Abstract
Background
IL-17 is the defining cytokine of the Th17, Tc17, and γδ T cell populations that plays a critical role in mediating inflammation and autoimmunity. Psoriasis vulgaris is an inflammatory skin disease mediated by Th1 and Th17 cytokines with relevant contributions of IFN-γ, TNF-α, and IL-17. Despite the pivotal role IL-17 plays in psoriasis, and in contrast to the other key mediators involved in the psoriasis cytokine cascade that are capable of inducing broad effects on keratinocytes, IL-17 was demonstrated to regulate the expression of a limited number of genes in monolayer keratinocytes cultured in vitro.
Methodology/Principal Findings
Given the clinical efficacy of anti-IL-17 agents is associated with an impressive reduction in a large set of inflammatory genes, we sought a full-thickness skin model that more closely resemble in vivo epidermal architecture. Using a reconstructed human epidermis (RHE), IL-17 was able to upregulate 419 gene probes and downregulate 216 gene probes. As possible explanation for the increased gene induction in the RHE model is that C/CAAT-enhancer-binding proteins (C/EBP) -β, the transcription factor regulating IL-17-responsive genes, is expressed preferentially in differentiated keratinocytes.
Conclusions/Significance
The genes identified in IL-17-treated RHE are likely relevant to the IL-17 effects in psoriasis, since ixekizumab (anti-IL-17A agent) strongly suppressed the “RHE” genes in psoriasis patients treated in vivo with this IL-17 antagonist.
Introduction
Psoriasis is a chronic inflammatory skin disorder characterized by a dense dermal inflammatory infiltrate and altered keratinocyte (KC) differentiation [1]. Leukocytes that infiltrate the dermis produce many pro-inflammatory mediators that set up the cycle of pathogenic inflammation. Interleukin (IL)-17 has emerged as one of the most crucial players in the current model of psoriasis pathogenesis. IL-17 was thought to be produced mainly by Th17 cells, a subset of CD4+ T helper cells that is distinct from the Th1 and Th2 lineages [2], [3], but it is becoming increasingly appreciated that it is also produced by CD8+ T cells (Tc1) and γδ T cells [4], [5], and potentially by some non-T cells, including mast cells and neutrophils [6]. IL-17 signaling activates the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and the C/CAAT-enhancer-binding proteins (C/EBP) family, particularly C/EBPβ and C/EBPδ [7], [8] to enhance expression of pro-inflammatory cytokines and chemokines, intercellular adhesion molecules, and anti-microbial peptides (AMPs) by numerous cell types, including granulocytes, chondroblasts, fibroblasts, and epithelial cells (keratinocytes, endothelial cells, and mucosal epithelial cells) [9]–[16].
A crucial role of IL23/Th17 axis in the pathogenesis of psoriasis was proposed based on several recent studies: (i) dermal IL-17-producing CD4+ T cell and γδ T cell infiltrate as well as (ii) IL-17-producing CD8+ T cells within psoriatic epidermis; (iii) high expression levels of IL-23, IL-17, and IL-22 in psoriatic lesional skin; (iv) high serum levels of IL-22 and IL-17 that correlated with disease severity score [4], [5], [17]–[21]. Moreover, some of the Th17 pathway-related genes, IL-23A subunit, IL-23R, IL23B subunit, have been identified as psoriasis susceptibility genes [22]–[24]. Responses to tumor necrosis factor (TNF)α-blocking therapy and narrow-band ultraviolet B light therapy are correlated with the suppression of Th17 pathway [20], [25]–[27]. More recently, therapeutic approaches suppressing the IL-23/Th17 axis have proved highly effective in the treatment of psoriasis [28]–[33].
Keratinocytes are the key-responding cells to the psoriatic pro-inflammatory and pro-proliferative microenvironment since they bear receptors for key inflammatory cytokines, including IL-17 [12], [34], [35]. Surprisingly, although anti-IL-17 therapies showed astonishing clinical efficacy in improving psoriasis, in vitro studies of cultured monolayer keratinocytes identified a restricted number of genes induced by IL-17 [10], [12]. This discrepancy between the biological effects of neutralizing IL-17 and the IL-17–induced gene expression is even more pronounced considering other key-cytokines, such as TNFα and interferon (IFN)-γ, are capable of broadly regulating genomic expression in keratinocytes (502 and 3549 gene transcripts induced by TNFα and IFN-γ, respectively) [12]. Hence, in order to investigate this discrepancy further, we analyzed expression of C/EBPβ, a downstream signaling molecule of IL-17, and found that it localizes to the uppermost layers of the human epidermis in non-lesional and lesional psoriatic skin, indicating that perhaps only mature keratinocytes fully respond to IL-17 stimulation. We, therefore, sought to create in vitro conditions that could more closely mimic the in vivo epidermal architecture. Using Reconstructed Human Epidermis (RHE), a 3D epidermal skin model composed of a keratinocyte multi-layer supported by connective tissue, we evaluated the genomic response to IL-17.
Our results suggest IL-17 acts as a regulator of inflammatory gene expression preferentially in differentiated keratinocytes and induced genes include many psoriasis-related transcripts.
Results
Increased expression of the IL-17-specific transcription factor, C/EBPβ, by terminally differentiated keratinocytes
Normal human keratinocytes were found to constitutively bear the IL-17 receptor (IL-17R) and they are able to produce several IL-17-induced inflammatory and immune-related mediators implicated in psoriasis pathogenesis (e.g. IL-8, CCL20, S100A12, CXCL1, and CXCL2).
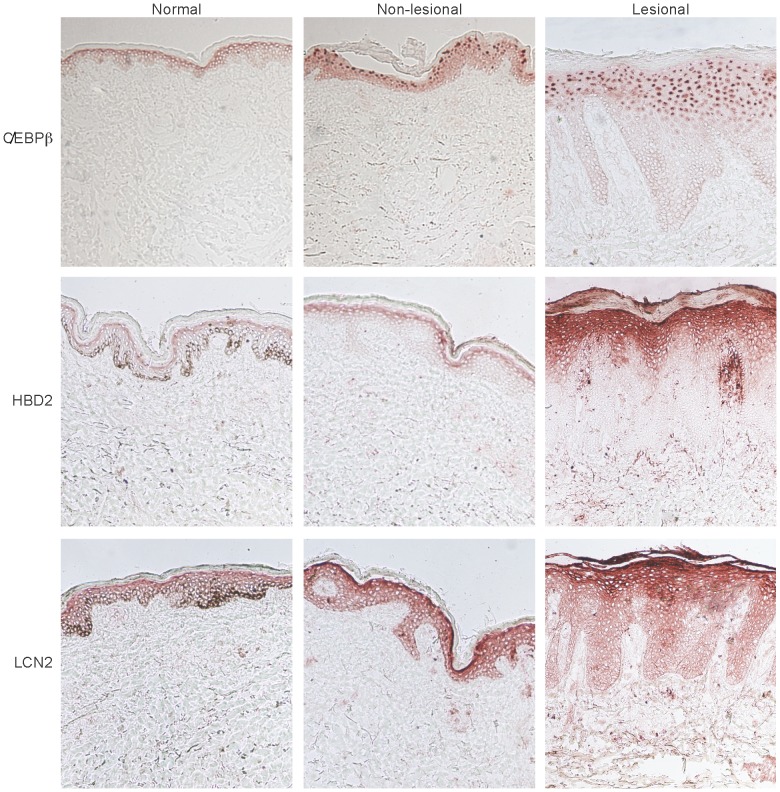
Unlike the constitutively expressed IL-17R, C/EBPβ, a critical transcriptional factor in the IL-17 signaling cascade, was detected only within more mature keratinocytes localized to the upper spinous-granular layers of the epidermis (Figure 1). C/EBPβ staining showed a nuclear pattern that was slightly detectable in the uppermost layers of the epidermis in normal skin, while it was very evident in the epidermis of non-lesional and lesional psoriatic skin. In addition to C/EBPβ, IL-17-regulated proteins, such as human β-defensin 2 (HBD2) and lipocalin 2 (LCN2), also localized to the outermost spinous-granular layers (Figure 1). Immunofluorescence staining further illustrated the co-localization of C/EBPβ and HBD2 in psoriatic skin (Figure S1).
Figure 1. IL-17-regulated C/EBPβ, human β-defensin 2, and lipocalin are expressed by terminally differentiated keratinocytes.
Immunohistochemistry for C/EBPβ (top), human β-defensin 2 (HBD2, middle), and lipocalin (LCN-2, bottom) in normal, non-lesional or lesional psoriatic skin showing predominant expression in the spinous-granular layer of the epidermis.
In order to verify whether this staining pattern was specific for C/EBPβ, we examined the expression patterns of additional epidermal transcriptional factors: RFX5, which is not usually overexpressed in lesional psoriatic skin, and STAT (Signal Transducers and Activators of Transcription) −1, a crucial mediator of IFN-γ signaling that has also been implicated in psoriasis pathogenesis. In contrast to the localization C/EBPβ to the spinous-granular layers of the epidermis, RFX5 was only localized to the basal layer of the epidermis (Figure S2). On the other hand, STAT1 was expressed by all viable keratinocytes as shown by pan-epidermal staining (Figure S2). These distinct staining patterns suggest that transcription factors may be activated in different types of KCs (e.g. basal KCs versus granular KCs).
As further confirmation of increased expression of C/EBPβ in differentiated keratinocytes, we accessed data from a new study that measured mRNAs in human dermis, basal epidermis, and suprabasal epidermis after laser-capture microdissection [36] C/EBPβ mRNA was increased about 4–fold in suprabasal epidermis compared to the basal layer (Figure S3).
Furthermore, attempting to fully differentiate monolayer in vitro normal human epidermal keratinocytes (NHEKs) using different calcium concentrations, we obtained a significantly higher C/EBPβ expression in high-calcium-treated NHEKs compared to low-calcium condition. Together with C/EBPβ, we tested the expression of some keratinocyte-terminal differentiation genes such as involucrin (IVL), transglutaminase-1 (TGM1), and filaggrin-2 (FLG2). Keratinocytes differentiated after culturing in high Ca++ plus 2% fetal bovine serum (FBS) medium, showed enhanced expression of C/EBPβ (p = 0.03) as well as high expression of FLG2, TGM1, and IVL mRNAs (p<0.002 for all) (Figure S4).
IL-17 induces a large number of genes in RHE
The fact that C/EBPβ and some IL-17-regulated proteins are expressed mainly by terminally differentiated KCs may explain why IL-17 induces such a limited number of genes in primary KCs cultured in vitro, as these KCs maintain a more undifferentiated, basal phenotype.
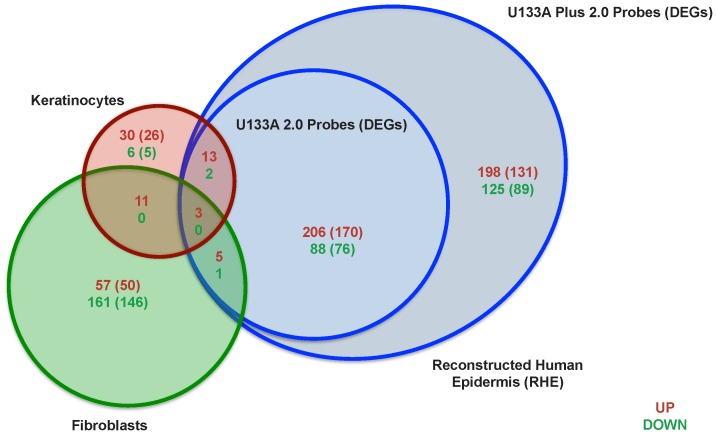
Therefore, in order to fully characterize the genes induced by IL-17 in KCs, we used a Reconstructed Human Epidermis (RHE) model, a full thickness epidermal skin structure, consisting of normal human-derived epidermal KCs organized into basal, spinous, granular, and cornified layers, analogously to those found in vivo. This epidermal structure is supported by connective tissue including fibroblasts. RHE was incubated with IL-17 (200 ng mL−1), IL-22 (200 ng mL−1), or IFN-γ (20 ng mL−1) for 48 hours and the cytokine-induced gene expression levels were measured with AffymetrixU133A Plus 2.0 arrays. In order to align the gene array analysis with the previously published data [12], we compared gene expression levels in IL-17-treated RHE versus untreated RHE using the selection criteria of fold change (FCH) >1.5 and false discovery rate (FDR)<0.1 that were used for the monolayer KC gene expression analysis [12]. In contrast to monolayer KCs [12] in which IL-17 altered the expression of only 65 probe-sets (60 unique differentially expressed gene, DEGs, using ENTREZ identifiers), in RHE, IL-17 induced the expression of many more genes (641 probe-sets, representing 490 DEGs) (Table S1), of which 425 probe-sets (322 DEGs) resulted upregulated and 216 probe-sets (168 DEGs) downregulated (Figure 2).
Figure 2. IL-17 induces a large number of genes in RHE.
Venn diagram illustrates the number of up-regulated (red) and down-regulated (green) probe-sets with the number of unique DEGs in parentheses of IL-17-treated keratinocytes, fibroblasts or RHE compared to the respective untreated conditions. U133A 2.0 arrays were used for KC and fibroblasts, while U133A Plus 2.0 arrays were used for RHE (FCH >1.5 and FDR<0.1 were used for all arrays). The additional semi-circle of RHE genes represents the probe-sets (DEGs) that were not present in the U133A 2.0 arrays.
To demonstrate that the IL-17 response in RHE did not merely represent the sum of the genes induced by IL-17 in fibroblasts and KCs, the RHE gene set was compared to gene sets obtained from in vitro cultured KCs or fibroblasts treated with IL-17. Differential expression induced by IL-17 in KCs or fibroblasts was compared to the respective untreated conditions. A subset of transcripts (323 probe-sets) was only detected in RHE with U133A plus 2.0 arrays, as these probe-sets were not present on the U133A 2.0 arrays, which were used for treated fibroblasts and KCs (Figure 2, semi-circle). Even when the analysis was restrictedly performed with U133A 2.0 arrays, the number of upregulated RHE gene transcripts (227 probe-sets) resulted about 3-fold and 4-fold higher than the number of upregulated gene detected in fibroblasts and KCs, respectively. There was very little overlap in the IL-17-regulated genes between the three conditions (9 DEGs were detected in both RHE and fibroblasts and 18 DEGs in both KCs and RHE). While there were some KC or fibroblast-specific genes, a large number of genes were uniquely expressed in RHE (Figure 2 and Table S1).
IL-17 induces the expression of C/EBPβ and many inflammatory genes in RHE
We also verified the expression of C/EBPβ in RHE model, eventually reflecting the in vivo condition. In the IL-17-treated RHE compared with untreated RHE, we detected an upregulation of C/EBPβ gene transcripts by polymerase chain reaction (PCR), and also, protein expression of C/EBPβ within the granular layer cells by immunohistochemistry (Figure S5). The expression of C/EBPβ induced by IL-17 in RHE correlates with the upregulation of Th17 pathway genes, such as IL23A, STAT3, and DEFB4 (Table 1 and Table S1). IL-17 also induced a number of anti-microbial peptides, including S100A12, S100A7A, SERPINB4, SERBINB3, which are highly expressed in psoriasis (Table 1) [37], as has been described previously [12]. Some other characteristic IL-17-regulated genes, such as IL8, IL6, CCL20, CXCL2, CXCL3, CXCL5, and LCN2 were up-regulated to a lesser extent (1.3–2.3 fold induction), but these elevations did not pass significance thresholds (Table S1). Interestingly, IL-1 family members, IL1A, IL1B, IL1F8, and IL1F9 were significantly activated by IL-17 in this model (Table 1). Signaling through these cytokines activates NF-κB, which may synergize with IL-17-induced C/EBPs to enhance transcription of many IL-17-regulated genes, further amplifying the inflammatory loops in psoriasis. In addition, there was up-regulation of cytokines, which limit NF-κB activation, such as IL1F5 and IL11, suggesting that IL-17 may also induce control mechanisms to prevent excessive inflammation [38], [39].
Table 1. Selected immune-related genes expressed in IL-17-treated RHE.
| Symbol | Description | FCH1 | FDR2 |
| DEFB4A | defensin, beta 4A | 24.37 | 0.005 |
| IL1F9 | interleukin 1 family, member 9 | 16.37 | 0.001 |
| IL19 | interleukin 19 | 12.82 | 0.007 |
| S100A7A | s100 calcium binding protein A7A | 12.70 | 0.011 |
| IL23A | interleukin 23, alpha subunit, p19 | 11.53 | 0.001 |
| SERPINB4 | serpin peptidase inhibitor, clade B, member 4 | 7.03 | 0 |
| TGFA | transforming growth factor, alpha | 5.04 | 0.035 |
| S100A12 | s100 calcium binding protein A12 | 4.85 | 0.013 |
| IL1F8 | interleukin 1 family, member 8 (eta) | 4.34 | 0.086 |
| C/EBPA | CCAAT/enhancer binding protein (C/EBP), alpha | 4.28 | 0.006 |
| IL1B | interleukin 1, beta | 3.94 | 0.013 |
| IL1B | interleukin 1, beta | 3.89 | 0.038 |
| RNASE7 | ribonuclease, RNase A family, 7 | 3.48 | 0.005 |
| IL11 | interleukin 11 | 3.42 | 0.045 |
| RNASE7 | ribonuclease, RNase A family, 7 | 3.23 | 0.013 |
| SERPINA1 | serpin peptidase inhibitor, clade A, member 1 | 3.08 | 0.058 |
| SERPINA1 | serpin peptidase inhibitor, clade A, member 1 | 2.88 | 0.089 |
| TGFA | transforming growth factor, alpha | 3.08 | 0.046 |
| IL1A | interleukin 1, alpha | 2.85 | 0.038 |
| IL1F5 | interleukin 1 family, member 5 (delta) | 2.68 | 0.01 |
| MAP3K9 | mitogen-activated protein kinase kinasekinase 9 | 2.58 | 0.044 |
| RNASE7 | ribonuclease, RNase A family, 7 | 2.30 | 0.031 |
| STAT3 | signal transducer and activator of transcription 3 | 2.18 | 0.021 |
| MAP2K3 | mitogen-activated protein kinase kinase 3 | 1.97 | 0.03 |
| SERPINB3 | serpin peptidase inhibitor, clade B, member 3 | 1.88 | 0.01 |
FCH, fold change;
FDR, false discovery rate.
IL-17 modulates keratinocyte mitogens and cell-cycle genes
Intriguingly, we observed an induction of cytokines that induce epidermal hyperplasia, including IL19 and heparin-binding EGF-like growth factor (HBEGF). Thus, IL-17 might indirectly stimulate KC proliferation and epidermal hyperplasia via paracrine cytokine secretion. There was also over-expression of cell cycle-related genes, such as cyclin E1 (CCNE1), cell division cycle associated 5 (CDCA5), and cell division cycle 25 homolog A (CDC25A). IL-17 likely stimulates epidermal keratinocyte differentiation, modulating the expression of KC differentiation genes including transglutaminase (TGM)-1, TGM3, small proline-rich protein 2C and 4 (SPRR2C, SPRR4), kallikrein-related peptidase 6, 10, and 13 (KLK6, KLK10, KLK13), and cornifelin (CNFN). Furthermore, IL-17 also modulates the expression of genes, including sphingomyelin phosphodiesterase 1, acid lysosomal (SMPD1) and serine palmitoyltransferase, long chain base subunit 2(SPTLC2), which are related to lamellar bodies and epidermal lipid barrier formation (Table 2).
Table 2. Selected genes involved in keratinocyte proliferation and differentiation.
| Symbol | Description | FCH1 | FDR2 | Biological Function |
| IL19 | interleukin 19 | 12.82 | 0.01 | Epidermal hyperplasia inducers |
| HBEGF | herparin-binding EGF-like growth factor | 3.29 | 0.03 | |
| SPRR2C | smallproline-rich protein 2C (pseudogene) | 27.07 | 0.00 | |
| TGM3 | transglutaminase 3 | 7.15 | 0.01 | |
| SPRR4 | smallproline-rich protein 4 | 5.21 | 0.01 | |
| KLK13 | kallikrein-related peptidase 13 | 4.75 | 0.10 | |
| KLK13 | kallikrein-related peptidase 13 | 4.57 | 0.08 | KC differentiation-related genes |
| TGM1 | transglutaminase 1 | 3.01 | 0.02 | |
| KLK6 | kallikrein-related peptidase 6 | 2.89 | 0.04 | |
| CNFN | cornifelin | 2.80 | 0.05 | |
| KLK10 | kallikrein-related peptidase 10 | 2.13 | 0.01 | |
| SMPD1 | sphingomyelinphosphodiesterase 1, acid lysosomal | 2.61 | 0.01 | Laminar bodies/extra-cellular lipids formation |
| SPTLC2 | serinepalmitoyltransferease, long chain base subunit 2 | 2.54 | 0.08 | |
| CCNE1 | cyclin E1 | 2.50 | 0.02 | |
| CDCA5 | cell division cycle associated 5 | 1.88 | 0.04 | Cell cycle-related genes |
| CDC25A | cell division cycle 25 homolog A (S. pombe) | 1.68 | 0.01 |
FCH, fold change;
FDR, false discovery rate.
RHE showed a specific genomic response to IL-17 stimulation
To verify whether the RHE genomic response to IL-17 stimulation was specific to IL-17, we stimulated RHE with IFN-γ or IL-22, cytokines thought to be involved in the psoriasis inflammatory cascade. The IL-22 response in KCs or RHE was minimal with only 35 probe-sets induced in RHE and 23 in KCs (data not shown). The response to IFN-γ was strong, with upregulation of 294 transcripts (Figure S6). As illustrated in Figure S6A, there was minimal overlap in the genes induced by IL-17 or IFN-γ. Furthermore, scatter plots comparing the genes induced in RHE versus in vitro cultured KCs showed that IL-17 induced a large number of genes only in RHE, while on the contrary, IFN-γ, induced a larger number of genes in monolayer KCs compared to RHE (Figure S6B), consistent with the constitutive expression of STAT1 by all KCs at all stages of differentiation (Figure S2).
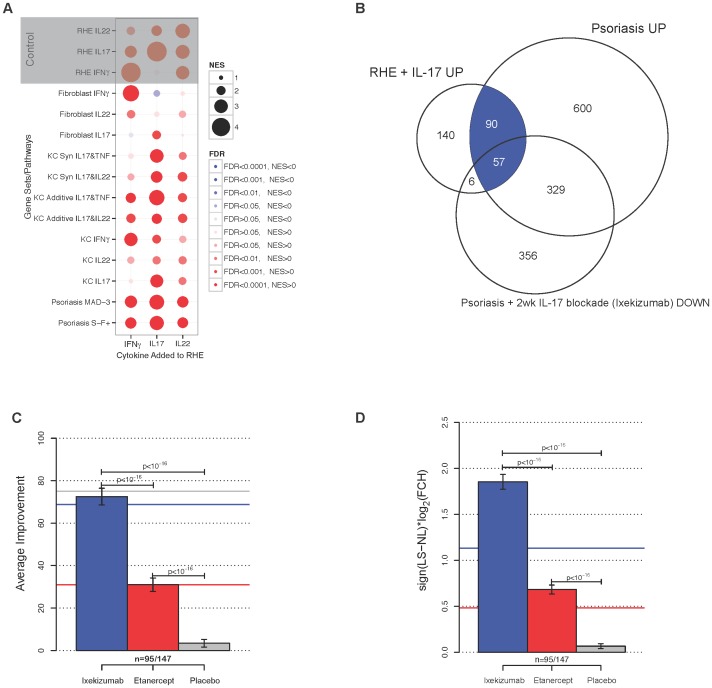
As confirmation of the specific RHE response to cytokine stimulation, we performed Reverse transcriptase-PCR (RT-PCR) (Figure S6C) testing some genes including IL23A, S100A7A, IL19, and IL1F8, which appeared to be upregulated by IL-17 in RHE, as listed in Table S1. We also correlated RHE gene expression induced by IL-17, IL-22, or IFN-γ with various gene sets using gene set enrichment analysis (GSEA) (Figure 3A). The phenotype induced by IL-17 on RHE (as defined by the FCH between IL17 treated vs. control), was strongly enriched of genes in the psoriasis transcriptomes, and, to a lesser strength, with additive and synergistic TNF-α/IL-17 KC genes. RHE profile response to IL-22 or IFN-γ showed lower normalized enrichment scores (NESs), compared to IL-17 stimulation.
Figure 3. Improvement of psoriasis with IL-17 blockade is associated with reduced expression of IL-17-induced RHE genes.
(A) Correlation between various gene sets and RHE gene profile response to cytokine stimulation (IL-17, IFN-γ, or IL-22) using GSEA. NES: normalized enrichment score; FDR: false discovery rate. (B) Venn diagram summarizing the number of DEGs among those in the psoriasis transcriptome or IL-17-treated RHE with improvement of at least 75% at two weeks post-ixekizumab. (C) Proportion of genes in IL-17-treated RHE that were differentially regulated in psoriasis (blue shaded area of (A)) and on the U133A 2.0 arrays (n = 95 out of 147 total DEGs which included additional genes only seen on the U133A Plus 2.0 arrays) that responded to treatment with IL-17 blockade (Ixekizumab, blue), TNF blockade (etanercept, red) or placebo (gray) at 2 weeks. Colored lines are changes in all MAD-3 psoriasis genes after both treatments. (D) The average change in expression (log2FCH) of RHE+IL-17 genes toward recovery with ixekizumab, etanercept, or placebo at 2 weeks.
In order to determine if this IL-17 response is a reasonable model for the in vivo role of IL-17 in psoriasis, we compared the transcriptome of RHE treated with IL-17 to a previously published MAD-3 psoriasis transcriptome [40]. As shown in Figure 3B, half of the genes induced by IL-17 in RHE are also included in the psoriasis transcriptome, indicating that the response of RHE to IL-17 may reflect gene activation common in psoriasis lesion.
We next examined to what extent IL-17 blockade suppressed the genes highly induced by IL-17 in RHE. We hypothesized that if the genes regulated by IL-17 in the RHE model are relevant to psoriasis, then there should be a corresponding reduction in the same genes after therapeutic blockade of IL-17. Therefore, we compared the transcriptome of IL-17-treated RHE with the transcriptome of lesional skin two weeks after treatment with ixekizumab, an anti-IL-17 antibody [29] (Figure 3B). We have previously defined a “residual disease genomic profile,” which included the genes that do not improve by at least 75% compared to NL levels by the end of successful treatment [40]. In the recent ixekizumab study, 72.5% of the 95 genes in the IL-17 treated RHE model that were also part of the MAD-3 psoriasis transcriptome improved by over 75% at two weeks post-treatment compared with 68% of all psoriasis genes (Figure 3C) [41]. For comparison, only 31% of either the RHE genes or the psoriasis genes had recovered with etanercept at the same time point. There was a 3.45 FCH (1,79 log2) average expression of these RHE genes towards recovery with ixekizumab at two weeks, compared with 1.62 FCH (0.70 log2) for etanercept and no change (1.01 FCH, 0.01 log2) with placebo (Figure 3D). Overall, these results suggest that IL-17-induced genes in the RHE model coincide with genes that are suppressed in vivo by blocking IL-17 signaling with a neutralizing antibody to IL-17A.
Discussion
Previous experiments have shown a limited number of genes induced by IL-17 in KCs despite ubiquitous epidermal expression of the IL-17 receptor [10], [12], [42]. In this study, we found that the distinct expression pattern of C/EBPβ, a crucial IL-17-related transcriptional factor, in the upper spinous-granular layers composed of more differentiated keratinocytes, may explain why undifferentiated, monolayer KCs respond less to IL-17 stimulation. Thus, in order to more fully investigate the genomic effects of IL-17 on KCs, we used a full-thickness skin model that more closely mimicked epidermal architecture and KC differentiation process. Using RHE, we identified a much larger number of genes induced by IL-17.
IL-17 elicits its pro-inflammatory effects in RHE, stimulating the expression of several genes including IL23A, IL1β, and IL36B (IL1F8) that have been implicated in psoriasis pathogenesis and found to be over-expressed in psoriatic lesional skin. The production of inflammatory cytokines by epidermal KCs likely perpetuates and sustains skin inflammation driven by T cells, especially given the essential roles of IL-23 and IL-1β in stimulating IL-17 production. Moreover, as key-regulator of innate immunity, IL-17 was shown to modulate antimicrobial peptides (AMPs) such as S100A7A, DEFB4, RNASE7, and Serpins A1, B3, and B4. Johnston et al. [43], demonstrated that IL-1F8 significantly induced several AMPs in an RHE model, including LCN2, defensins, HBD-2 and HBD-3, CAMP, elafin, serpinB1, and IL-8, and thus, the IL-1F8 expression induced by IL-17 stimulation may lead to a feed-forward loop amplifying AMP expression, which represents a distinct feature of lesional psoriatic skin.
Many abnormalities in keratinocyte differentiation are highly evident in psoriasis, including the expansion of the spinous-granular layer of the epidermis and alterations in terminal differentiation of KCs leading to a defective epidermal barrier with increased transepidermal water loss. Expression of the IL-17-specific transcription factor, C/EBPβ, in the spinous-granular layer may implicate IL-17 in these processes. Along these lines, Rizzo et al. [44], showed that IL-17A, like IL-22, was a downstream mediator of the changes induced by IL-23 injection in murine skin, and that both of these Th17 cytokines are necessary to produce IL-23–mediated psoriasis-like skin pathology. Indeed, the blockade of IL-17 or IL-22 in this model inhibits epidermal hyperplasia, indicating that either IL-17 or IL-22 can increase keratinocyte proliferation. Accordingly, in RHE, IL-17 induced IL19, a pro-proliferative cytokine belonging to IL-10 family, that is overexpressed in lesional psoriatic skin and has been implicated in epidermal hyperplasia [45]. Moreover, IL-17 induced several genes involved in terminal differentiation, including S100 proteins, S100A12 and S100A7A, and transglutaminases, TGM1 and TGM3, suggesting a role for IL-17 in this process. Additionally, the up-regulation of cell cycle-related genes such as CCNE1, CDCA5, and CDCA25A, suggests a direct contribution of IL-17 to epidermal KC proliferation. Therefore, the results of the current study suggest that IL-17 may have a much broader role than previously thought in stimulating the epidermal changes seen in psoriasis.
We defined the in vivo correlations of the novel gene set induced by IL-17 in RHE by analyzing the results of a recent clinical trial in psoriasis patients with a potent IL-17A antagonist, ixekizumab. IL-17 blockade is highly effective in reversing psoriasis, impressively resolving clinical, histological, and genomic facets of the disease [29]. Our results suggest that the genes regulated by IL-17 in RHE are likely relevant to the effects of IL-17 in psoriasis, since ixekizumab strongly suppressed these “RHE” genes in psoriasis patients treated in vivo with this IL-17 antagonist.
Psoriasis is thought to develop and be maintained as a result of cooperative efforts of several T cell cytokines in addition to IL-17, namely IFN-γ and IL-22, which augments cellular recruitment through chemokine induction and stimulates epidermal hyperplasia, respectively. However, the complete reversal of the psoriasis phenotype by ixekizumab and other IL-17 antagonists [29], suggests that this model may need to be revised to account for the centrality of IL-17 in driving the inflammatory circuits in psoriasis. While epidermal acanthosis is not highly evident in histological sections of IL-17 treated RHE, many cyclins associated with increased cell proliferation are elevated in IL-17 treated cultures and growth factors associated with keratinocyte proliferation, e.g. IL-19, TGFα, HBEGF, are increased. Thus, IL-17 might contribute to epidermal hyperplasia in vivo through indirect effects on keratinocytes. In addition, leukocytes that are recruited by cytokines induced in keratinocytes by IL-17 could also be important in producing epidermal hyperplasia in vivo, either through elaborated interleukins or by migration through the epithelium [38], [43], [45]–[47]. Furthermore, the induction of genes such as CCL20 (a chemoattractant for CCR6-bearing cells, such as Th17 cells), IL1β, and IL23 (both involved in the Th17 differentiation process) suggests that IL-17 may create feed-forward loops that perpetuate Th17-polarized inflammatory processes. Along these lines, the induction of anti-microbial peptides may also sustain inflammation as IL-17-induced cathelicidin (LL-37) has been shown to complex with nucleic acids to activate DC stimulation of broader and more non-specific T cell activation [48], [49]. Overall, these data strongly implicate IL-17 as a central player in the pathogenic mechanism underlying the pathogenesis of psoriasis. Additionally, this study provides insight into the IL-17-induced expression of inflammatory genes belonging to damage-associated molecular pattern molecules (DAMP) or inflammasome that could be potentially identified as novel therapeutic targets. Indeed, the blockade of these IL-17-downstream genes may represent a further step in the therapeutic strategy to be more selective in inhibiting the inflammatory cascade.
IL-17 is most closely associated with the pathogenesis of psoriasis, but it could also contribute to other inflammatory skin disease, e.g., atopic dermatitis (AD). Significant IL-17 expression is seen in skin lesions of intrinsic AD (low IgE sub-type) and increased expression of several S100A genes (S100A7, S100A8, S100A9, S100A12), that are synergistically regulated by IL-17 and IL-22 is also detected [50].
Additionally, relative to normal skin, AD lesions have increased expression of AMPs (LCN, β-defensins, etc.) that are IL-17-regulated, although the measured levels are much lower than seen in psoriasis. Since the range of products regulated by IL-17 in AD are largely overexpressed in the upper spinous and granular layers of AD epidermis, the selective expression of C/EBPβ in more differentiated KCs is also likely to be relevant to AD pathogenesis.
Materials and Methods
Skin samples
Skin punch biopsies (6 mm diameter) were obtained from normal volunteers and patients with moderate-to-severe chronic plaque psoriasis under a Rockefeller University Institutional Review Board-approved protocol. Written, informed consent was obtained from all subjects, and adhered to the Declaration of Helsinki Principles.
The biopsy specimens were frozen in OTC (Sakura, Torrance, CA, U.S.A.) and stored at −80°C for immunohistochemistry and immunofluorescence.
Immunohistochemistry and Immunofluorescence
Frozen tissue sections of psoriatic lesional, non-lesional, and normal skin were stained using standard procedures for both IHC and IF as previously described [51].
Immunohistochemistry
Staining was performed with antibody targeting C/EBPβ, LCN2, HBD2, STAT1, RFX5 (Table S2). According to the primary antibody species, either biotin-labeled horse anti-mouse antibodies (Vector Laboratories, Burlingame, CA, U.S.A.) or biotin-labeled rabbit anti-goat antibodies (Vector Laboratories, Burlingame, CA, U.S.A.) were amplified with avidin-biotin complex (Vector Laboratories) and developed using chromogen 3-amino-9-ethylcarbazole (Sigma Aldrich, St Louis, MO, U.S.A.). For the staining in the RHE, a black line denotes the dermoepidermal junction. Appropriate negative controls were used.
Immunofluorescence
Frozen skin sections from non-lesional and lesional psoriasis patients were fixed with acetone and blocked in 10% normal chicken serum (Vector Laboratories) for 30 minutes. Primary antibodies for C/EBPβ and HBD2 (Table S2) were incubated overnight at 4°C and amplified with the appropriate secondary antibody goat anti-mouse IgG1 conjugated to Alexa Fluor 488 and chicken anti-goat Alexa Flour 594 (Invitrogen, Eugene, OR) respectively, for 30 minutes.
IF images were acquired using the appropriate filters of a Zeiss Axioplan 2 wide-field fluorescence microscope (Thornwood, NY) with a Plan Neofluar 20×0.7 numerical aperture lens and a Hamamatsu Orca Er-cooled charge-coupled device camera (Bridgewater, NJ), controlled by the METAVUE software (MDS Analytical Technologies, Downington, PA). Images in each figure are presented both as single-color stains (green and red) located above the merged image, so that localization of two markers on similar or different cells can be appreciated. Cells that co-express the two markers in a similar location are yellow in color. A white line denotes the dermoepidermal junction. Dermal collagen fibers gave green autofluorescence, and antibodies conjugated with a fluorochrome often gave background epidermal fluorescence.
Cell cultures
We cultivated primary human skin fibroblast lines (HF40 and HFF-1) (n = 2 each) that were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultivated in Dulbecco's minimum essential medium supplemented with 10% fetal calf serum and, when confluent, medium was supplemented with or without recombinant human (rh)-IL-17 (R&D System, Minneapolis, MN) of 200 ng ml−1 (same IL-17 source and concentration used in prior experiments with human keratinocytes) [10], [12]. After 24-hour incubation, fibroblasts were harvested for further analyses.
We also cultivated NHEKs obtained from PromoCell, in the Keratinocyte Growth Medium 2 supplemented with 0.004 ml/ml BPE, 0.125 ng/ml EGF, 5 ug/ml Insulin, 0.33 ug/ml Hydrocortisone, 0.39 ug/ml Epinephrine, 10 ug/ml Transferrin, and 0.06 mM Ca++ (all items purchased from PromoCell GmbH, Heidelberg, Germany). The experiment was performed in triplicate.
Once 70–80% confluent, the medium was changed with full media containing 0.06 mM Ca++, 1.2 mM Ca++, or 1.2 mM Ca++ plus 2.0% FBS, for 24 and 48 hours before harvesting for other analyses.
Human full-thickness skin model (RHE)
Full-thickness human skin models (MatTek Corp., Ashland, MA, U.S.A.) (n = 4) were incubated in assay media (MatTek Corp.) supplemented with or without rh-IL-17 (R&D Systems, Minneapolis, MN, U.S.A.) 200 ng mL−1, rh-IL-22 (Peprotech Inc., Rocky Hill, NJ, U.S.A.) 200 ng mL−1 200, or rh-IFN-γ (R&D Systems, Minneapolis, MN, U.S.A.) 20 ng mL−1, for 2 days. On day 2, the skin models were harvested for microarray analyses. The same concentrations used for treating in vitro monolayer keratinocytes were applied for RHE, as they were proved effective in gene modulation as previously described by our group [12].
Gene array
RNA was extracted from RHE using the RNeasy Mini Kit (Qiagen, Valencia, CA, U.S.A.) and on-column DNAse digestion (RNAse-free DNAse Set, Qiagen), for either gene array or RT-PCR procedures.
For each Affymetrix genechip, 4 µg total RNA was reverse transcribed, amplified, and labeled as described previously using BioArray High Yield RNA Transcription Labeling Kit (Enzo Biochem Inc., Farmingdale, NY, U.S.A.) [52]. Fifteen micrograms of the biotinylated cRNA were then hybridized to Affymetrix Human Genome U133A Plus 2.0 Array (Affymetrix, Santa Clara, CA, U.S.A.). The chips were washed, stained with streptavidin-phycoerythin, and scanned with a Hewlett-Packard HP GeneArray Scanner (Hewlett-Packard, Palo Alto, CA, U.S.A.).
Reverse transcriptase–polymerase chain reaction
To perform RT-PCR, the RNA extracted from fibroblasts and RHE model was processed using EZ PCR core reagents, primers, and probes (Applied Biosystems, Foster City, CA) as previously published [53], whilst total RNA was extracted from NHEKs using RNeasy micro kit (QIAGEN Inc, Valencia, CA).
The following sequences of primers and probes were used in this study: IL-19 (Hs00604655_m1), C/EBPβ (Hs 00270923_s1), S100A7A (Hs00752780_s1), IL-1F8 (Hs00758166_m1), IL-23A (Hs00372324_m1). The data were analyzed by the Applied Biosystems PRISM 7700 software (Sequence Detection Systems, ver. 1.7) and normalized to human acidic ribosomal protein (hARP) housekeeping gene (primer sequences Forward: CGCTGCTGAACATGCTCAA, Reverse: TGTCGAACACCTGCTGGATG, Probe: 6-FAM-TCCCCCTTCTCCTTTGGGCTGG- TAMRA).
Statistical analysis
Preprocessing and statistical analysis was conducted in R (http://www.rproject.org/).
Microarray GeneChip CEL data files were scanned for spatial artifacts using Harshlight package (http://asterion.rockefeller.edu/Harshlight/index2.html) [54]. Expression values were pre-processed using GCRMA algorithm [55]. ArrayQualityControl was used for standard QC.
Probes with at least one sample showing expression values greater than 3 and SD >0.1 were selected for further analyses.
Significance of cytokine induction in RHE gene expression was assessed by using a moderated paired t-test, comparing untreated RHE with cytokine-treated RHE. Subsequently, p-values were adjusted using Benjamini-Hochberg correction, which controls the FDR.
Genes were considered DEGs if FDR>0.1 and FCH>1.5, accordingly to the same cut-offs used for gene array data derived from previous IL-17/keratinocyte experiments [12]. IL-17 effects on gene expression was evaluated in RHE and compared with keratinocyte [12] and fibroblast responses to IL-17 exposure. These data are available in the Gene Expression Omnibus (GEO) repository under accession No. GSE52361.
To assess the biological meaning of IL-17-induced RHE genes in psoriasis, a comparison with the MAD-3 psoriasis transcriptome (defined by a meta-analysis of 3 published transcriptomes) [40] was performed. To evaluate the effect of antipsoriatic therapies on RHE genes induced by IL-17, previously published genomic responses to different therapeutic agents, namely ixekizumab [29] and etanercept [27], were analyzed. Comparisons included only IL17-induced RHE probe sets in hgu133a2 chips, since the gene array data for both treatments were performed using the same kind of chips. For those probes, whose expression differed after 2-week treatment compared to baseline, the mean variation was calculated. Similarly, the improvement at 2 weeks of treatment under both treatments was summarized, meaning as improvement the treatment effect divided by the level of disregulation at baseline, which was measured as LS vs NL differences estimated through the MAD-3 transcriptome.
Gene Set Enrichment Analysis (GSEA) was used to evaluate the enrichment of various gene sets in the gene response profile of the RHE treated with IL-17, IL-22, or IFN-γ [56].
Supporting Information
Co-localization of C/EBPβ and HBD2 in psoriatic skin. Immunofluorescence staining for IL-17 transcription factor, C/EBPβ (green), and downstream target, human β-defensin 2 (HBD2, red), in non-lesional (left) or lesional (right) psoriatic skin. Both proteins are localized to the spinous-granular layer, which is especially evident in non-lesional skin.
(TIFF)
Distinct staining patterns of epidermal transcription factors. Immunohistochemistry for transcription factors, RFX-5 and STAT1, in normal, non-lesional, and lesional psoriatic skin. RFX5 stains basal keratinocytes, while STAT1 has pan-epidermal expression.
(TIFF)
C/EBPβ gene expression in normal skin. C/EBPβ gene expression in reticular dermis, basal epidermis, and suprabasal epidermis, obtained by laser capture microdissection of normal human skin (Gulati et al., 2013).
(TIFF)
Expression levels of terminal differentiation genes in monolayer in vitro NHEKs. Increased expression of terminal differentiation genes was detected in high-calcium-treated NHEKs: (A) C/EBPβ, (B) FLG2, (C) TGM1, (D) IVL. Differences with low-calcium condition were statistically significant. Gene expression was normalized by hARP.
(TIFF)
C/EBPβ expression in RHE model. Differential C/EBPβ expression in untreated RHE (A) versus IL-17-treated RHE (B). Black line shapes the dermoepidermal junction, while arrows mark the light staining displayed in differentiated keratinocytes localized in the upper layers of the epidermis. (C) Detection of C/EBPβ gene expression in untreated and IL-17-treated-RHE by PCR.
(TIFF)
IL-17, IL-22, and IFN-γ induce unique RHE gene signatures. (A) Venn diagram illustrates the number of probe-sets regulated in RHE by IL-17, IL-22, or IFN-γ treatment. (B) Scatter plots comparing the genes induced in RHE versus in vitro cultured KCs showing that IL-17 induced a large number of genes only in RHE, while IFN-γ induced a larger number of genes in monolayer KCs compared to RHE. (C) Gene expression levels of some IL-17 signature genes detected by RT-PCR, confirmatory of the gene array results.
(TIFF)
Differentially expressed genes in IL-17-treated keratinocytes and/or RHE and/or fibroblasts.
(PDF)
Antibodies used for immunohistochemistry and immunofluorescence.
(DOCX)
Acknowledgments
We thank Artemis Khatcherian and Inna Cueto for the precious support in immunohistochemical procedures. Andrea Chiricozzi is supported by Leo-Pharma Research Foundation Silver Award 2012.
Funding Statement
These authors have no support or funding to report.
References
- 1. Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG (2013) The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol 34: 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, et al. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–32. [DOI] [PubMed] [Google Scholar]
- 3. Park H, Li Z, Yang XO, Chang SH, Nurieva R, et al. (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6: 1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai Y, Shen X, Ding C, Qi C, Li K, et al. (2011) Pivotal role of dermal IL-17-producing gamma/delta T cells in skin inflammation. Immunity 35: 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, et al. (2009) IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukocyte Biol 86: 435–43. [DOI] [PubMed] [Google Scholar]
- 6. Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, et al. (2011) Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 187: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaffen SL (2009) Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9: 556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, et al. (2004) Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem 279: 2559–67. [DOI] [PubMed] [Google Scholar]
- 9. Albanesi C, Cavani A, Girolomoni G (1999) IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol 162: 494–502. [PubMed] [Google Scholar]
- 10. Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, et al. (2011) Integrative Responses to IL-17 and TNF-alpha in Human Keratinocytes Account for Key Inflammatory Pathogenic Circuits in Psoriasis. J Invest Dermatol 131: 677–87. [DOI] [PubMed] [Google Scholar]
- 11. Laan M, Lotvall J, Chung KF, Linden A (2001) IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol 133: 200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, et al. (2008) Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 159: 1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickens SR, Volin MV, Mandelin AM 2nd, Kolls JK, Pope RM, et al. (2010) IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol 184: 3233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roussel L, Houle F, Chan C, Yao Y, Berube J, et al. (2010) IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol 184: 4531–7. [DOI] [PubMed] [Google Scholar]
- 15. Ruddy MJ, Shen F, Smith JB, Sharma A, Gaffen SL (2004) Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J Leukocyte Biol 76: 135–44. [DOI] [PubMed] [Google Scholar]
- 16. Shen F, Ruddy MJ, Plamondon P, Gaffen SL (2005) Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukocyte Biol 77: 388–99. [DOI] [PubMed] [Google Scholar]
- 17. Arican O, Aral M, Sasmaz S, Ciragil P (2005) Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, et al. (2004) Increased Expression of Interleukin 23 p19 and p40 in Lesional Skin of Patients with Psoriasis Vulgaris. J Exp Med 199: 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, et al. (2008) Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 128: 1207–11. [DOI] [PubMed] [Google Scholar]
- 20. Zaba L, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suárez-Fariñas M, et al. (2007) Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 204: 3183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caproni M, Antiga E, Melani L, Volpi W, Del Bianco E, et al. (2009) Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol 29: 210–4. [DOI] [PubMed] [Google Scholar]
- 22. Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, et al. (2007) Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet 122: 201–6. [DOI] [PubMed] [Google Scholar]
- 23. Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, et al. (2007) A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 80: 273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nair RP, Ruether A, Stuart PE, Jenisch S, Tejasvi T, et al. (2008) Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol 128: 1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson-Huang LM, Suarez-Farinas M, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, et al. (2010) Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol 130: 2654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piskin G, Tursen U, Sylva-Steenland RM, Bos JD, Teunissen MB (2004) Clinical improvement in chronic plaque-type psoriasis lesions after narrow-band UVB therapy is accompanied by a decrease in the expression of IFN-gamma inducers – IL-12, IL-18 and IL-23. Exp Dermatol 13: 764–72. [DOI] [PubMed] [Google Scholar]
- 27. Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, et al. (2009) Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol 124: 1022–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, et al. (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2: 52ra72. [DOI] [PubMed] [Google Scholar]
- 29. Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, et al. (2012) IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol 130: 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, et al. (2012) Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366: 1190–9. [DOI] [PubMed] [Google Scholar]
- 31. Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, et al. (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371: 1665–74. [DOI] [PubMed] [Google Scholar]
- 32. Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, et al. (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371: 1675–84. [DOI] [PubMed] [Google Scholar]
- 33. Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, et al. (2012) Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366: 1181–9. [DOI] [PubMed] [Google Scholar]
- 34. Scheynius A, Fransson J, Johansson C, Hammar H, Baker B, et al. (1992) Expression of interferon-gamma receptors in normal and psoriatic skin. J Invest Dermatol 98: 255–8. [DOI] [PubMed] [Google Scholar]
- 35. Trefzer U, Brockhaus M, Loetscher H, Parlow F, Kapp A, et al. (1991) 55-kd tumor necrosis factor receptor is expressed by human keratinocytes and plays a pivotal role in regulation of human keratinocyte ICAM-1 expression. J Invest Dermatol 97: 911–6. [DOI] [PubMed] [Google Scholar]
- 36. Gulati N, Krueger JG, Suárez-Fariñas M, Mitsui H (2013) Creation of Differentiation-Specific Genomic Maps of Human Epidermis Through Laser Capture Microdissection. J Invest Dermatol. 133: 2640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morizane S, Gallo RL (2012) Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 39: 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, et al. (2007) Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med 204: 2603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trepicchio WL, Ozawa M, Walters IB, Kikuchi T, Gilleaudeau P, et al. (1999) Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest 104: 1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tian S, Krueger JG, Li K, Jabbari A, Brodmerkel C, et al. (2012) Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PloS One 7: e44274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG (2011) Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol 131: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nograles KE, Suarez-Farinas M, Shemer A, Fuentes-Duculan J, Chiricozzi A, et al. (2010) Atopic dermatitis keratinocytes exhibit normal T(H)17 cytokine responses.J Allergy Clin Immunol 125: 744–6, 6 e1–6 e2. [DOI] [PMC free article] [PubMed]
- 43. Johnston A, Xing X, Guzman AM, Riblett M, Loyd CM, et al. (2011) IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol 186: 2613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, et al. (2011) IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol 186: 1495–502. [DOI] [PubMed] [Google Scholar]
- 45. Sa SM, Valdez PA, Wu J, Jung K, Zhong F, et al. (2007) The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178: 2229–40. [DOI] [PubMed] [Google Scholar]
- 46. Muhr P, Zeitvogel J, Heitland I, Werfel T, Wittmann M (2011) Expression of interleukin (IL)-1 family members upon stimulation with IL-17 differs in keratinocytes derived from patients with psoriasis and healthy donors. Br J Dermatol 165: 189–93. [DOI] [PubMed] [Google Scholar]
- 47. Tohyama M, Hanakawa Y, Shirakata Y, Dai X, Yang L, et al. (2009) IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur J Immunol 39: 2779–88. [DOI] [PubMed] [Google Scholar]
- 48. Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, et al. (2009) Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 206: 1983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, et al. (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449: 564–9. [DOI] [PubMed] [Google Scholar]
- 50. Suárez-Fariñas M, Dhingra N, Gittler J, Shemer A, Cardinale I, et al. (2013) Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 132: 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA (2007) Normal human dermis contains distinct populations of CD11cBDCA-1 dendritic cells and CD163FXIIIA macrophages. J Clin Invest 117: 2517–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou X, Krueger JG, Kao MC, Lee E, Du F, et al. (2003) Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol Genomics 13: 69–78. [DOI] [PubMed] [Google Scholar]
- 53. Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, et al. (2005) Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci U S A. 102: 2075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suárez-Fariñas M, Pellegrino M, Wittkowski KM, Magnasco MO (2005) Harshlight: a “corrective make-up” program for microarray chips. BMC Bioinformatics 6: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F (2004) A Model-Based Background Adjustment for Oligonucleotide Expression Arrays. J Am Stat Assoc 99: 909–917. [Google Scholar]
- 56. Suarez-Farinas M, Lowes MA, Zaba LC, Krueger JG (2010) Evaluation of the psoriasis transcriptome across different studies by gene set enrichment analysis (GSEA). PLoS One 5: e10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Co-localization of C/EBPβ and HBD2 in psoriatic skin. Immunofluorescence staining for IL-17 transcription factor, C/EBPβ (green), and downstream target, human β-defensin 2 (HBD2, red), in non-lesional (left) or lesional (right) psoriatic skin. Both proteins are localized to the spinous-granular layer, which is especially evident in non-lesional skin.
(TIFF)
Distinct staining patterns of epidermal transcription factors. Immunohistochemistry for transcription factors, RFX-5 and STAT1, in normal, non-lesional, and lesional psoriatic skin. RFX5 stains basal keratinocytes, while STAT1 has pan-epidermal expression.
(TIFF)
C/EBPβ gene expression in normal skin. C/EBPβ gene expression in reticular dermis, basal epidermis, and suprabasal epidermis, obtained by laser capture microdissection of normal human skin (Gulati et al., 2013).
(TIFF)
Expression levels of terminal differentiation genes in monolayer in vitro NHEKs. Increased expression of terminal differentiation genes was detected in high-calcium-treated NHEKs: (A) C/EBPβ, (B) FLG2, (C) TGM1, (D) IVL. Differences with low-calcium condition were statistically significant. Gene expression was normalized by hARP.
(TIFF)
C/EBPβ expression in RHE model. Differential C/EBPβ expression in untreated RHE (A) versus IL-17-treated RHE (B). Black line shapes the dermoepidermal junction, while arrows mark the light staining displayed in differentiated keratinocytes localized in the upper layers of the epidermis. (C) Detection of C/EBPβ gene expression in untreated and IL-17-treated-RHE by PCR.
(TIFF)
IL-17, IL-22, and IFN-γ induce unique RHE gene signatures. (A) Venn diagram illustrates the number of probe-sets regulated in RHE by IL-17, IL-22, or IFN-γ treatment. (B) Scatter plots comparing the genes induced in RHE versus in vitro cultured KCs showing that IL-17 induced a large number of genes only in RHE, while IFN-γ induced a larger number of genes in monolayer KCs compared to RHE. (C) Gene expression levels of some IL-17 signature genes detected by RT-PCR, confirmatory of the gene array results.
(TIFF)
Differentially expressed genes in IL-17-treated keratinocytes and/or RHE and/or fibroblasts.
(PDF)
Antibodies used for immunohistochemistry and immunofluorescence.
(DOCX)