Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling.
Abstract
Many plants respond to competition signals generated by neighbors by evoking the shade avoidance syndrome, including increased main stem elongation and reduced branching. Vegetation-induced reduction in the red light:far-red light ratio provides a competition signal sensed by phytochromes. Plants deficient in phytochrome B (phyB) exhibit a constitutive shade avoidance syndrome including reduced branching. Because auxin in the polar auxin transport stream (PATS) inhibits axillary bud outgrowth, its role in regulating the phyB branching phenotype was tested. Removing the main shoot PATS auxin source by decapitation or chemically inhibiting the PATS strongly stimulated branching in Arabidopsis (Arabidopsis thaliana) deficient in phyB, but had a modest effect in the wild type. Whereas indole-3-acetic acid (IAA) levels were elevated in young phyB seedlings, there was less IAA in mature stems compared with the wild type. A split plate assay of bud outgrowth kinetics indicated that low auxin levels inhibited phyB buds more than the wild type. Because the auxin response could be a result of either the auxin signaling status or the bud’s ability to export auxin into the main shoot PATS, both parameters were assessed. Main shoots of phyB had less absolute auxin transport capacity compared with the wild type, but equal or greater capacity when based on the relative amounts of native IAA in the stems. Thus, auxin transport capacity was unlikely to restrict branching. Both shoots of young phyB seedlings and mature stem segments showed elevated expression of auxin-responsive genes and expression was further increased by auxin treatment, suggesting that phyB suppresses auxin signaling to promote branching.
The development of shoot branches is a multistep process with many potential points of regulation. After the formation of an axillary meristem in the leaf axil, an axillary bud may form through the generation of leaves and other tissues. The axillary bud may grow out to form a branch, or may remain dormant or semidormant for an indefinite period of time (Bennett and Leyser, 2006). In Arabidopsis (Arabidopsis thaliana), the position of the bud in the rosette is a strong determinant of its fate, with upper buds displaying greater outgrowth potential than lower buds. In fact, the varying potential of buds at different positions is maintained even in buds that are activated to form branches, with the upper buds growing out first and most robustly, and lower buds growing out after a time lag and with less vigor (Hempel and Feldman, 1994; Finlayson et al., 2010).
The disparate fate of buds at different rosette positions is mediated, at least in part, by the process of correlative inhibition, whereby remote parts of the plant inhibit the outgrowth of the buds (Cline, 1997). Correlative inhibition is typically associated with the bud-inhibiting effects of auxin sourced in the shoot apex and transported basipetally in the polar auxin transport stream (PATS). Auxin in the PATS does not enter the bud and thus must act indirectly; however, the exact mechanism by which auxin inhibits bud outgrowth is not well understood, despite many years of intensive study (Waldie et al., 2010; Domagalska and Leyser, 2011). Evidence supports divergent models by which auxin may regulate branching. One model contends that the PATS modulates a bud outgrowth inhibiting second messenger (Brewer et al., 2009). Another model postulates a mechanism whereby competition between the main shoot and the axillary bud for auxin export in the PATS regulates bud activity (Bennett et al., 2006; Prusinkiewicz et al., 2009; Balla et al., 2011).
In addition to intrinsic developmental programming, branching is also modulated by environmental signals, including competition signals generated by neighboring plants. The red light:far-red light ratio (R:FR) is an established competition signal that is modified (reduced) by neighboring plants and sensed by the phytochrome family of photoreceptors. A low R:FR evokes the shade avoidance syndrome with plants displaying, among other phenotypes, enhanced shoot elongation and reduced branching (Smith, 1995; Ballaré, 1999; Franklin and Whitelam, 2005; Casal, 2012). Phytochrome B (phyB) is the major sensor contributing to R:FR responses, and loss of phyB function results in a plant that displays a phenotype similar to constitutive shade avoidance. It should be noted that actual shade avoidance is mediated by additional phytochromes and that the complete absence of functional phyB in the loss-of-function mutant may also result in a phenotype that does not exactly mirror shade avoidance. Loss of phyB function leads to reduced branching and altered expression of genes associated with hormone pathways and bud development in the axillary buds (Kebrom et al., 2006; Finlayson et al., 2010; Kebrom et al., 2010; Su et al., 2011). In Arabidopsis, phyB deficiency differentially affects the outgrowth of buds from specific positions in the rosette and thus demonstrates an important function in the regulation of correlative inhibition (Finlayson et al., 2010; Su et al., 2011), a process known to be influenced by auxin. Many aspects of auxin signaling are dependent on AUXIN RESISTANT1 (AXR1), which participates in activating the Skip-Cullin-F-box auxin signaling module (del Pozo et al., 2002). Reduced auxin signaling resulting from AXR1 deficiency enabled phyB-deficient plants to branch profusely and reduced correlative inhibition, thus establishing auxin signaling downstream of phyB action (Finlayson et al., 2010). Although a link between auxin signaling and phyB regulation of branching was demonstrated, the details of the interaction were not discovered.
The relationship between auxin and shade avoidance responses has been investigated in some detail. Auxin signaling was implicated in shade avoidance responses mediated by ARABIDOPSIS THALIANA HOMEOBOX PROTEIN2 in young Arabidopsis seedlings (Steindler et al., 1999). Rapid changes in leaf development resulting from canopy shade were also shown to involve TRANSPORT INHIBITOR RESPONSE1-dependent auxin signaling (Carabelli et al., 2007). A link between auxin abundance and the response to the R:FR was demonstrated in Arabidopsis deficient for the TRP AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1) auxin biosynthetic enzyme (Tao et al., 2008). Young wild-type seedlings respond to a decreased R:FR by increasing indole-3-acetic acid (IAA) biosynthesis, accumulating IAA, increasing hypocotyl and petiole elongation, and increasing leaf elevation. However, these responses are reduced in plants deficient in TAA1. Subsequent studies confirmed the importance of auxin in responses to the R:FR (Pierik et al., 2009; Kozuka et al., 2010; Keller et al., 2011), and also identified the auxin transporter PIN-FORMED3 as essential for hypocotyl elongation responses in young seedlings (Keuskamp et al., 2010). In addition to the roles of auxin abundance and transport in the process, auxin sensitivity has also been implicated in shade avoidance. Several auxin signaling genes are direct targets of the phytochrome signaling component PHYTOCHROME INTERACTING FACTOR5 (PIF5), and these genes are misregulated in Arabidopsis deficient in either PHYTOCHROME INTERACTING FACTOR4 (PIF4) or PIF5 (Hornitschek et al., 2012; Sun et al., 2013). Auxin-responsive hypocotyl elongation and auxin-induced gene expression were also reduced in young seedlings of the pif4pif5 double mutant (Hornitschek et al., 2012), which show defects in shade avoidance responses (Lorrain et al., 2008).
Although some aspects of the regulation of branching are now understood, there are still many gaps in our knowledge of the process, especially as related to the regulation of branching by light signals. Because auxin is known to play a major role in regulating branch development, and because recent studies have implicated auxin in general shade avoidance responses and specifically in the regulation of branching by phyB, the hypothesis that auxin homeostasis, transport, and/or signaling may contribute to the hypobranching phenotype of phyB-deficient plants was generated and tested, using a variety of physiological and molecular approaches.
RESULTS
Reduced Branching in phyB Is Alleviated by Decapitation and Treatment with a Polar Auxin Transport Inhibitor
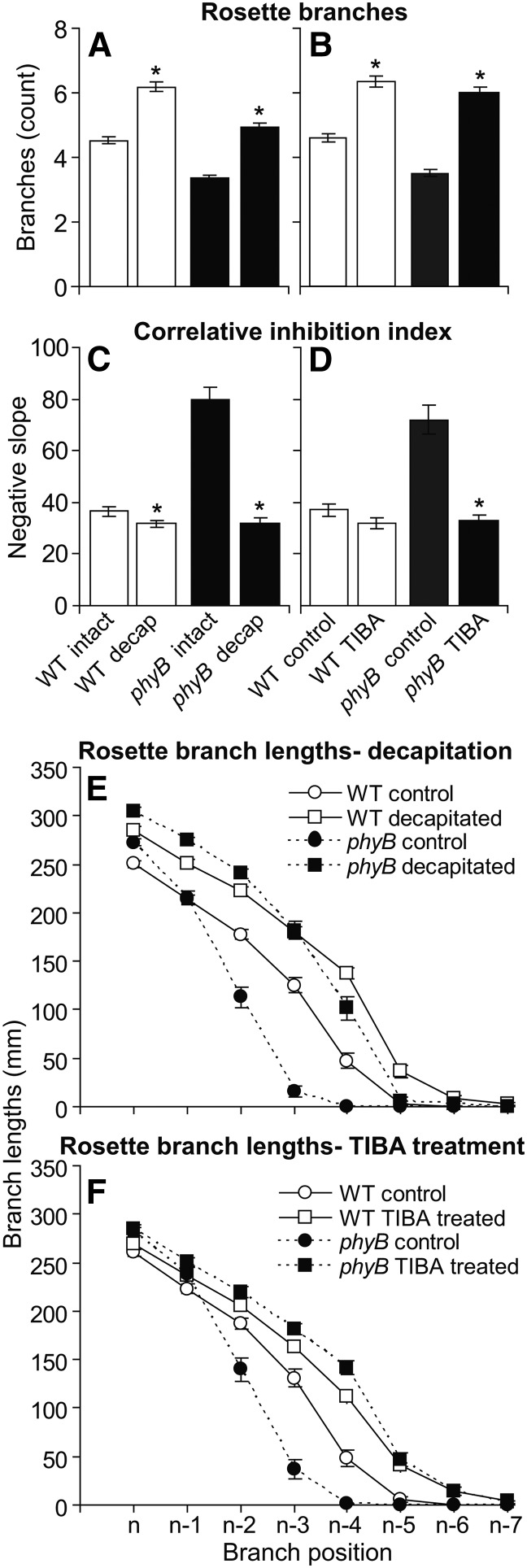
phyB deficiency was previously shown to reduce branching (Finlayson et al., 2010). Because auxin is known to exert pronounced effects on branch development, the effect of disrupting the PATS was investigated to determine whether some of the phyB branching phenotype could be attributed to this phenomenon. Intact phyB produced fewer branches than the wild type (Fig. 1A), but also produced fewer rosette leaves (8.8 versus 11.7 leaves for phyB and the wild type, respectively). The defect in phyB branching was previously shown to result from reduced bud outgrowth rather than bud initiation, because there were always more buds than branches (Finlayson et al., 2010), and this phenomenon was also apparent in this study (Fig. 1, A and B; Supplemental Fig. S1). Decapitating the main inflorescence below the lowermost cauline branch at 1 d before anthesis (predicted) removed all apical auxin sources, and increased the number of buds growing out to form branches in both phyB and the wild type (Fig. 1A). In the phyB mutant, this promotion was at least as great as in the wild type, despite the fact that it has fewer leaves and hence a reduced branching potential. Although branch numbers provide an estimate of the numbers of buds initiating outgrowth, the subsequent fate of the bud is also very relevant, because buds may initiate outgrowth but subsequently exhibit divergent elongation patterns. The lengths of the top three rosette branches were used to calculate a correlative inhibition index (CII), as previously described (Finlayson et al., 2010; Su et al., 2011). This index integrates the timing of the initiation of bud outgrowth and the elongation rate of branches from these upper positions, providing a quantitative estimate of branching vigor. Decapitation had a profound effect on the CII of phyB, but had a relatively weak effect on that of the wild type (Fig. 1C). A more detailed examination of the branch lengths at each position on the rosette showed that phyB responded more strongly to decapitation than the wild type, with much greater promotion of outgrowth occurring in the elongation of the lower buds (n-2, n-3, and n-4; Fig. 1E). Decapitation permitted increased elongation of the branches of both genotypes (Fig. 1E). Application of the auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA) to the main inflorescence stems at 1 d before anthesis (predicted) had virtually the same effect as decapitation, with stronger effects observed in phyB than in the wild type (Fig. 1, B, D, and F). Overall, the data suggested that suppressed branching in phyB could result from elevated signaling through the PATS.
Figure 1.
The number of rosette branches (A and B), CIIs (C and D), and branch lengths (E and F) of intact and decapitated (A, C, and E) or control and TIBA-treated (B, D, and F) wild type and phyB at 10 DPA. Data are means ± se with n = 72 (decapitation) or n = 50 (TIBA). Asterisks indicate a significant difference between intact and decapitated values or control and TIBA-treated values within a genotype at α = 0.05. WT, Wild type.
The Main Inflorescence Stems of phyB Have Less IAA Compared with the Wild Type
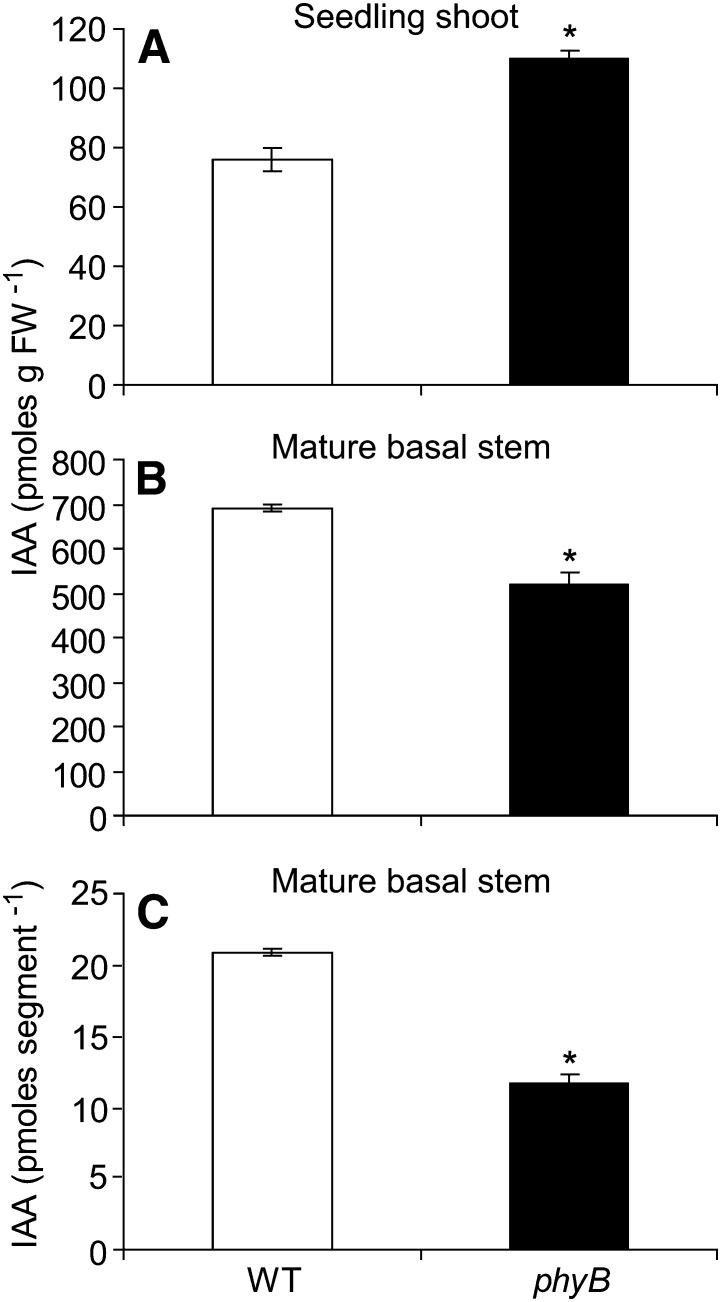
Because decapitation strongly promoted branching in phyB, and previous studies indicated that a low R:FR promotes IAA biosynthesis in young seedlings (Tao et al., 2008; Keuskamp et al., 2010; Hornitschek et al., 2012), we tested the hypothesis that phyB has elevated levels of IAA. As predicted from previous studies demonstrating increased IAA abundance in seedlings exposed to a low R:FR, IAA levels were elevated in whole shoots of 14-d-old phyB compared with the wild type (Fig. 2A). However, because the PATS of the main shoot was expected to exert the most profound effects on branching, IAA abundances were also assessed in 15-mm basal sections of the main inflorescence stem of the wild type and phyB. IAA levels were actually reduced in phyB compared with the wild type (Fig. 2B). The stems of phyB are thinner than those of the wild type; therefore, when IAA abundance is considered on a per-stem-segment basis, the discrepancy between the two genotypes is increased (Fig. 2C). Because each segment represents an individual plant, this comparison provides an estimate of the absolute amount of IAA in similar tissues of each genotype. In summary, a relationship between elevated IAA abundance and reduced branching in phyB could not be established.
Figure 2.
IAA abundance in the wild-type and phyB shoots of 14-d-old seedlings (A) and basal stem segments of mature plants, expressed on a per-weight basis (B) and per 15-mm stem segment (C). Data are means ± se with n = 4. Asterisks indicate a significant difference between the wild type and phyB at α = 0.05. WT, Wild type.
phyB Axillary Bud Outgrowth Shows Elevated Sensitivity to Auxin
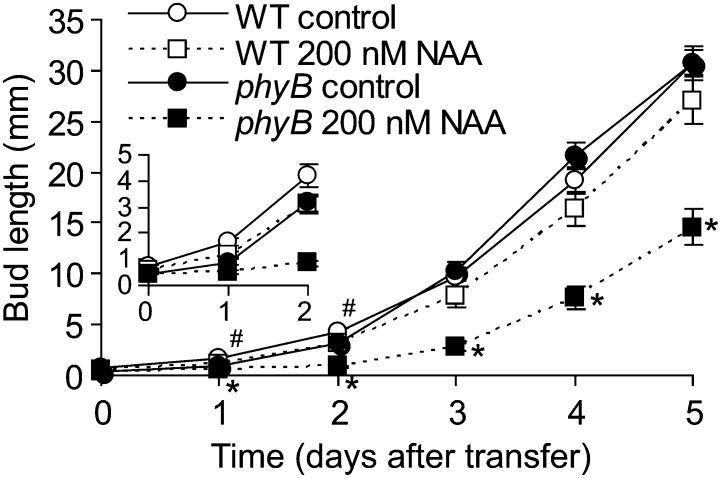
To explore whether the branching defect in phyB was a result of increased auxin responsiveness, a split plate bud outgrowth assay (Chatfield et al., 2000) was conducted. The assay supplied auxin to the apical end of a segment of main inflorescence stem spanning a leaf with an unelongated bud in the axil. Previous studies demonstrated that auxin applied in this manner inhibits the outgrowth of the isolated axillary bud (Chatfield et al., 2000). The obvious strategy of applying auxin to decapitated, but otherwise intact, plants was not pursued because Arabidopsis branching is not responsive to this treatment (Cline, 1996; Cline et al., 2001). Buds of phyB and the wild type elongated at similar rates in the absence of supplemental auxin (Fig. 3). The outgrowth of phyB buds was inhibited by 200 nm 1-naphthaleneacetic acid (NAA) supplied to the apical end of the stem, but the wild-type buds were inhibited only slightly at the earliest time points (Fig. 3). The NAA-treated wild-type buds were slightly larger than those of phyB at the start of the treatment (0.616 mm versus 0.465 mm). However, this small difference did not affect auxin responsiveness, because the average elongation of wild-type buds that were very small (0.4 or 0.5 mm) at the start of the assay was actually greater than the average of the entire set including slightly larger buds. Overall, the data indicate that phyB bud outgrowth was more sensitive than the wild type to auxin.
Figure 3.
Elongation of the wild-type and phyB axillary buds with and without apically supplied auxin (200 nm NAA), using a split plate in vitro assay. Data are means ± se with n = 18 to 20. Number signs and asterisks indicate significant differences between control and treated buds at α = 0.05 for the wild type and phyB, respectively. WT, Wild type.
The Main Inflorescence Stems of phyB Have Reduced Absolute, But Similar Relative, Auxin Transport Capacity Compared with the Wild Type
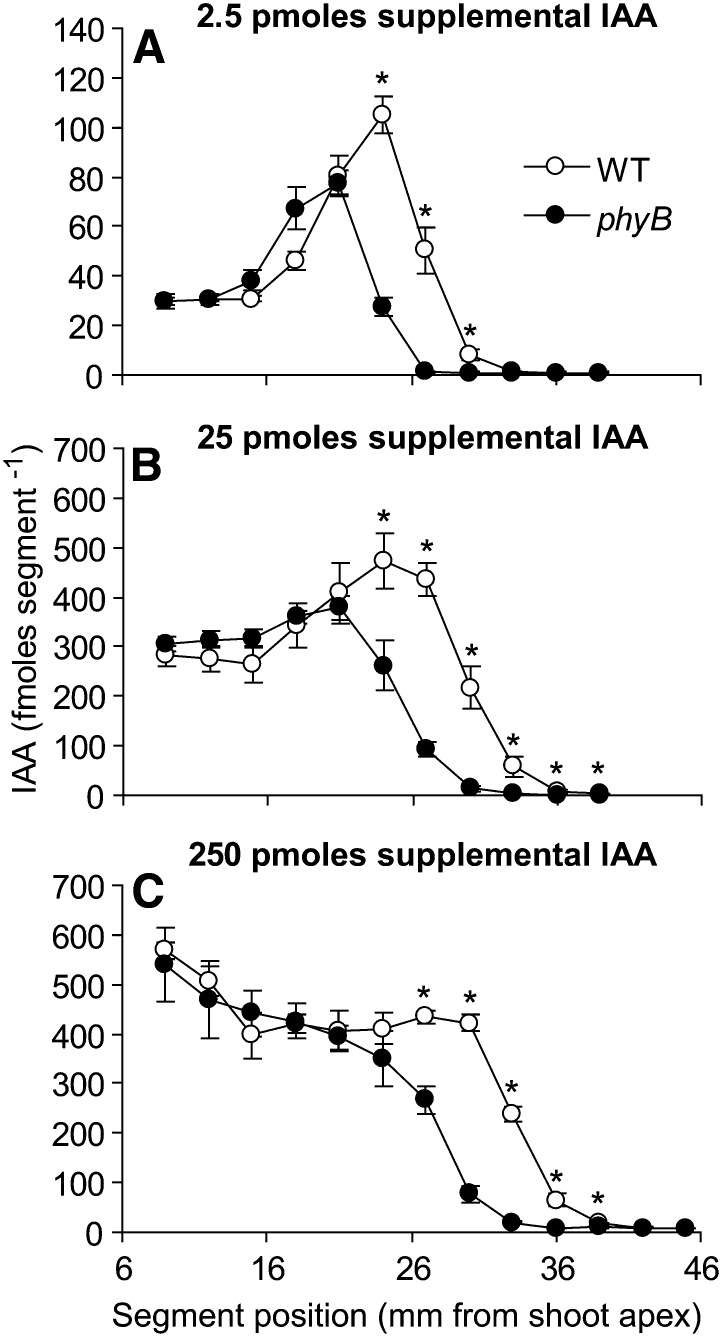
Parameters of auxin transport were then measured using radiolabeled IAA (Brewer et al., 2009) to determine whether an association exists between the rate or capacity of auxin transport and the branching phenotype of phyB. The rate of auxin transport was lower in phyB compared with the wild type at all three IAA concentrations tested, as evidenced by the distance that the peak front had traveled at harvest (Fig. 4). The height of the IAA peak demonstrated that the ultimate capacity of the IAA transport system was also reduced in phyB. Differences in capacity were not caused by differential uptake, because labeled IAA levels were equivalent in sections nearer to the treatment site in both genotypes, at all three concentrations. The reduced transport capacity of the phyB main shoot PATS could potentially limit the amount of auxin the system is able to accept from other auxin sources such as axillary buds. However, phyB was able to transport about 375 fmol of additional IAA per 3-mm segment (maximum peak value in Fig. 4B), which amounts to 0.125 pmol/mm. This amount is equal to more than 15% of the IAA measured per millimeter of stem in phyB (0.79 pmol mm−1; Fig. 2C). The wild type was able to transport approximately an additional 470 fmol per 3-mm segment, which corresponds to only 11% of the total IAA measured per millimeter of stem in the wild type (1.39 pmol mm−1; Fig. 2C). Therefore, although auxin transport was reduced in untreated phyB, it was not saturated and was able to transport as much or more additional IAA on a relative basis compared with the wild type. An underlying assumption for the calculations above is that all of the endogenous IAA measured in the stem is accessible to the PATS. Studies in pea (Pisum sativum) indicate that most of the endogenous stem IAA is available to the PATS, because IAA rapidly decreases to low levels in subtending portions of the stem after decapitation (Morris et al., 2005). However, in the case that only a fraction of the IAA is actually accessible to the PATS in Arabidopsis, then the relative transport capacity would be greater than that calculated, further reducing the likelihood that PATS capacity restricts branching in phyB.
Figure 4.
Auxin transport in main shoots of the wild type and phyB supplied with 2.5 pmol (A), 25 pmol (B), and 250 pmol (C) supplemental IAA. Data are means ± se with n = 5. Asterisks indicate a significant difference between the wild type and phyB at α = 0.05. WT, Wild type.
Auxin Signaling Is Elevated in phyB
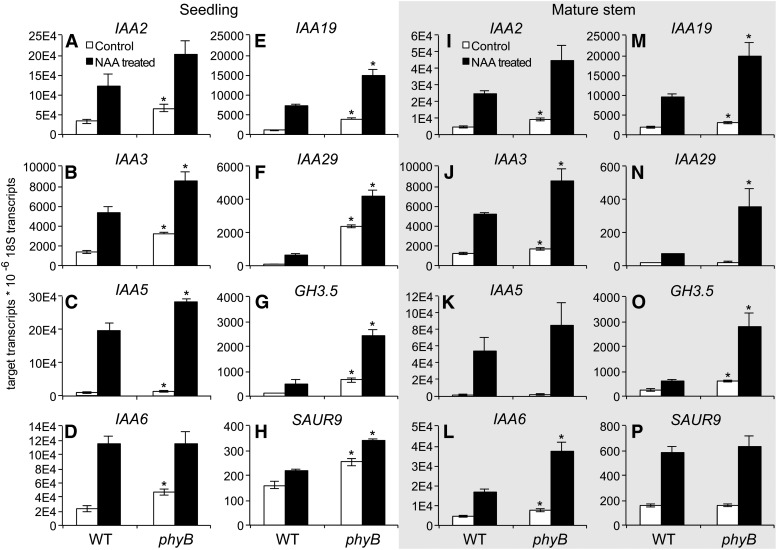
The effects of decapitation and auxin transport inhibitor application implied that auxin in the PATS differentially affected branching of phyB, but experiments examining IAA abundance provided data that conflicted with this hypothesis. However, the split plate assay of bud outgrowth responses to exogenous auxin did show that phyB has increased auxin sensitivity. Because the relative capacity of the PATS was similar in phyB and the wild type, the possibility remained that the auxin-dependent effect resulted from enhanced auxin signaling in phyB. The expression of a panel of eight auxin-responsive genes was therefore assessed in 14-d-old seedlings to estimate auxin signaling. The expression of all eight genes was significantly elevated in phyB compared with the wild type (Fig. 5, A–H). Basal expression differences of phyB compared with the wild type varied from 1.58-fold for SMALL AUXIN UPREGULATED RNA9 (SAUR9) (Fig. 5H) to 20-fold for IAA29 (Fig. 5G). Treatment with the auxin NAA increased the expression of all eight genes, and induced expression to greater levels in phyB compared with the wild type in six of the eight genes (Fig. 5, B, C, E–H). The expression trends of GH3.5 and SAUR9 were similar those of the various AUXIN/Indole-3-acetic acid (AUX/IAA) genes, demonstrating a general increase in auxin signaling rather than a more specific effect on a single gene family. Therefore, constitutive auxin signaling was elevated in phyB seedlings compared with the wild type, and this signaling could be further increased with the application of exogenous auxin.
Figure 5.
Expression of auxin-responsive genes in shoots of 14-d-old seedlings (A–H) and in basal stem segments of mature plants (at anthesis, I–P) of the wild type and phyB with and without auxin (50 μm NAA) treatment. Data are means ± se with n = 4. Asterisks indicate a significant difference between corresponding treatments of the wild type and phyB at α = 0.05. WT, Wild type.
The expression of auxin-responsive genes was also assessed in basal segments of the main inflorescence stem adjacent to the rosette leaves of mature plants at anthesis, the presumed site of PATS effects on branching (Fig. 5, I–P). Five of the eight genes showed significantly elevated expression in untreated phyB compared with the wild type (Fig. 5, I, J, L, M, O), with differences in expression ranging from 1.39-fold for IAA3 to 2.29-fold for GH3.5. Treatment with NAA increased the expression of all of the genes, and the expression of five of the eight increased to significantly higher levels in phyB compared with the wild type (Fig. 5, J, L–O). The data indicated that the main stems of phyB also exhibited elevated auxin signaling, but specific patterns were different from those observed in seedlings and expression differences were less pronounced.
DISCUSSION
As previously described, branching in phyB Arabidopsis was suppressed compared with the wild type (Finlayson et al., 2010; Su et al., 2011). This suppression was associated with elevated expression of the branching repressor BRANCHED1 (BRC1) in the axillary buds, and loss of BRC1 function alleviated the hypobranching phenotype (Finlayson et al., 2010; González-Grandío et al., 2013). Furthermore, both loss of function of components of the MAX pathway, and compromised auxin signaling through AXR1 also alleviated the deficiency in phyB branching (Finlayson et al., 2010). Presumably, all of these components act downstream of the R:FR signals transduced by phyB. In this study, the role of auxin in generating the phyB branching phenotype was specifically tested using a variety of approaches assessing the roles of auxin sources, auxin abundance in the stem, bud outgrowth sensitivity to auxin, auxin transport, and auxin signaling.
Decapitation and auxin transport inhibitor treatment permitted extra branches to grow out in both genotypes, but the effect was stronger in phyB. In addition, the CII was strongly influenced by decapitation in phyB, but was only weakly responsive in the wild type. The results suggested that auxin transported from the shoot apices in the PATS regulated the coordination of bud outgrowth reflected by the CII, and that this pathway was stronger in phyB than in the wild type. The weak effect of decapitation on the CII observed in the wild type suggested that either the CII is largely set before decapitation occurred, or that other factors contribute to regulating this parameter. On the other hand, the shoot apices appeared to strongly limit branch outgrowth and the coordination of branch outgrowth in phyB, presumably through the action of the PATS. These results support earlier studies demonstrating that auxin signaling is required for the phyB hypobranching phenotype (Finlayson et al., 2010).
Prior research showed that IAA levels increased in young Arabidopsis seedlings in a TAA1- dependent manner soon after the application of a low R:FR (Tao et al., 2008). In addition, early shade avoidance responses including increased elevation of leaves and more rapid hypocotyl elongation were dependent on TAA1 function. Subsequent studies also demonstrated increased IAA levels in young Arabidopsis seedlings provided with a low R:FR (Keuskamp et al., 2010; Hornitschek et al., 2012). In this study, we found that IAA levels were elevated in whole shoots of phyB compared with the wild type at 14 d after planting, consistent with previous reports showing increased IAA accumulation in response to competition signals and confirming a role for phyB. In contrast with the case for seedlings, IAA levels were reduced in the stem of the main inflorescence of mature phyB plants compared with the wild type, indicating that the effect of phyB function on IAA abundance is dependent on the stage of growth and/or the specific tissues examined. Thus, it appears that reduced branching in phyB was not the result of increased auxin abundance in the PATS.
Although elevated PATS auxin abundance could not explain the branching phenotype of phyB, a role for auxin was clearly demonstrated using the split plate assay that showed increased inhibition of bud outgrowth in phyB with low levels of auxin. Although the effect was obvious, the indirect nature of auxin action on bud outgrowth prevented a clear mechanistic interpretation because the results could not differentiate between effects caused by sensitivity in terms of auxin signaling, or effects related to the bud’s ability to export auxin into the stem segment in the presence of exogenous NAA. The effects of phyB deficiency on auxin transport and auxin signaling were therefore directly tested.
At first glance, the results of the auxin transport study indicated a reduced capacity for auxin transport in phyB. The lower capacity of auxin transport in phyB might suggest that axillary buds could have more difficulty exporting auxin into the PATS, which could inhibit bud outgrowth in a manner consistent with the auxin transport competition theory (Li and Bangerth, 1999; Domagalska and Leyser, 2011). However, phyB was capable of transporting additional IAA in the PATS, and in fact could transport at least as much additional IAA as the wild type relative to the native IAA content of the tissue. Therefore, given the reduced IAA levels in phyB, the axillary buds should be able to establish auxin export into the main shoot PATS at least as easily as in the wild type, and it is unlikely that the overall reduced auxin transport capacity limits its branching.
The reduced branching of phyB-deficient plants was associated with enhanced auxin signaling in both seedling shoots and mature stem segments, as reported by the expression of various auxin-responsive genes. The specific expression patterns were dissimilar in the seedling shoots versus mature stems, indicating tissue- and/or stage-specific effects of phyB deficiency on auxin signaling. Whereas axillary buds were not obvious in the younger seedlings, axillary bud initiation in long days (16 h) was documented as early as 10 d after germination in ecotype Landsberg erecta of Arabidopsis (Hempel and Feldman, 1994). It is possible that the elevated auxin signaling could affect future branching responses by programming meristematic tissues prior to their differentiation into buds. Because auxin treatment increased expression of most of the tested genes to higher levels in phyB than in the wild type, it is apparent that basal auxin signaling as well as auxin-induced signaling were elevated in phyB. Members of the PIF family of proteins directly interact with phyB in the nucleus to regulate gene expression (Leivar and Quail, 2011). Activated phyB targets PIFs for degradation through an ubiquitin-proteasome-mediated process. Chromatin immunoprecipitation assays have shown that both auxin biosynthesis and signaling genes are direct targets of PIF5 (Hornitschek et al., 2012). Furthermore, auxin-responsive gene expression and picloram-induced hypocotyl elongation were suppressed in pif4pif5 double mutants (Hornitschek et al., 2012). The increased auxin sensitivity of phyB-deficient plants may therefore result from enhanced auxin signaling conferred by PIF5 (and PIF4) function in the absence of repressive phyB. Expression of Aux/IAA gene family members is rapidly induced by auxin (Mockaitis and Estelle, 2008) and has therefore often been used to gauge auxin sensitivity. It is interesting to note, however, that IAA19 and IAA29, which are direct targets of PIF5 transcriptional regulation, are presumed to act as repressors of auxin signaling (Tatematsu et al., 2004; Sun et al., 2013). This functionality is not easily reconciled with the discovery that although PIF5 promotes their expression, and the pif4pif5 double mutant has reduced expression, this mutant exhibits reduced auxin sensitivity (Hornitschek et al., 2012). Recent studies have highlighted the complexity of auxin receptor function through interactions between multiple TRANSPORT INHIBITOR RESPONSE/AUXIN SIGNALING F-BOX proteins and Aux/IAA proteins (Calderón Villalobos et al., 2012; Havens et al., 2012). Given this complexity, it is possible that specific interactions might alter auxin sensitivity in a nonintuitive manner. It is also possible that additional pathways exist whereby phyB, and perhaps PIFs, can modulate auxin signaling.
The effects of AXR1-dependent auxin signaling are transduced to alter the expression and/or activity of components of the bud autonomous machinery, including BRC1, that more directly affect bud development (Finlayson, 2007). Although several studies investigated the expression of branching-related genes in Arabidopsis buds (Finlayson et al., 2010; Su et al., 2011; Reddy et al., 2013), the full complement of the bud autonomous machinery remains unknown, as does the integration of bud autonomous and nonbud autonomous components. A variety of studies using a wide selection of species previously implicated abscisic acid (ABA) as a potential regulator of shoot branching (Arney and Mitchell, 1969; Tucker and Mansfield, 1972; Tucker, 1977; Tamas et al., 1979; Le Bris et al., 1999; Chatfield et al., 2000; Cline and Oh, 2006; Arend et al., 2009; González-Grandío et al., 2013; Ortiz-Morea et al., 2013). Recent work providing genomic, biochemical, and genetic evidence has established a role for ABA in restricting bud outgrowth in Arabidopsis grown under a low R:FR (Reddy et al., 2013), potentially by acting within the bud. Plants deficient in phyB exhibit a phenotype very similar to that of plants grown under a low R:FR, including inhibition of branching (Finlayson et al., 2010). Thus, it appears that activated phyB functions to both generally repress auxin signaling and to suppress ABA accumulation in buds to promote branching, whereas inactivation of phyB has the opposite effects. Determining how auxin-mediated and ABA-mediated pathways interact to regulate branching is likely to provide further insight into the mechanisms underlying this important developmental program.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The ecotype Columbia of Arabidopsis (Arabidopsis thaliana) was used throughout. Wild-type ecotype Columbia 0 (CS60000) and phyB-9 seed was obtained from the Arabidopsis Biological Resource Center at Ohio State University.
Seeds were stratified for 3 d at 4°C and sown in inserts filled with Metro-Mix 200 potting mixture. Plants were grown under 18-h-light/6-h-dark photoperiods with 24°C/18°C day/night temperatures in a growth chamber providing 180 μmol m−2 s−1 photosynthetic photon flux density (R:FR of 5.4) and were fertilized weekly with 4 mL 1× Hoagland solution. Light was provided using a mixture of fluorescent (F48T12/CW/VHO; Philips Lighting) and compact fluorescent (CF30EL/TWIST; Osram Sylvania Products) lamps. Light was measured with a Li-1800 spectroradiometer (Licor Biosciences). The R:FR was calculated as the quantum flux density from 655 to 665 nm divided by the quantum flux density from 725 to 735 nm. The spectrum of the light source is provided in Supplemental Figure S2.
Manipulation of Apical Auxin Supply and Architectural and Branch Elongation Analyses
The apical source of auxin in the main shoot PATS was removed by decapitating plants below the lowest cauline branch at 1 d before the predicted occurrence of anthesis. The PATS was also disrupted by applying a ring of lanolin with 2% (w/w) TIBA in 20% (v/w) ethanol around the main shoot stem below the lowest cauline branch at 1 d before the predicted occurrence of anthesis. Architectural characteristics and branch elongation were measured at 10 DPA as described by Finlayson et al. (2010) except that the CII was calculated for each record individually. Means for each treatment were calculated by pooling the results of three experiments with a total of 72 replicates for decapitation and 50 replicates for TIBA treatment.
Analysis of Hormone Abundance
IAA abundances were determined in whole seedling shoots (10 shoots per replicate) at 14 d after sowing, and in basal 1.5-cm segments of the main inflorescence stem (8 to 10 segments per replicate) at anthesis. IAA was extracted and quantified using isotope dilution selected ion monitoring gas chromatography-mass spectroscopy as described by Reddy et al. (2013). Four biological replicates were measured for each genotype.
Split Plate Assay of Bud Growth
The split plate assay of axillary bud outgrowth was conducted based on the method of Chatfield et al. (2000). Plants were grown in culture on 0.75% (w/v) agar with 0.4× Murashige and Skoog salts and 0.2% (w/v) Suc. Stem sections spanning buds were excised from the main inflorescence prior to the initiation of bud elongation and were inserted into the split plate system, containing 0.8% (w/v) agar with 0.4× Murashige and Skoog salts and 0.2% (w/v) Suc, with or without 200 nm NAA infused into the top (apical) section of the agar. Bud lengths were measured daily. Eighteen to 20 biological replicates were measured for each genotype/treatment combination.
Analysis of Auxin Transport
The measurement of IAA transport in mature main inflorescence stems was conducted according to the method of Brewer et al. (2009). One μL of the desired IAA concentration (2.5, 25, and 250 μM) containing 3H-labeled IAA (20 Ci/mmol; American Radiolabeled Chemicals) was applied to the shoot apex of plants at 2 or 3 DPA. Plants were harvested from 3 to 3.5 h after treatment began and 3-mm shoot segments were dissected, incubated in scintillation cocktail with shaking for 24 h, and the radioactivity in each sample was determined using a liquid scintillation counter. Five replicates were measured for each genotype/treatment combination.
Analysis of Gene Expression
For seedlings, 14-d-old plants were sprayed until lightly wetted with 50 μm NAA in a solution containing 1% (v/v) ethanol and 0.025% (v/v) Silwet, or with a control solution lacking the NAA. Whole shoots were harvested 45 min after the treatment was applied, with 10 shoots comprising one replicate. Mature plants were treated in a similar manner at the time of anthesis. Basal 1.5-cm segments of the main inflorescence stem adjacent to the rosette were harvested 45 min after treatment, with 8 to 10 segments comprising one replicate. Total RNA was extracted and gene expression was measured by quantitative PCR using the methods of Su et al. (2011). Primers for IAA19, GH3.5, and SAUR9 were taken from Effendi et al. (2011). Primers were as follows: IAA2, 5′-AGCTATGTCTTGGATTACCCGGAA-3′ and 5′-ACTGGTGGCCAACCAACGATTT-3′; IAA3, 5′-GGTGATTGGATGCTCATTGGTGAT-3′ and 5′-CAACCCAAGCACAGACAGAGATTT-3′; IAA5, 5′-TCTGCAAATTCTGTTCGGATGCT-3′ and 5′-CTCTTGCACGATCCAAGGAACATT-3′; IAA6, 5′-AATCTCTTCGGCTGTCTTGGCATA-3′ and 5′-TGGAGACCAAAACCAGTTGCAT-3′; and IAA29, 5′-AAGGGAAAGAGGGTGACTGGCTA-3′ and 5′-ATGGTCCGATTTGAACGCCTAT-3′. Four biological replicates were measured for each genotype/treatment combination.
Statistical Analyses
Comparisons between means were made using a two-tailed Student’s t test with α < 0.05.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The number of rosette buds (unelongated buds plus branches) of the intact and decapitated (A) or control and TIBA-treated (B) wild type and phyB at 10 DPA.
Supplemental Figure S2. Spectrum of the light source used for plant growth.
Acknowledgments
The authors thank Jorge J. Casal, Tesfamichael H. Kebrom, and Christian Fankhauser for helpful discussions regarding this article.
Glossary
- PATS
polar auxin transport stream
- IAA
indole-3-acetic acid
- R:FR
red light:far-red light ratio
- CII
correlative inhibition index
- TIBA
2,3,5-triiodobenzoic acid
- NAA
1-naphthaleneacetic acid
- ABA
abscisic acid
Footnotes
This work was supported by the National Science Foundation (grant no. IOS-0719414) and Texas AgriLife Research (to S.A.F.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Arend M, Schnitzler JP, Ehlting B, Hänsch R, Lange T, Rennenberg H, Himmelbach A, Grill E, Fromm J. (2009) Expression of the Arabidopsis mutant ABI1 gene alters abscisic acid sensitivity, stomatal development, and growth morphology in gray poplars. Plant Physiol 151: 2110–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arney SE, Mitchell DL. (1969) The effect of abscisic acid on stem elongation and correlative inhibition. New Phytol 68: 1001–1015 [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S. (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65: 571–577 [DOI] [PubMed] [Google Scholar]
- Ballaré CL. (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4: 97–102 [DOI] [PubMed] [Google Scholar]
- Bennett T, Leyser O. (2006) Something on the side: axillary meristems and plant development. Plant Mol Biol 60: 843–854 [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón Villalobos LI, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao H, et al. (2012) A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat Chem Biol 8: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I. (2007) Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21: 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2012) Shade avoidance. The Arabidopsis Book 10: e0157, /10.1199/tab.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O. (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24: 159–169 [DOI] [PubMed] [Google Scholar]
- Cline MG. (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot 78: 255–266 [Google Scholar]
- Cline MG. (1997) Concepts and terminology of apical dominance. Am J Bot 84: 1064–1069 [PubMed] [Google Scholar]
- Cline MG, Chatfield SP, Leyser O. (2001) NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Ann Bot 87: 61–65 [Google Scholar]
- Cline MG, Oh C. (2006) A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann Bot 98: 891–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M. (2002) AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis Cullin AtCUL1 is required for auxin response. Plant Cell 14: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Effendi Y, Rietz S, Fischer U, Scherer GFE. (2011) The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J 65: 282–294 [DOI] [PubMed] [Google Scholar]
- Finlayson SA. (2007) Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol 48: 667–677 [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ. (2010) Phytochrome regulation of branching in Arabidopsis. Plant Physiol 152: 1914–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC. (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96: 169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P. (2013) BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25: 834–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens KA, Guseman JM, Jang SS, Pierre-Jerome E, Bolten N, Klavins E, Nemhauser JL. (2012) A synthetic approach reveals extensive tunability of auxin signaling. Plant Physiol 160: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ. (1994) Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192: 276–286 [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA. (2010) Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ 33: 48–58 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. (2006) Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 140: 1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MM, Jaillais Y, Pedmale UV, Moreno JE, Chory J, Ballaré CL. (2011) Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J 67: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A. (2010) Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bris M, Michaux-Ferrière N, Jacob Y, Poupet A, Barthe P, Guigonis JM, Le Page-Degivry MT. (1999) Regulation of bud dormancy by manipulation of ABA in isolated buds of Rosa hybrida cultured in vitro. Aust J Plant Physiol 26: 273–281 [Google Scholar]
- Leivar P, Quail PH. (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Bangerth F. (1999) Autoinihibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant 106: 415–420 [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Estelle M. (2008) Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24: 55–80 [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA. (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol 138: 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Morea FA, Vicentini R, Silva GFF, Silva EM, Carrer H, Rodrigues AP, Nogueira FTS. (2013) Global analysis of the sugarcane microtranscriptome reveals a unique composition of small RNAs associated with axillary bud outgrowth. J Exp Bot 64: 2307–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LACJ. (2009) Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and della proteins in Arabidopsis. Plant Physiol 149: 1701–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O. (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA. (2013) Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol 163: 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (1995) Physiological and ecological function within the phytochrome family. Ann Rev Plant Physiol Plant Mol Biol 46: 289–315 [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. (1999) Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- Su H, Abernathy SD, White RH, Finlayson SA. (2011) Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant Cell Environ 34: 1986–1998 [DOI] [PubMed] [Google Scholar]
- Sun J, Qi L, Li Y, Zhai Q, Li C. (2013) PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell 25: 2102–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas IA, Ozbun JL, Wallace DH, Powell LE, Engels CJ. (1979) Effect of fruits on dormancy and abscisic acid concentration in the axillary buds of Phaseolus vulgaris L. Plant Physiol 64: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT. (2004) MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell 16: 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DJ. (1977) The effects of far-red light on lateral bud outgrowth in decapitated tomato plants and the associated changes in the levels of auxin and abscisic acid. Plant Sci Lett 8: 339–344 [Google Scholar]
- Tucker DJ, Mansfield TA. (1972) Effects of light quality on apical dominance in Xanthium strumarium and the associated changes in endogenous levels of abscisic acid and cytokinins. Planta 102: 140–151 [DOI] [PubMed] [Google Scholar]
- Waldie T, Hayward A, Beveridge CA. (2010) Axillary bud outgrowth in herbaceous shoots: How do strigolactones fit into the picture? Plant Mol Biol 73: 27–36 [DOI] [PubMed] [Google Scholar]