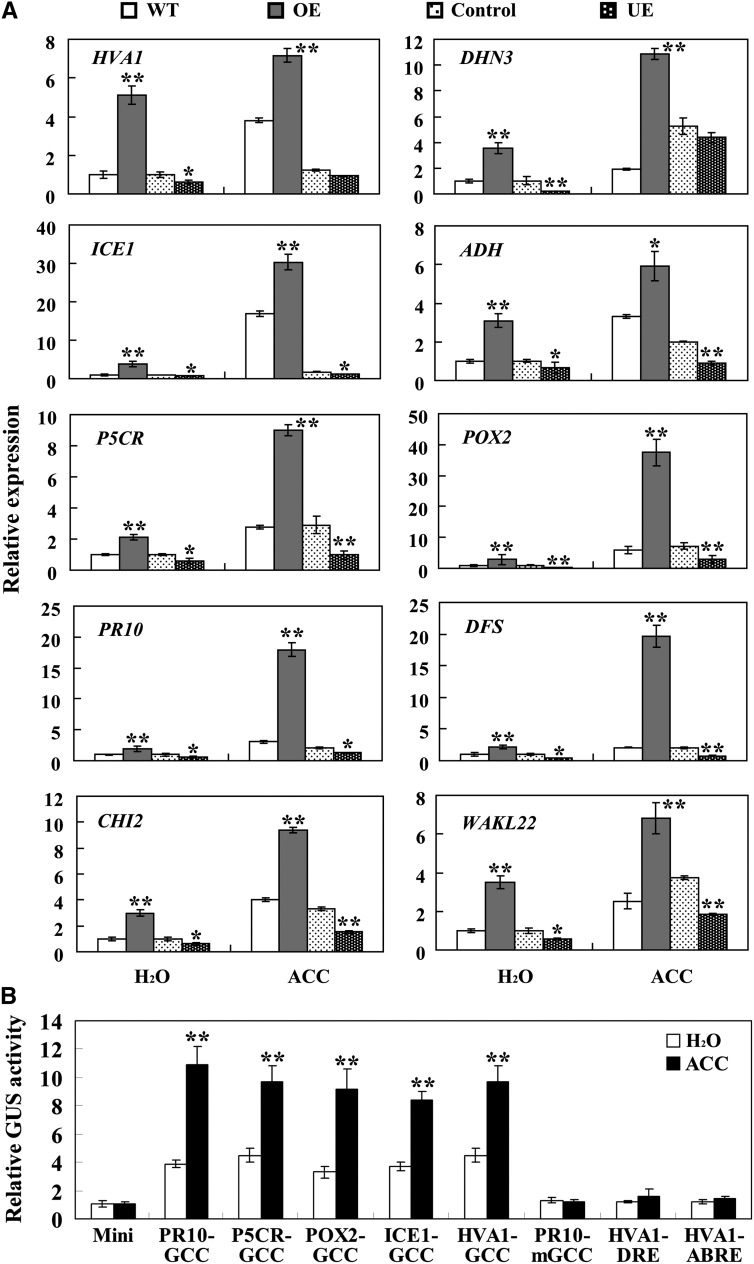
ERF TFs have been implicated in biotic and abiotic stress responses through their regulation of defense- and stress-related gene expression. As an ERF transcription activator (Dong et al., 2010), TaPIE1 protein should activate a subset of defense- and stress-related genes in wheat. To identify the genes up-regulated by TaPIE1, microarray analyses were first conducted with an Affymetrix Wheat GeneChip Array hybridized with probes from TaPIE1 overexpressors (OE22 and OE28) and wild-type cv Yangmai 12 plants treated by freezing (–5°C for 12 h) and then inoculated with R. cerealis (21 dpi). As shown in Supplemental Table S2, 1,078 genes in TaPIE1-overexpressing lines were up-regulated at least 3-fold higher (P ≤ 0.05) relative to the wild-type plants, among which 131 were categorized for their involvement in pathogen and cold/freezing stress responses based on a Gene Ontology analysis. Interestingly, most of the promoters in these 131 genes contain at least one GCC box (Supplemental Table S3). Of these 131 genes, 38 were annotated for their involvement in pathogen defense responses, 58 were involved in freezing stress responses, and 35 participated in both the cold/freezing response and the defense response (Supplemental Table S3; Supplemental Fig. S4A). These genes encoded TFs including INDUCER OF CBF EXPRESSION1 (ICE1), WRKY45, and NAC2; kinases including the calcium-dependent protein kinases CPK3 and CPK6 and WALL-ASSOCIATED KINASE-LIKE protein (WAKL); ROS-scavenging genes including the peroxidase POX2; ALCOHOL DEHYDROGENASE (ADH; a marker gene for low-oxygen stress); heat shock proteins; transporter-related proteins; Pro biosynthesis-related enzymes, including PYRROLINE-5-CARBOXYLATE REDUCTASE (P5CR); ethylene biosynthesis-related enzyme (1-AMINOCYCLOPROPANE-1-CARBOXYLIC ACID OXIDASE2 [ACO2]); cold-related effectors including the late embryogenesis-abundant (LEA) proteins Abscisic Acid-Induced Protein1 (HVA1), Responsive to Abscisic Acid Protein18 (RAB18), and DEHYDRIN3 [DHN3]); defense-related proteins including Pathogenesis-Related Protein10 (PR10), DEFENSIN (DFS), CHITINASE2 (CHI2), and GLUCANASE (Glu8); and others (Supplemental Table S3). The limitation of these data is that TaPIE1 overexpression may lead to the up-regulation of genes that are not normally influenced by TaPIE1. To address this concern, we analyzed the transcription of 17 stress- and defense-related genes (HVA1, RAB18, DHN3, ADH, ICE1, CPK3, CPK6, ACO2, POX2, Jasmonate-Induced Protein1 (JR1), P5CR, Lipid Transfer Protein (LTP), PR10, DFS, CHI2, WAKL22, and Glu8) using qRT-PCR in TaPIE1-underexpressing and -overexpressing plants and control wheat plants. As shown in Figure 4 and Supplemental Figure S4B, with the exceptions of LTP and Glu8, the transcription of the other 15 genes in TaPIE1-underexpressing plants clearly decreased relative to the control, whereas TaPIE1-overexpressing plants accumulated more transcripts of the 16 genes (excluding Glu8) than did the wild-type plants, demonstrating that most of these stress- and defense-related genes identified by microarray analyses were indeed up-regulated by TaPIE1.
Figure 4.
TaPIE1-activated defense- and stress-related genes were up-regulated in TaPIE1-overexpressing (OE) and down-regulated in TaPIE1-underexpressing (UE) plants. Total RNA was extracted from leaves of wild-type (WT), OE (OE22 and OE28), UE, and control plants at the four-leaf stage under normal conditions, freezing treatments (–9°C, 12 h for wild-type and OE plants; –5°C, 12 h for UE and control plants), or R. cerealis infection for 21 d. The expression levels of those genes in wild-type plants under normal conditions were set to 1. The amplification of the wheat ACTIN gene was used as an internal control to normalize all the data. Significant differences between the OE and wild-type plants or the UE and control plants under the same conditions were derived from the results of three independent replications (Student’s t test: *P < 0.05, **P < 0.01). Error bars indicate se.
We further investigated 10 stress- and defense-related genes (HVA1, DHN3, ADH, ICE1, P5CR, POX2, PR10, DFS, CHI2, and WAKL22). Following freezing stimulus, the expression of nine stress- and defense-related genes (excluding DFS) was significantly elevated (Fig. 4). The expression of eight genes (excluding ICE1 and P5CR) was significantly induced by R. cerealis infection (Fig. 4). These inductions in TaPIE1-overexpressing lines reached a higher level than those of wild-type plants, but the levels in TaPIE1-underexpressing plants were compromised more than in the control plants (Fig. 4). These data indicated that TaPIE1 activated the transcription of these stress- and defense-related genes, which were induced by R. cerealis and/or freezing stresses.
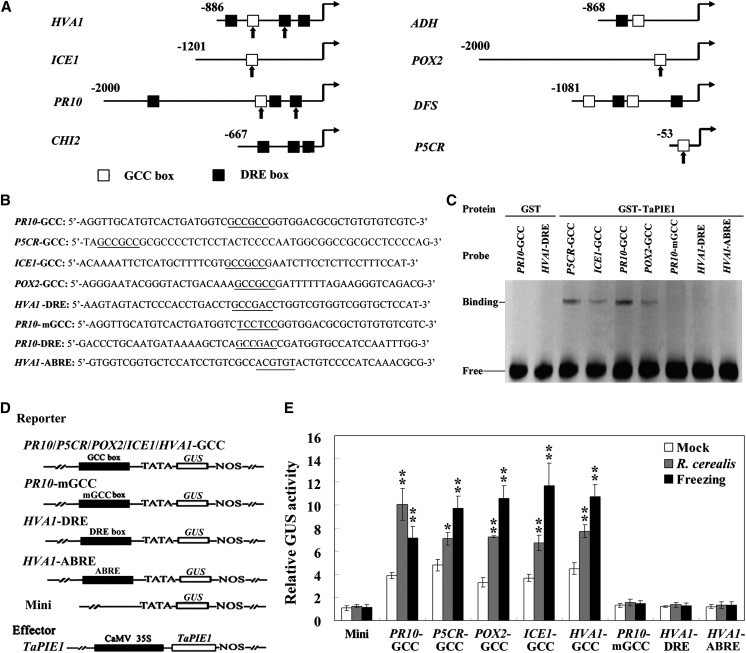
TaPIE1 Binds to GCC Boxes in Defense- and Stress-Related Genes
In model plants, ERF TFs regulate the expression of defense and stress-related genes by interacting with GCC box and/or non-GCC motifs (DRE/C-repeat; Chakravarthy et al., 2003; Zhang et al., 2012a; Cheng et al., 2013). For example, Arabidopsis ERF1 bound preferentially to different cis-elements of downstream genes under different stress treatments (Cheng et al., 2013). To explore how TaPIE1 regulates the expression of the 10 aforementioned defense- and stress-related genes, we searched the cis-elements present in the promoter sequences that were upstream from the start codon of each of these genes. As shown in Figure 5A and Supplemental Table S4, the queried promoter sequences for PR10, HVA1, ADH, ICE1, POX2, P5CR, and DFS genes separately contained at least one GCC box, and no GCC box was found in the partial promoters of CHI2 (667 bp) or DHN3 (334 bp), possibly because both queried sequences were not complete; the WAKL22 promoter was not found in the present databases. Additionally, several DREs and/or abscisic acid-responsive elements (ABREs) were also found in the queried promoters of these genes (Supplemental Table S4).
Figure 5.
TaPIE1 binding affinity to the GCC box in the TaPIE1-activated defense- and stress-related gene promoters. A and B, In the promoters of the HVA1, ICE1, PR10, CHI2, ACO2, ADH, POX2, DFS, and P5CR genes, the fragments containing the GCC box and/or DRE (marked) were synthesized and used as probes for EMSA. The core sequences of cis-elements are underlined. C, EMSA indicated that TaPIE1 could specifically bind to the GCC boxes in the defense- and stress-related genes. The free probe and the binding band of recombinant GST-TaPIE1 to the GCC box are indicated. D, Scheme of the reporter and effector vectors used in the transient expression assay in tobacco. PR10/P5CR/POX2/ICE1-GCC, HVA1-DRE, and HVA1-ABRE indicate the GCC box, DRE, and ABRE sequences of these gene promoters, respectively, and were inserted upstream from the minimal promoter of 35S in the pBI121 vector. Mini indicates the reporter vector containing the minimal promoter upstream of the GUS reporter gene. TaPIE1 indicates the effector vector expressing of full-length TaPIE1. E, TaPIE1 activates the expression of the GUS gene controlled by the GCC box. A. tumefaciens cells containing the reporter and effector vectors were simultaneously infiltrated into the leaves of 4-week-old tobacco plants, which were treated by R. cerealis or 4°C treatment for 12 h. Relative GUS activity is the ratio of activation of the GUS reporter gene driven by TaPIE1 relative to that in the empty vector. Significant differences between normal conditions (mock) and freezing or R. cerealis treatment were derived from the results of three independent replications (Student’s t test: *P < 0.05, **P < 0.01). Error bars indicate se.
The partial fragments containing GCC boxes in PR10, P5CR, ICE1, and POX2, or the mutant GCC (mGCC) box in PR10, or the DRE motif in HVA1, or the ABRE in HVA1 (Fig. 5, A and B) were separately used as probes for EMSA to investigate whether the TaPIE1 protein binds to these cis-elements in these specific defense- and stress-related genes. As shown in Figure 5C, the recombinant GLUTATHIONE S-TRANSFERASE (GST)-TaPIE1 protein could bind to GCC boxes in PR10, P5CR, ICE1, and POX2 but not to DRE or ABRE in HVA1 and the mGCC box, whereas GST protein failed to bind to the GCC box or DRE cis-elements. These results indicated that TaPIE1 could interact with the GCC boxes in these defense- and stress-related genes. Furthermore, the GCC box-, mGCC box-, DRE-, or ABRE-containing fragments of the PR10, P5CR, ICE1, POX2, and HVA1 promoters were separately subcloned into the upstream part of the minimal promoter (TATA box), and the GUS reporter gene in the pBI121 vector (Fig. 5D) then was used to perform transient expression assays on TaPIE1 transactivation activity following by binding to the GCC box in tobacco with or without R. cerealis and freezing treatments. The results showed that the TaPIE1 protein strongly activated the expression of the GCC box-driving GUS genes, whereas the GUS activity driven by the mGCC box, DRE, or ABRE was markedly lower relative to that driven by the GCC box and exhibited no obvious differences from that driven by the minimal promoter (Fig. 5E). These results indicated that the GCC box motif is the most important for the activation of TaPIE1 in the expression of PR10, P5CR, ICE1, POX2, and HVA1 genes. Interestingly, the transactivation activity of TaPIE1 following by the GCC box interaction was markedly elevated in tobacco challenged with R. cerealis and freezing, further suggesting that R. cerealis and freezing challenges might enhance the TaPIE1-activated transcription of these specific defense- and stress-related genes with the GCC boxes.
Ethylene Biosynthesis Positively Modulates TaPIE1-Mediated Responses
Ethylene plays an important role in biotic and abiotic stress responses, in which certain ERF TFs play important roles (Zhang et al., 2009; Zhang and Huang, 2010). TaPIE1 transcript abundance in wheat was up-regulated by exogenous ethylene and 1-aminocyclopropane-1-carboxylic acid (ACC), an ethylene biosynthesis precursor, but was down-regulated by CoCl2, an ethylene biosynthesis inhibitor (Dong et al., 2010; Supplemental Fig. S5). qRT-PCR analyses showed that the transcript abundance of 10 TaPIE1-activated stress- and defense-related genes (HVA1, DHN3, ADH, ICE1, P5CR, POX2, PR10, DFS, CHIT2, and WAKL22) significantly increased after exogenous ACC treatment but decreased after CoCl2 treatment in the leaves of wild-type cv Yangmai 12 wheat treated for 1 h (Supplemental Fig. S5). These data suggested that TaPIE1 and TaPIE1-activated genes were positively regulated by ethylene biosynthesis, and they may be downstream of the ethylene response. Notably, following exogenous ACC treatment for 1 h, the transcript abundance of these defense- and stress-related genes increased to a greater extent in the TaPIE1-overexpressing plants than in the wild-type plants but were lowest in TaPIE1-underexpressing plants (Fig. 6A), indicating that TaPIE1 acted as an important molecular link to mediate the responses of these defense- and stress-related genes to ethylene. Moreover, the transient expression assays in tobacco showed that exogenous ACC application significantly enhanced the relative GUS activity driven by the GCC box that was identified by TaPIE1 (Fig. 6B). These data indicated that ethylene biosynthesis may positively regulate the activation of TaPIE1 in the expression of the specific stress- and defense-related genes.
Figure 6.
Ethylene biosynthesis and signaling positively regulated the stress- and defense-responsive genes activated by TaPIE1. A, qRT-PCR analysis of 10 stress- and defense-related genes in wild-type (WT), TaPIE1-overexpressing (OE) and TaPIE1-underexpressing (UE), and control wheat cv Yangmai 12 plants treated by 50 μm ACC for 1 h. The expression levels of those genes in the wild-type plants treated with water were set to 1. Significant differences between the OE and wild-type plants or the UE and control plants under the same treatments were derived from the results of three independent replications (Student’s t test: *P < 0.05, **P < 0.01). B, Relative GUS activity in 4-week-old tobacco leaves transformed with a TaPIE1 effector and GUS reporter followed by applying water (as a control) or 50 μm ACC for 12 h. Relative GUS activity was determined as described in the legend for Figure 5E. Significant differences between ACC and water treatment were analyzed based on three independent replications (Student’s t test: *P < 0.05, **P < 0.01). Error bars indicate se.
To address the relationship between ethylene and R. cerealis resistance or freezing tolerance caused by TaPIE1 overexpression, wild-type, TaPIE1-overexpressing and -underexpressing, and control seedlings were pretreated with ACC or CoCl2 for 24 h. Following –5°C treatment for 12 h, electrolyte leakage from TaPIE1 overexpressors was significantly increased by CoCl2 pretreatment but decreased by ACC pretreatment (Fig. 7A), suggesting that the inhibition of ethylene biosynthesis decreased TaPIE1-mediated freezing tolerance and that ethylene biosynthesis promoted the tolerance. The ACC and CoCl2 pretreatments did not significantly alter electrolyte leakage in TaPIE1 underexpressors, whereas electrolyte leakage in control plants was significantly reduced by ACC pretreatment and increased after CoCl2 pretreatment (Fig. 7B), indicating that TaPIE1 underexpression decreased the ethylene effect on TaPIE1-mediated freezing tolerance and that TaPIE1 was downstream of ethylene biosynthesis. Furthermore, 7 d after R. cerealis inoculation, CoCl2 pretreatment significantly increased but ACC pretreatment decreased the relative biomass of R. cerealis (a negative indicator of disease resistance) in TaPIE1-overexpressing seedlings (Fig. 7C), and the effects of ACC and CoCl2 pretreatments on the disease resistance of TaPIE1-underexpressing seedlings were weaker than those on the control plants (Fig. 7D). These data indicated that inhibition of ethylene biosynthesis compromised TaPIE1-mediated resistance, whereas ethylene biosynthesis promoted resistance; moreover, the results also indicate that TaPIE1 was downstream from ethylene biosynthesis. Interestingly, the increased transcript abundance of TaPIE1 by freezing stimulus (–5°C for 12 h) was significantly suppressed by CoCl2 pretreatment but was enhanced after ACC pretreatment (Fig. 7E), indicating that the response of TaPIE1 to freezing stress was positively regulated by ethylene biosynthesis.
Figure 7.
Enhanced tolerance to freezing and R. cerealis in TaPIE1-overexpressing wheat is primarily modulated by ethylene biosynthesis and ethylene signaling. A and B, Electrolyte leakage in leaves of wild-type (WT), TaPIE1-overexpressing (OE22 and OE28) and TaPIE1-underexpressing (UE), and control plants was measured after pretreatments with water, CoCl2 (0.1 mm), and ACC (50 μm) for 24 h followed by exposure to –5°C for 12 h. C and D, R. cerealis relative biomasses of TaPIE1-overexpressing (OE22 and OE28), TaPIE1-underexpressing (UE), and control wheat plants were assayed after pretreatments with water, CoCl2 (0.1 mm), and ACC (50 μm) for 24 h and then R. cerealis inoculation for 7 d. E, Transcript induction of TaPIE1 by freezing is regulated by ACC (50 μm) or CoCl2 (0.1 mm) treatment. For C to E, the total RNA was extracted from the leaf sheaths (C and D) or leaves (E) of plants at the four-leaf stage pretreated with water, CoCl2, and ACC for 24 h, followed by R. cerealis treatment for 7 d (C and D) or –5°C freezing treatment for the indicated times (E). The amplification of the wheat ACTIN gene was used as an internal control to normalize all the data. Significant differences between CoCl2 or ACC treatment and water treatment were analyzed based on three replications (Student’s t test: *P < 0.05, **P < 0.01). Error bars indicate se.
DISCUSSION
In model plants, the functional roles and underlying mechanisms of many ERFs in stress responses have been studied, but little is known about the functional roles of ERFs and their mechanisms in wheat. R. cerealis infection and freezing stress are important limitations in wheat production. In this study, we discovered novel roles for the wheat ERF TaPIE1 in the positive regulation of defense responses to freezing (abiotic) and necrotrophic fungal pathogen R. cerealis (biotic) stresses, and we unraveled the molecular and physiological mechanisms underlying the functions of TaPIE1 in wheat.
TaPIE1 Mediates Resistance to Both R. cerealis and Freezing Stresses in Wheat
The expression of TaPIE1 was significantly induced by freezing and pathogen challenges. To understand the putative molecular basis of TaPIE1 in these responses, we analyzed cis-elements that were within 2,161 bp upstream from the start codon of TaPIE1. The cis-elements in the TaPIE1 promoter include four GT-1 motifs (response to pathogen; Park et al., 2004), two low temperature-responsive element boxes, and two ethylene-responsive elements (Supplemental Fig. S6). These motifs may partially be attributed to TaPIE1 responses to pathogens, freezing, and ethylene stimuli. The stress resistance phenotype assays of TaPIE1-overexpressing and -underexpressing wheat plants revealed that TaPIE1 acted as a positive regulator for the synergistic cross talk between R. cerealis and freezing stress signals in wheat. TaPIE1 overexpression significantly enhanced resistance to both R. cerealis and freezing stresses in transgenic wheat, while the resistance to these stresses was compromised by TaPIE1 underexpression, revealing that TaPIE1 is required for wheat defense responses to both R. cerealis and freezing stresses. To our knowledge, our report is the first to uncover the positive regulation of an ERF TF in cross talk between freezing (abiotic) and necrotrophic fungal pathogen R. cerealis (biotic) stresses in wheat. In Arabidopsis, NTL6 (an NAC TF) participates in cold stress and bacterial pathogen P. syringae responses (Seo et al., 2010). The discovery of new molecules in wheat biotic and abiotic stress responses should be pursued to broaden insights into the stress-responsive pathways in various plant species.
Interestingly, TaPIE1 overexpression in the T4 and T5 wheat lines did not obviously affect growth or major agronomic traits (including plant height, tiller number, spike length, spikelet number, grain number per spike, and 1,000-grain weight) in wheat under normal conditions (Supplemental Table S5), suggesting that TaPIE1 has potential as a target for improving R. cerealis resistance and freezing stress tolerance in wheat.
Putative Molecular Mechanism Underlying the Function of TaPIE1
In model plants, ERFs can activate the expression of specific defense- or stress-related genes by binding to different cis-elements (i.e. GCC box or DRE) in response to different stress signals (Solano et al., 1998; Chakravarthy et al., 2003; Zhang et al., 2007, 2012a; Cheng et al., 2013) or by interacting with other TFs (Zhang et al., 2007). Recent investigations revealed a novel mechanism of Arabidopsis ERF1 whereby ERF1 preferentially bound to different cis-elements of downstream genes under different stress treatments (Cheng et al., 2013).
To elucidate the molecular mechanisms underlying the function of TaPIE1, we first used microarray analysis to identify TaPIE1-up-regulated genes by comparing the transcriptomic profiles of TaPIE1-overexpressing and wild-type wheat plants. Microarray analysis showed that TaPIE1 overexpression resulted in a comprehensive transcriptomic modification in transgenic wheat. The TaPIE1-activated defense- and stress-related genes included regulatory genes (i.e. ICE1, CPK3, and CPK6) and effector genes (i.e. HVA1 and DHN3) for freezing responses (Winfield et al., 2010) as well as PR10, DFS, and CHITINASE for plant defense (Gao et al., 2000; Vasavirama and Kirti, 2012). Transcriptional analyses using qRT-PCR in TaPIE1-underexpressing and -overexpressing wheat plants confirmed that TaPIE1 up-regulated the transcription of these defense- and stress-responsive genes, including HVA1, RAB18, DHN3, ADH, ICE1, CPK3, CPK6, ACO2, POX2, JR1, P5CR, PR10, DFS, CHI2, and WAKL22. Furthermore, these defense- and stress-related genes were induced by R. cerealis (excluding ICE1 and P5CR) and freezing (excluding DFS) stimuli, indicating that some genes in the R. cerealis and freezing stress responses overlapped, as indicated by other reports about other biotic and freezing/cold stress responses (Seo et al., 2010; Zhang et al., 2012b). Our results also showed that ICE1 and P5CR primarily responded to freezing and DFS responded primarily to R. cerealis challenge in wheat. The transcriptional up-regulation of these genes may contribute incrementally to TaPIE1-mediated stress resistance. TaPIE1 also down-regulated some genes (Supplemental Table S2), some of which encoded additional molecular factors underlying the function of TaPIE1.
EMSAs and transient expression assays in tobacco demonstrated that TaPIE1, an ERF transcription activator, did interact with GCC boxes in these defense- and stress-responsive genes in vitro and in planta, thereby contributing to the increased transcription of these genes. The GCC box motif is critical for the transactivation of TaPIE1. Direct binding of TaPIE1 to the GCC box may be one of the molecular mechanisms to explain the regulation of the defense- and stress-responsive genes by TaPIE1 at the transcriptional level. Future chromatin immunoprecipitation analysis might provide additional support for this hypothesis. Furthermore, in tobacco, TaPIE1 would only bind to the GCC boxes rather than to DRE or ABRE, and the binding activity of TaPIE1 was elevated in response to either R. cerealis or freezing stress. However, in Arabidopsis, ERF1 bound to GCC boxes in defense-related genes in response to biotic stresses; conversely, under abiotic stresses, ERF1 bound to DRE in stress-related genes rather than GCC boxes (Cheng et al., 2013). These results suggest that the activation mechanism of wheat TaPIE1 may be distinct from that of Arabidopsis ERF1. The different activation mechanisms of TaPIE1 and ERF1 may be partially attributed to their low degree of shared sequence identity (34.64%). Another explanation is that they may be controlled by different regulators in different species. Certain ERFs revealed different possible roles and mechanisms in response to biotic and abiotic stresses in monocots and dicots (Zhang et al., 2007, 2012a; Quan et al., 2010). It will be very interesting to further identify with which cis-elements TaPIE1 protein will preferentially interact during wheat responses to freezing treatment and R. cerealis infection.
TFs have been implicated in the regulation of cold/freezing responses (Yi et al., 2004; Yamaguchi-Shinozaki and Shinozaki, 2006; Seo et al., 2010; Zhang and Huang, 2010). In Arabidopsis, the C-repeat-binding factor CBF3 is transcriptionally regulated by the TF ICE1, which in turn induces the expression of COLD-RESPONSIVE (COR) genes, consequently resulting in a cold tolerance response. The ICE1-CBF-COR cascade is one of the primary signaling pathways (Seo et al., 2010; Yang et al., 2010). Our data demonstrate that TaPIE1 can interact with the GCC box in a promoter of the wheat ICE1 homolog and up-regulate its expression, suggesting that TaPIE1 may function upstream of the ICE1 homolog. The TaPIE1-ICE1 functional module in wheat may differ from the ICE1-CBF-COR module in Arabidopsis.
Possible Physiological Mechanisms of TaPIE1-Mediated Stress Resistance
This study demonstrated possible physiological mechanisms to explain the stress resistance phenotypes caused by TaPIE1 overexpression. First, under freezing or R. cerealis stress conditions, TaPIE1 overexpression reduced and TaPIE1 underexpression increased levels of stress-induced ROS. These data suggested that TaPIE1 may play a crucial role in ROS homeostasis under both stresses, which may be important for wheat responses to R. cerealis and freezing stresses. TaPIE1 was induced by H2O2 stimulus (Supplemental Fig. S7A), and the induction peak of TaPIE1 was earlier in response to ACC (at 30 min) than to H2O2 (at 1 h; Supplemental Fig. S7B), suggesting that the action of H2O2 might be located downstream of the ethylene regulation. TaPIE1 overexpressors displayed enhanced expression of POX2 and 14 additional ROS-scavenging genes, which contributed to ROS detoxification (Supplemental Table S3). TaPIE1 was shown to interact with the GCC box in a POX2 promoter and thereby to activate POX2 transcription. These results support the view that TaPIE1 regulates the expression of ROS-scavenging genes and ROS accumulation, in turn contributing to TaPIE1-mediated resistance. Emerging evidence also suggested that the regulation of ERF in ROS-dependent signaling pathways plays key roles in the cross talk between biotic and abiotic stress signaling (Wu et al., 2008; Schmidt et al., 2013). Second, following freezing stimulus, both Pro and soluble sugar contents were significantly elevated in TaPIE1-overexpressing wheat but decreased in TaPIE1-underexpressing wheat. The accumulation of Pro in plant cells contributes to drought, salt, and temperature tolerance via multiple mechanisms (Sharma and Verslues, 2010; Cheng et al., 2013). Interestingly, P5CR (an important gene involved in Pro synthesis; Verbruggen and Hermans, 2008) was markedly and consistently activated by TaPIE1. Moreover, CPK3 and CPK6 that promote Pro accumulation (Mori et al., 2006) and five additional Pro synthetase-like genes (Ta.24372.2.S1_a_at, Ta.21467.1.S1_at, TaAffx.109581.3.S1_s_at, Ta.21467.3.S1_a_at, and TaAffx.116865.2.S1_at) were more highly expressed in our microarray analysis of TaPIE1-overexpressing wheat. These data suggest that Pro accumulation is likely to be one contributor to the enhanced stress resistance caused by TaPIE1 overexpression. Third, following freezing and R. cerealis stimuli, TaPIE1 overexpression may reduce plasma membrane damage, contributing to enhanced resistance to both R. cerealis and freezing stresses.
Ethylene Biosynthesis Modulates TaPIE1-Mediated Responses
ERF TFs play crucial roles in ethylene signaling pathways and stress responses. For example, the tomato (Solanum lycopersicum) ERF protein TERF2/LeERF2 was an important expression regulator of ethylene biosynthesis genes (including ACO and 1-aminocyclopropane-1-carboxylic acid synthesis) and for the production of ethylene (Zhang et al., 2009). TERF2/LeERF2-enhanced freezing tolerance was modulated through ethylene biosynthesis and the ethylene signaling pathway (Zhang and Huang, 2010). Overexpression of Arabidopsis ERF1 activates the transcription of downstream effector genes, such as defense- and stress-related genes, to promote the ethylene response and tolerance to certain soil-borne pathogen, drought, and salt stresses (Solano et al., 1998; Berrocal-Lobo et al., 2002; Cheng et al., 2013).
Our study showed that the transcript abundance of TaPIE1 was induced by ethylene and freezing treatments. Importantly, the increased transcript abundance of TaPIE1 by freezing stimulus was significantly suppressed after CoCl2 pretreatment, whereas it was enhanced after ACC pretreatment. These results indicated that the response of TaPIE1 to freezing stress was associated with ethylene biosynthesis. Recent studies showed that exogenous hormone treatments can alter the transactivation activity of ERF (Zhou et al., 2008). Our data showed that exogenous ethylene application increased the transactivation activity of TaPIE1 and the transcript levels of TaPIE1-activated defense- and stress-responsive genes with GCC boxes, whereas an ethylene biosynthesis inhibitor repressed their transcript levels. Notably, inhibiting ethylene biosynthesis reduced freezing tolerance and R. cerealis resistance caused by TaPIE1 overexpression. Moreover, ACC application cannot recover the reduced tolerance of TaPIE1-underexpressing plants to freezing and R. cerealis stresses. These data suggested that ethylene biosynthesis positively modulated TaPIE1-mediated responses to freezing and R. cerealis stresses and that TaPIE1 was downstream from ethylene biosynthesis.
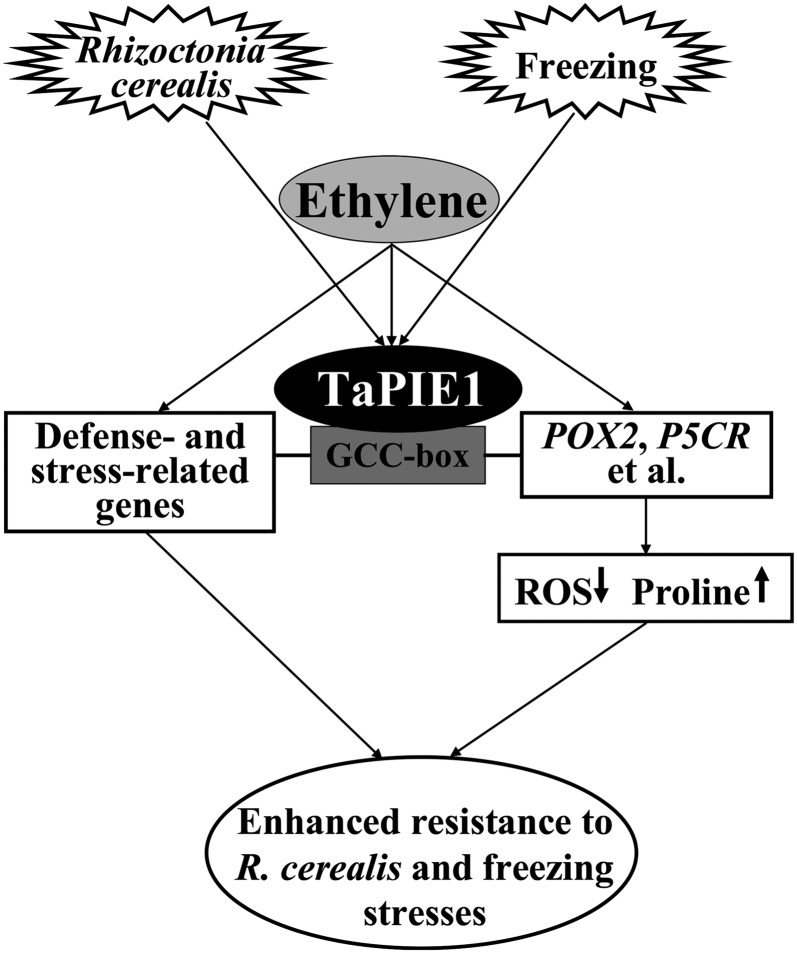
Based on our data, we propose a model to explain the potential role of TaPIE1 in wheat responses to R. cerealis and freezing stresses (Fig. 8). TaPIE1 acts as a positive regulator to mediate wheat responses of ethylene, R. cerealis, and freezing stimuli. TaPIE1 overexpression can activate the expression of POX2, P5CR, and additional defense- and stress-related genes downstream of ethylene biosynthesis, which regulates physiological changes, finally leading to enhanced resistance to both R. cerealis and freezing stresses. Ethylene positively regulated TaPIE1-mediated responses (Fig. 8). This study provides new insights into the ERF regulation mechanism of biotic and abiotic stress responses in wheat and presents TaPIE1 as an ideal candidate to target in the effort to improve freezing and R. cerealis resistance in wheat and perhaps in other crop plants.
Figure 8.
Proposed model of TaPIE1 function in R. cerealis and freezing stress responses in wheat.
MATERIALS AND METHODS
Plant Material, Treatments, and Primers
The seeds of a spring wheat (Triticum aestivum ‘Yangmai 12’) were provided by Shunhe Cheng (Lixiahe Agricultural Institute of Jiangsu). The cv Yangmai 12 is moderately susceptible to Rhizoctonia cerealis and freezing stresses and was used as the host of transforming and silencing TaPIE1 in the study.
All wheat plants were grown in a glasshouse at 22°C/14-h-light (intensity of 300 μmol m−2 s−1) and 12°C/10-h-dark conditions. To investigate the effects of ACC, CoCl2, and H2O2 on the transcription of TaPIE1 and defense- and stress-related genes, seedlings at the four-leaf stage of wild-type, TaPIE1-overexpressing and -underexpressing, and BSMV:00-infected (control) cv Yangmai 12 plants were sprayed with 50 μm ACC, 10 mm H2O2, 0.1 mm CoCl2, or water (as a control) for the indicated times following Zhang et al. (2012b). For the effects of ACC and CoCl2 on TaPIE1-mediated tolerance to R. cerealis and freezing stress, the plants were first pretreated for 24 h with 50 μm ACC, 0.1 mm CoCl2, or water (as a control) and then exposed to –5°C or infected with R. cerealis for the indicated times.
All primers used for vector construction, PCR, and qRT-PCR assays for all target genes are listed in Supplemental Table S6.
DNA and RNA Extraction and Complementary DNA Synthesis
The genomic DNA of each sample was isolated from wheat leaves by using the cetyl-trimethyl-ammonium bromide method (Saghai-Maroof et al., 1984). Total RNA was extracted from the leaves or stems of wheat plants with TRIzol and subjected to RNase-free DNase I digestion and purification.
Five micrograms of purified RNA from each sample was used as the template for synthesizing the first-strand complementary DNA by using the SuperScript II First-Strand Synthesis Kit (Invitrogen).
Virus-Induced Gene Silencing Construct and Generation of TaPIE1-Underexpressing Wheat Plants
To prepare the recombinant BSMV:TaPIE1 construct, one fragment of TaPIE1 (nucleotides 107–375 of the TaPIE1 coding sequence) was subcloned in the reverse orientation into the NheI restriction site in an RNAγ of BMSV:00 (Supplemental Fig. S2). Following the protocol of Zhou et al. (2007), BSMV:TaPIE1 was inoculated onto the second leaf of cv Yangmai 12 wheat plants at the two-leaf stage. Seedlings of the same age were inoculated with the BSMV:00 virus as a control. The inoculated plants were grown at conditions of 25°C/14 h of light (300 μmol m−2 s−1) and 16°C/10 h of dark, 90% relative humidity for 2 d, followed by 22°C/14 h of light (300 μmol m−2 s−1) and 12°C/10 h of dark.
PCR and Southern-Blot Analyses of TaPIE1-Overexpressing Wheat Plants
The introduced TaPIE1 in TaPIE1-overexpressing transgenic wheat plants was monitored for the presence of a PCR fragment (296 bp) using transgene-specific primers, with a program of initial denaturation at 94°C for 5 min, followed by 35 cycles of amplification (45 s at 94°C, 45 s at 56.8°C, and 25 s at 72°C), and 72°C for 10 min.
Southern blotting was conducted according to the modified protocols of Sharp et al. (1989) and Zhang et al. (2012b), in which 20 μg of genomic DNA digested by the restriction enzyme SpeI was hybridized to the probe derived from the TaPIE1 transgene-specific fragment (296 bp).
RT-PCR and qRT-PCR
RT-PCR and qRT-PCR were used to analyze the expression of target genes. The RT-PCR consisted of 28 to 35 cycles of amplification (45 s at 94°C, 45 s at 56°C–60°C, and 45 s at 72°C) with primers specific to target genes. qRT-PCR was performed by using SYBR Green I Mix (Takara) with an ABI 7300 RT-PCR system (Applied Biosystems) according to the protocols of Dong et al. (2010). The relative expression levels of each gene were calculated by using the 2–ΔΔCT method (Livak and Schmittgen, 2001). Three biological replicates were performed for each qRT-PCR.
Assessments of R. cerealis Responses in Wheat Plants
At the tillering stage, the base stems of at least 90 plants for each TaPIE1-overexpressing line in T4 and T5 generations and wild-type cv Yangmai 12 were inoculated with R. cerealis according to the method described by Chen et al. (2008). At least 60 BSMV:TaPIE1-infected (TaPIE1 underexpressing) or BSMV:00-infected cv Yangmai 12 plants at 20 d post virus infection were inoculated with R. cerealis. All assays were repeated three times. The disease symptoms of wheat plants were rated at 55 dpi. Infection types were categorized using a 0 to 5 scale, and the disease indices were calculated for each line according to Chen et al. (2008).
To further investigate the responses of TaPIE1 overexpressors and underexpressors to R. cerealis ingress, qRT-PCR was used to assess the relative biomass of R. cerealis in the base stems of the wheat plants, and the assessment was based on the transcript level of R. cerealis ACTIN in reference to wheat ACTIN.
Freezing Tolerance Assays
TaPIE1-overexpressing and wild-type cv Yangmai 12 seedlings at the four-leaf stage (4 weeks old) were subjected to cold acclimation at 4°C for 6 h and then exposed to –9°C. The survival rates of the plants were observed at –9°C for 0, 12, and 18 h following a recovery period at 22°C for 7 d. TaPIE1-underexpressing and BSMV:00-infected plants at 15 dpi with the virus were transferred to 4°C for 6 h and then exposed to –5°C. Survival rates were observed at –5°C for 0, 12, and 18 h. At least 30 plants per line in each evaluation were tested with three experimental replications.
Measurements of Electrolyte Leakage and Pro and Soluble Sugar Contents
Electrolyte leakage was measured on the basis of the relative conductivity of the leaves according to Zhang and Huang (2010). The Pro content in the leaves was measured with 0.5 g of fresh leaves extracted in 5 mL of 3% aqueous sulfosalicylic acid (Cheng et al., 2013). The total soluble sugars were extracted from 0.5 g of fresh leaves and measured by using an anthrone colorimetric assay at 620 nm with a spectrophotometer, using Glc as a standard (Dubois et al., 1956). All the measurements were repeated three times, and Student’s t test was used for inferential statistical analysis.
ROS Assay
The in situ formations of H2O2 and O2− in leaves were detected by 3,3-diaminobenzidine and nitroblue tetrazolium staining, respectively, following Lee et al. (2002). About 0.5 g of fresh leaves for each sample was harvested and used to measure the H2O2 concentration according to Warm and Laties (1982).
Microarray Assay
Total RNA for each sample was extracted from the leaves of 10 seedlings from two TaPIE1-overexpressing lines (OE22 and OE28) or wild-type cv Yangmai 12, both of which were exposed to –5°C for 12 h at the four-leaf stage and then challenged with R. cerealis for 21 d. After purification, the pooled RNA of the TaPIE1 overexpressors and the RNA of wild-type cv Yangmai 12 were used for the preparation of Cy5- and Cy3-labeled complementary RNA, and these were subjected to microarray experiments according to the manufacturer’s protocols (Affymetrix). Again using the manufacturer’s protocols, the labeled probes, Cy5-(OE22 + OE28) and Cy3-Yangmai 12, were hybridized with an Affymetrix Wheat GeneChip Array, which contained 61,127 probe sets. The hybridized arrays were conducted with three replications. Data analysis was conducted by using Expressionist software (version Pro 3.1; Genedata) and the MAS 5.0 algorithm (Affymetrix; Caldo et al., 2004; Boddu et al., 2006). Up-regulated or down-regulated transcript probe sets in TaPIE1 overexpressors compared with wild-type cv Yangmai 12 were filtered out by a fold change threshold (OE22 + OE28 signal value versus cv Yangmai 12 signal value) of 3.0 or greater or 0.33 or less, respectively, using a stringent cutoff at false discovery rate ≤ 0.05 corresponding to P ≤ 0.05.
Putative functions were assigned to the differentially expressed genes using HarvEST (Affymetrix Wheat 1 Chip version 1.58; e ≤ 10−10) and based on the best BLASTX search in a nonredundant protein sequence database at the National Center for Biotechnology Information. Gene Ontology analysis was performed using the SBS server of Ebioservice (http://www.ebioservice.com), which was connected to the Gene Ontology Web site (http://www.geneontology.org).
Promoter Analysis of TaPIE1 and TaPIE1-Activated Genes
Promoter sequences upstream from the ATG of TaPIE1 and TaPIE1-activated genes were obtained from Wheat Genomics (http://www.cerealsdb.uk.net/) and the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/guide/), and cis-elements in the promoters were searched in the PLACE database (http://www.dna.affrc.go.jp/PLACE/).
Analysis of TaPIE1 Binding to the GCC Box and DRE in TaPIE1-Activated Genes
Partial sequences (approximately 50 bp) containing the GCC box, DRE, or ABRE in promoters of the specific wheat defense- and stress-related genes were synthesized (Fig. 5, A and B) and used as probes in EMSA. EMSA was used to determine whether TaPIE1 can bind to these probes in vitro according to a modified protocol by Dong et al. (2010). Each reaction mixture including 1 μg of each probe and 1 μg of GST-TaPIE1/GST was incubated on ice for 8 h. The GST-TaPIE1 binding and free probes were resolved on an 8% polyacrylamide gel (90 V for 60 min) and then visualized after ethidium bromide staining.
To perform transient expression assays, the GCC box-, mGCC box-, DRE-, or ABRE-containing fragments of the PR10, P5CR, ICE1, POX2, and HVA1 promoters were separately inserted upstream from the TATA box (−46 to +10; minimal promoter) to replace the 35S promoter in the pBI121 vector, resulting in the reporter vectors. In the reporter vectors, the GUS gene was driven by a minimal promoter and the GCC box, mGCC box, DRE, or ABRE cis-elements. In the effector construct, the GUS gene was replaced by the full coding region of TaPIE1 and controlled by the 35S promoter. The effector and reporter vectors (1:1 ratio) were cointroduced into Agrobacterium tumefaciens (LBA4404) and used for a transient expression assay in the leaves of 30-d-old tobacco (Nicotiana tabacum) following Zhang et al. (2009). For exploring the effects of freezing, R. cerealis, and ACC treatments on the transactivation activity of TaPIE1, the transient expression tobacco plants were treated for 12 h with 4°C, or R. cerealis inoculum, or 50 μm ACC and then were collected for the relative GUS activity assay.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Transcription of TaPIE1 was markedly induced in overexpressing transgenic wheat after freezing and R. cerealis stimuli.
Supplemental Figure S2. Scheme of genomic RNAs of BSMV:00 and the recombinant BSMV:TaPIE1 virus.
Supplemental Figure S3. Analysis of H2O2 and O2− accumulation in wheat.
Supplemental Figure S4. Venn diagram and validation of selected microarray data.
Supplemental Figure S5. Ethylene biosynthesis positively regulates the 10 stress-related genes in the leaves of wild-type wheat.
Supplemental Figure S6. The cis-elements in the TaPIE1 promoter.
Supplemental Figure S7. TaPIE1 transcription is induced by ACC and H2O2 treatments.
Supplemental Table S1. Sharp eyespot disease indices in TaPIE1-overexpressing, -underexpressing, and control wheat plants after R. cerealis inoculation.
Supplemental Table S2. Differential expression genes in the TaPIE1-overexpressing lines compared with wild-type wheat cv Yangmai 12.
Supplemental Table S3. Stress- and defense-related genes up-regulated by TaPIE1
Supplemental Table S4. The cis-elements in the promoters of eight TaPIE1-activated genes.
Supplemental Table S5. Major agronomic traits in TaPIE1-overexpressing and wild-type wheat plants.
Supplemental Table S6. Sequences of primers used in this study.
Acknowledgments
We are grateful to Sarah Hancock (Kansas Agricultural Experiment Station, Kansas State University) and Dr. Zhijin Zhang (Biotechnology Research Institute, Chinese Academy of Agricultural Sciences) for their critical readings of the manuscript and to Lipu Du and Dr. Na Dong (Institute of Crop Science, Chinese Academy of Agricultural Sciences) and Qiaofeng Zhang (Jiangsu Academy of Agricultural Sciences) for their technical assistance.
Glossary
- TF
transcription factor
- H2O2
hydrogen peroxide
- qRT
quantitative reverse transcription
- EMSA
electrophoretic mobility shift assay
- RT
reverse transcription
- BSMV
Barley stripe mosaic virus
- dpi
days post inoculation
- ROS
reactive oxygen species
- O2−
superoxide anion
- DRE
dehydration-responsive element
- ABRE
abscisic acid-responsive element
- mGCC
mutant GCC
- ACC
1-aminocyclopropane-1-carboxylic acid
Footnotes
This work was supported by the 863 program of China (grant no. 2012AA10A309 to Z.Z.) and the National Key Sci-Tech program of China (grant no. 2013ZX08002–001 to Z.Z.).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Berrocal-Lobo M, Molina A, Solano R. (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 [DOI] [PubMed] [Google Scholar]
- Boddu J, Cho S, Kruger WM, Muehlbauer GJ. (2006) Transcriptome analysis of the barley-Fusarium graminearum interaction. Mol Plant Microbe Interact 19: 407–417 [DOI] [PubMed] [Google Scholar]
- Caldo RA, Nettleton D, Wise RP. (2004) Interaction-dependent gene expression in Mla-specified response to barley powdery mildew. Plant Cell 16: 2514–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón O, González M, López Y, Portieles R, Pujol M, González E, Schoonbeek HJ, Métraux JP, Borrás-Hidalgo O. (2010) Over-expression of a protein kinase gene enhances the defense of tobacco against Rhizoctonia solani. Gene 452: 54–62 [DOI] [PubMed] [Google Scholar]
- Chakravarthy S, Tuori RP, D’Ascenzo MD, Fobert PR, Despres C, Martin GB. (2003) The tomato transcription factor Pti4 regulates defense-related gene expression via GCC box and non-GCC box cis elements. Plant Cell 15: 3033–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang Z, Liang H, Liu H, Du L, Xu H, Xin Z. (2008) Overexpression of TiERF1 enhances resistance to sharp eyespot in transgenic wheat. J Exp Bot 59: 4195–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wei L, Zhang XX, Zhang BQ, Yu HS, Chen HG. (2009) Composition and virulence of pathogen of wheat sharp eyespot in north latitude 33° of China. J Triticeae Crops 29: 1110–1114 (in Chinese with English abstract) [Google Scholar]
- Cheng MC, Liao PM, Kuo WW, Lin TP. (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162: 1566–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Liu X, Lu Y, Du LP, Xu HJ, Liu HX, Xin ZY, Zhang ZY. (2010) Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct Integr Genomics 10: 215–226 [DOI] [PubMed] [Google Scholar]
- Dong W, Ai X, Xu F, Quan T, Liu S, Xia G. (2012) Isolation and characterization of a bread wheat salinity responsive ERF transcription factor. Gene 511: 38–45 [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Frederiks TM, Christopher JT, Harvey GL, Sutherland MW, Borrell AK. (2012) Current and emerging screening methods to identify post-head-emergence frost adaptation in wheat and barley. J Exp Bot 63: 5405–5416 [DOI] [PubMed] [Google Scholar]
- Gao AG, Hakimi SM, Mittanck CA, Wu Y, Woerner BM, Stark DM, Shah DM, Liang JH, Rommens CM. (2000) Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat Biotechnol 18: 1307–1310 [DOI] [PubMed] [Google Scholar]
- Gill BS, Appels R, Botha-Oberholster AM, Buell CR, Bennetzen JL, Chalhoub B, Chumley F, Dvorák J, Iwanaga M, Keller B, et al. (2004) A workshop report on wheat genome sequencing: International Genome Research on Wheat Consortium. Genetics 168: 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Aguilar GA, Fortiz J, Cruz R, Baez R, Wang CY. (2000) Methyl jasmonate reduces chilling injury and maintains postharvest quality of mango fruit. J Agric Food Chem 48: 515–519 [DOI] [PubMed] [Google Scholar]
- Gutterson N, Reuber TL. (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7: 465–471 [DOI] [PubMed] [Google Scholar]
- Hein I, Barciszewska-Pacak M, Hrubikova K, Williamson S, Dinesen M, Soenderby IE, Sundar S, Jarmolowski A, Shirasu K, Lacomme C. (2005) Virus-induced gene silencing-based functional characterization of genes associated with powdery mildew resistance in barley. Plant Physiol 138: 2155–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32: 227–254 [DOI] [PubMed] [Google Scholar]
- Lee BH, Lee H, Xiong L, Zhu JK. (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- McGrath KC, Dombrecht B, Manners JM, Schenk PM, Edgar CI, Maclean DJ, Scheible WR, Udvardi MK, Kazan K. (2005) Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol 139: 949–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R. (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15: 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK. (2009) Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol 150: 1368–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate-Sánchez L, Anderson JP, Young J, Singh KB. (2007) AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol 143: 400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, Park CY, Jeong JC, Moon BC, Lee JH, et al. (2004) Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol 135: 2150–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R, Hu S, Zhang Z, Zhang H, Zhang Z, Huang R. (2010) Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J 8: 476–488 [DOI] [PubMed] [Google Scholar]
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81: 8014–8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Mieulet D, Hubberten HM, Obata T, Hoefgen R, Fernie AR, Fisahn J, San Segundo B, Guiderdoni E, Schippers JH, et al. (2013) Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 25: 2115–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield SR, Huang L, Brandt AS, Gill BS. (2005) Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol 138: 2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Kim MJ, Park JY, Kim SY, Jeon J, Lee YH, Kim J, Park CM. (2010) Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant J 61: 661–671 [DOI] [PubMed] [Google Scholar]
- Sharma P, Sharma N, Deswal R. (2005) The molecular biology of the low-temperature response in plants. Bioessays 27: 1048–1059 [DOI] [PubMed] [Google Scholar]
- Sharma S, Verslues PE. (2010) Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ 33: 1838–1851 [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Chao S, Desai S, Gale MD. (1989) The isolation, characterization and application in the Triticeae of a set of wheat RFLP probes identifying each homoeologous chromosome arm. Theor Appl Genet 78: 342–348 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streatfield SJ, Magallanes-Lundback ME, Beifuss KK, Brooks CA, Harkey RL, Love RT, Bray J, Howard JA, Jilka JM, Hood EE. (2004) Analysis of the maize polyubiquitin-1 promoter heat shock elements and generation of promoter variants with modified expression characteristics. Transgenic Res 13: 299–312 [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Kato W, Asada Y, Sako K, Sato T, Sonoda Y, Kidokoro S, Yamaguchi-Shinozaki K, Tamaoki M, Arakawa K, et al. (2009) DEAR1, a transcriptional repressor of DREB protein that mediates plant defense and freezing stress responses in Arabidopsis. J Plant Res 122: 633–643 [DOI] [PubMed] [Google Scholar]
- Vasavirama K, Kirti PB. (2012) Increased resistance to late leaf spot disease in transgenic peanut using a combination of PR genes. Funct Integr Genomics 12: 625–634 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. (2008) Proline accumulation in plants: a review. Amino Acids 35: 753–759 [DOI] [PubMed] [Google Scholar]
- Warm E, Laties GG. (1982) Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminol. Phytochemistry 21: 827–831 [Google Scholar]
- Winfield MO, Lu C, Wilson ID, Coghill JA, Edwards KJ. (2010) Plant responses to cold: transcriptome analysis of wheat. Plant Biotechnol J 8: 749–771 [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang Z, Zhang H, Wang XC, Huang R. (2008) Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol 148: 1953–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li LC, Zhao YX, Lu Y, Ni ZY, Liu L, et al. (2007) Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol 65: 719–732 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57: 781–803 [DOI] [PubMed] [Google Scholar]
- Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. (2010) A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. Plant J 63: 283–296 [DOI] [PubMed] [Google Scholar]
- Yi SY, Kim JH, Joung YH, Lee S, Kim WT, Yu SH, Choi D. (2004) The pepper transcription factor CaPF1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol 136: 2862–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li W, Chen J, Yang Y, Zhang Z, Zhang H, Wang XC, Huang R. (2007) Transcriptional activator TSRF1 reversely regulates pathogen resistance and osmotic stress tolerance in tobacco. Plant Mol Biol 63: 63–71 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang D, Chen J, Yang Y, Huang Z, Huang D, Wang XC, Huang R. (2004) Tomato stress-responsive factor TSRF1 interacts with ethylene responsive element GCC box and regulates pathogen resistance to Ralstonia solanacearum. Plant Mol Biol 55: 825–834 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huang R. (2010) Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol 73: 241–249 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang J, Zhang R, Huang R. (2012a) The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J 71: 273–287 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Quan R, Wang XC, Huang R. (2009) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150: 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Liu X, Wang XD, Zhou MP, Zhou XY, Ye XG, Wei XN. (2012b) An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense- and stress-related genes. New Phytol 196: 1155–1170 [DOI] [PubMed] [Google Scholar]
- Zhou H, Li S, Deng Z, Wang X, Chen T, Zhang J, Chen S, Ling H, Zhang A, Wang D, et al. (2007) Molecular analysis of three new receptor-like kinase genes from hexaploid wheat and evidence for their participation in the wheat hypersensitive response to stripe rust fungus infection. Plant J 52: 420–434 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Yang Y, Zhang Z, Zhang H, Hu X, Chen J, Wang XC, Huang R. (2008) Abscisic acid regulates TSRF1-mediated resistance to Ralstonia solanacearum by modifying the expression of GCC box-containing genes in tobacco. J Exp Bot 59: 645–652 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Chen JM, Yao QH, Xiong F, Sun CC, Zhou XR, Zhang J, Xiong AS. (2011) Discovery and expression profile analysis of AP2/ERF family genes from Triticum aestivum. Mol Biol Rep 38: 745–753 [DOI] [PubMed] [Google Scholar]