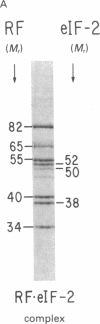
Abstract
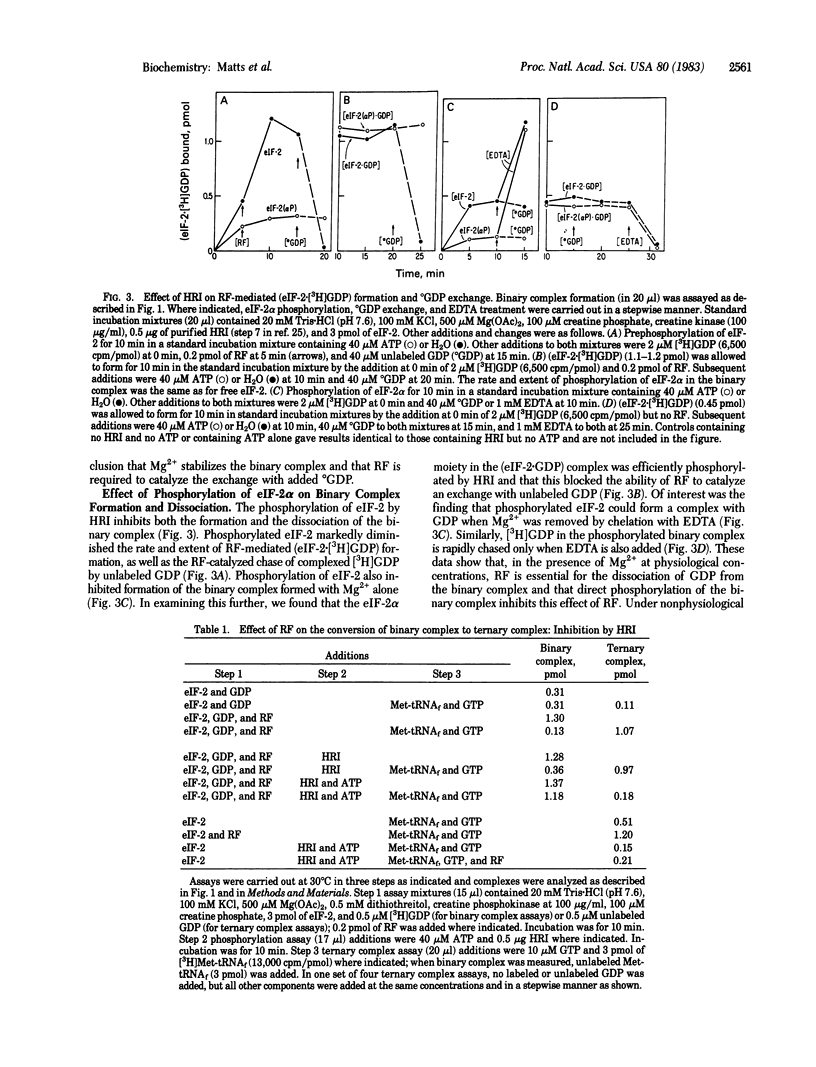
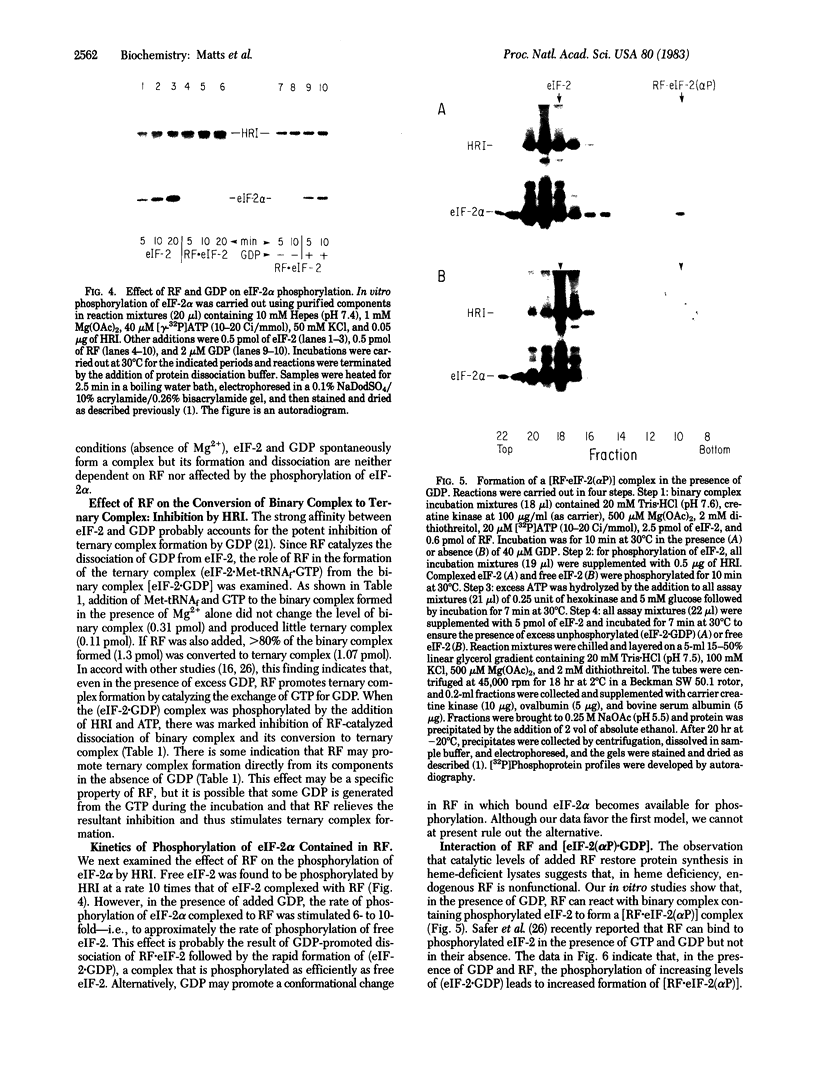
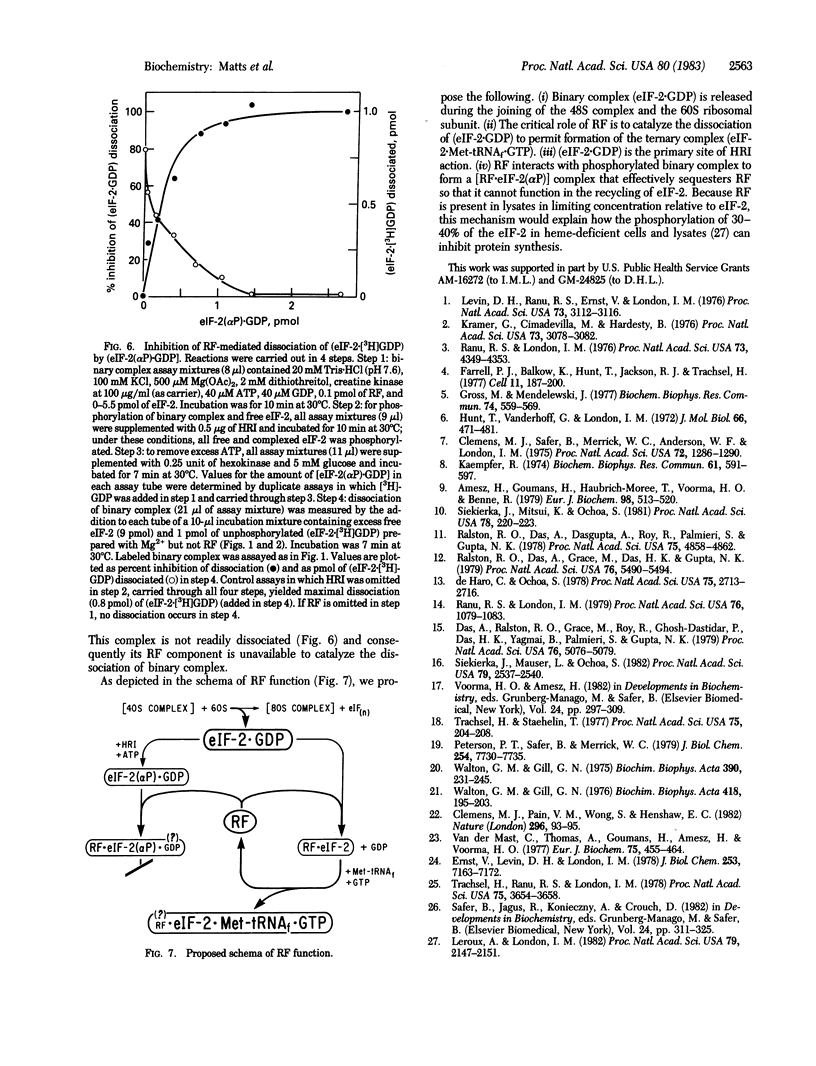
The reticulocyte reversing factor (RF) isolated as a complex with eukaryotic initiation factor 2 (eIF-2) acts catalytically in restoring protein synthesis in reticulocyte lysates inhibited by heme deficiency. In reconstituted in vitro assay mixtures containing Mg2+ (0.25-0.5 mM), RF catalyzes the formation of the binary complex (eIF-2-GDP) but this effect is inhibited when eIF-2 is phosphorylated by the heme-regulated kinase for the alpha-subunit of eIF-2 (HRI). More significantly, RF catalyzes the rapid dissociation of (eIF-2-GDP), which permits the exchange of GTP for GDP and, in the presence of Met-tRNAf, promotes the formation of the ternary complex (eIF-2-Met-tRNAf X GTP). However, phosphorylation of the binary complex by HRI prevents its dissociation by RF and, as a consequence, ternary complex formation is inhibited. Our results indicate that phosphorylated binary complex [eIF-2(alpha P).GDP] interacts with RF to form a [RF . eIF-2(alpha P)] that is not readily dissociable. This binding of RF renders it unavailable to catalyze the dissociation of unphosphorylated binary complex, thereby blocking the recycling of eIF-2. Since RF is present in lysates at a limited concentration relative to that of eIF-2, the sequestering of RF in this manner could account for the observation that the phosphorylation of a small proportion of eIF-2 in heme-deficient lysates is sufficient to inhibit protein synthesis.
Full text
PDF
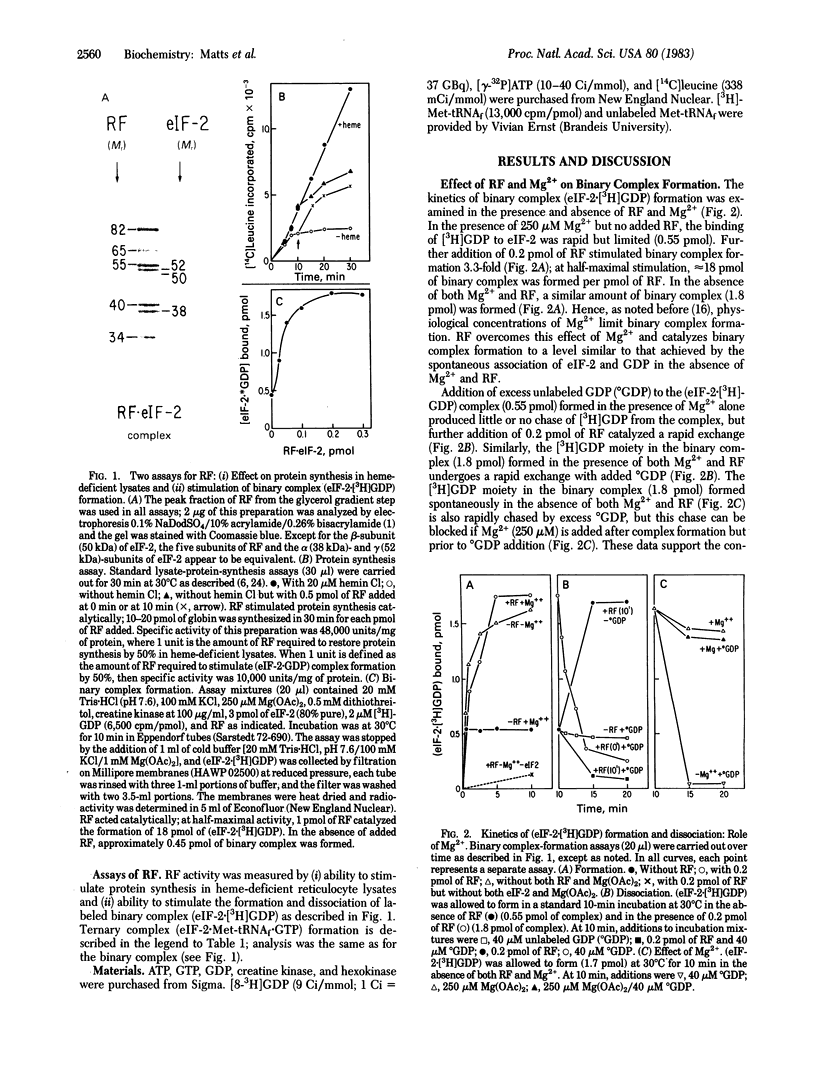
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz H., Goumans H., Haubrich-Morree T., Voorma H. O., Benne R. Purification and characterization of a protein factor that reverses the inhibition of protein synthesis by the heme-regulated translational inhibitor in rabbit reticulocyte lysates. Eur J Biochem. 1979 Aug 1;98(2):513–520. doi: 10.1111/j.1432-1033.1979.tb13212.x. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Ralston R. O., Grace M., Roy R., Ghosh-Dastidar P., Das H. K., Yaghmai B., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: mechanism of protein synthesis inhibition by heme-regulated inhibitor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5076–5079. doi: 10.1073/pnas.76.10.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Evidence that glucose 6-phosphate regulates protein synthesis initiation in reticulocyte lysates. J Biol Chem. 1978 Oct 25;253(20):7163–7172. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux A., London I. M. Regulation of protein synthesis by phosphorylation of eukaryotic initiation factor 2 alpha in intact reticulocytes and reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2147–2151. doi: 10.1073/pnas.79.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. T., Safer B., Merrick W. C. Role of eukaryotic initiation factor 5 in the formation of 80 S initiation complexes. J Biol Chem. 1979 Aug 25;254(16):7730–7735. [PubMed] [Google Scholar]
- Ralston R. O., Das A., Dasgupta A., Roy R., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: characteristics of a ribosomal factor that reverses inhibition of protein synthesis in heme-deficient lysates. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4858–4862. doi: 10.1073/pnas.75.10.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R. O., Das A., Grace M., Das H., Gupta N. K. Protein synthesis in rabbit reticulocytes: characteristics of a postribosomal supernatant factor that reverses inhibition of protein synthesis in heme-deficient lysates and inhibition of ternary complex (Met-tRNAfMet.eIF-2.GTP) formation by heme-regulated inhibitor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5490–5494. doi: 10.1073/pnas.76.11.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: additional initiation factor required for formation of ternary complex (eIF-2.GTP.Met-tRNAf) and demonstration of inhibitory effect of heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1079–1083. doi: 10.1073/pnas.76.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mitsui K. I., Ochoa S. Mode of action of the heme-controlled translational inhibitor: relationship of eukaryotic initiation factor 2-stimulating protein to translation restoring factor. Proc Natl Acad Sci U S A. 1981 Jan;78(1):220–223. doi: 10.1073/pnas.78.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and characterization of heme-reversible translational inhibitor. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3654–3658. doi: 10.1073/pnas.75.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Binding and release of eukaryotic initiation factor eIF-2 and GTP during protein synthesis initiation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):204–208. doi: 10.1073/pnas.75.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Mast C., Thomas A., Goumans H., Amesz H., Voorma H. O. Initiation of protein synthesis in eukaryotes. Binding to Sepharose-heparin and partial purification of initiation factors from Krebs II ascites cells. Eur J Biochem. 1977 May 16;75(2):455–464. doi: 10.1111/j.1432-1033.1977.tb11548.x. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Regulation of ternary (Met-tRNAf - GTP - eukaryotic initiation factor 2) protein synthesis initiation complex formation by the adenylate energy charge. Biochim Biophys Acta. 1976 Jan 19;418(2):195–203. doi: 10.1016/0005-2787(76)90069-1. [DOI] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]