Abstract
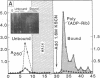
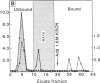
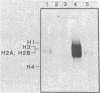
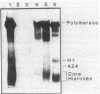
Antibody to poly(ADP-ribose) has been covalently coupled to Sepharose and utilized to isolate selectively oligonucleosomes undergoing the poly(ADP-ribosyl)ation reaction from the bulk of chromatin. Approximately 12% of the unfractionated oligonucleosomes were bound to the immunoaffinity column and these represented essentially 100% of the original poly(ADP-ribosyl)ated nucleosomal species in the unfractionated chromatin. Poly(ADP-ribosyl)ated chromatin was not bound by preimmune IgG columns. KSCN eluted the modified nucleosomes in the form of nucleoprotein complexes. The eluted chromatin components were shown to contain poly(ADP-ribosyl)ated histones as well as automodified poly(ADP-ribose) polymerase. By using [3H]lysine- and [3H]arginine-labeled chromatin, it was shown that the poly-(ADP-ribosyl)ated histones, attached to stretches of oligonucleosomes bound to the column, had a 6-fold enrichment of the modification compared to histones of the unfractionated chromatin. This indicated that non-poly(ADP-ribosyl)ated nucleosomes, connected and proximal to the modified regions, were copurified by this procedure. This allowed characterization of the oligonucleosomal DNA around poly(ADP-ribosyl)ated chromatin domains to be compared with the unbound bulk chromatin. The data indicated that immunofractionated poly(ADP-ribosyl)ated oligonucleosomal DNA contained significant amounts of internal single-strand breaks compared with bulk chromatin. The bound nucleo-protein complexes were found to be enzymatically active for poly(ADP-ribose) polymerase after elution from the antibody column. In contrast, the unbound nucleosomes, representing 90% of the unfractionated chromatin, were totally inactive in the poly(ADP-ribosyl)ation reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annunziato A. T., Schindler R. K., Thomas C. A., Jr, Seale R. L. Dual nature of newly replicated chromatin. Evidence for nucleosomal and non-nucleosomal DNA at the site of native replication forks. J Biol Chem. 1981 Nov 25;256(22):11880–11886. [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Butt T. R., DeCoste B., Jump D. B., Nolan N., Smulson M. Characterization of a putative poly(adenosine diphosphate ribose)-chromatin complex. Biochemistry. 1980 Nov 11;19(23):5243–5249. doi: 10.1021/bi00564a014. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Smulson M. Relationship between nicotinamide adenine dinucleotide concentration and in vitro synthesis of poly(adenosine diphosphate ribose) on purified nucleosomes. Biochemistry. 1980 Nov 11;19(23):5235–5242. doi: 10.1021/bi00564a013. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Taylor C. W., Baum R. M., Yeoman L. C., Olson M. O., Prestayko A. W., Busch H. Isolation and characterization of protein A24, a "histone-like" non-histone chromosomal protein. J Biol Chem. 1975 Sep 25;250(18):7182–7187. [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Butt T. R., Smulson M. Nuclear protein modification and chromatin substructure. 3. Relationship between poly(adenosine diphosphate) ribosylation and different functional forms of chromatin. Biochemistry. 1979 Mar 20;18(6):983–990. doi: 10.1021/bi00573a008. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Butt T. R., Smulson M. Reconstitution of HeLa cell poly(adenosine diphosphate ribose) polymerase with purified oligonucleosomal chromatin. Biochemistry. 1980 Mar 4;19(5):1031–1037. doi: 10.1021/bi00546a031. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Smulson M. Purification and characterization of the major nonhistone protein acceptor for poly(adenosine diphosphate ribose) in HeLa cell nuclei. Biochemistry. 1980 Mar 4;19(5):1024–1030. doi: 10.1021/bi00546a030. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Miwa M., Matsushima T., Sugimura T. Studies on anti-poly(adenosine diphosphate ribose) antibody. Biochem Biophys Res Commun. 1974 Jul 10;59(1):300–306. doi: 10.1016/s0006-291x(74)80206-8. [DOI] [PubMed] [Google Scholar]
- Malik N., Bustin M., Smulson M. Antibody to poly(adenosine diphosphate-ribose) polymerase and its use in chromatin analysis. Nucleic Acids Res. 1982 May 11;10(9):2939–2950. doi: 10.1093/nar/10.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak S. P., Beard P. Analysis of DNA double- and single-strand breaks by two dimensional electrophoresis: action of micrococcal nuclease on chromatin and DNA, and degradation in vivo of lens fiber chromatin. Nucleic Acids Res. 1980 Jun 25;8(12):2665–2678. doi: 10.1093/nar/8.12.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan N. L., Butt T. R., Wong M., Lambrianidou A., Smulson M. E. Characterization of poly(ADP-ribose)--histone H1 complex formation in purified polynucleosomes and chromatin. Eur J Biochem. 1980 Dec;113(1):15–25. doi: 10.1111/j.1432-1033.1980.tb06133.x. [DOI] [PubMed] [Google Scholar]
- Ogata N., Ueda K., Kawaichi M., Hayaishi O. Poly(ADP-ribose) synthetase, a main acceptor of poly(ADP-ribose) in isolated nuclei. J Biol Chem. 1981 May 10;256(9):4135–4137. [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Stone P. R., Lorimer W. S., 3rd, Kidwell W. R. Properties of the complex between histone H1 and poly(ADP-ribose synthesised in HeLa cell nuclei. Eur J Biochem. 1977 Nov 15;81(1):9–18. doi: 10.1111/j.1432-1033.1977.tb11921.x. [DOI] [PubMed] [Google Scholar]
- Sudhakar S., Tew K. D., Smulson M. E. Effect of 1-methyl-1-nitrosourea on poly(adenosine diphosphate-ribose) polymerase activity at the nucleosomal level. Cancer Res. 1979 Apr;39(4):1405–1410. [PubMed] [Google Scholar]