Abstract
To find out the most easily identifiable and anatomically consistent landmark for identification of facial nerve during parotid surgery. Ten cadaveric dissections and ten live parotid surgeries for different types of parotid tumours were done. Cadaveric dissection was performed in the Department of Anatomy and the surgeries were done in the Department of ENT and Head and Neck surgery of R. G. Kar Medical College of Kolkata. The distance of the facial nerve trunk from three most commonly used landmarks (viz., tympanomastoid suture, tragal pointer and posterior belly of digastric muscle) was measured in both cadaver and live patients. The ease of identification of the nerve trunk using each of the landmarks, particularly during live surgery was also assessed. The mean distance of the tympanomastoid suture from the facial nerve trunk was 3.5 mm (cadaver) and 3.87 mm (live surgery), the tragal pointer was found to be at a mean distance of 16.61 mm (cadaver) and 16.36 mm (live surgery) and in case of the posterior belly of digastric muscle it was 7.41 mm (cadaver) and 8.03 mm (live surgery). During live surgery the posterior belly of digastric was found to be the most easily identifiable landmark with a consistent anatomical relationship with the nerve trunk. The posterior belly of digastric muscle is the most easily identifiable and a very consistent landmark for facial nerve dissection during parotidectomy. When supplemented with the tragal pointer, accuracy in identifying the facial nerve trunk is very high, thereby avoiding inadvertent injury to the nerve trunk.
Keywords: Facial nerve, Parotidectomy, Posterior belly of digastric, Tragal pointer
Introduction
Parotidectomy is basically an anatomical dissection. Identification of the facial nerve trunk is essential during surgery of the parotid gland because facial nerve injury is the most daunting potential complication of parotid gland surgery owing to the close relation between the gland and the extratemporal course of facial nerve. There are two approaches to identify the facial nerve trunk during parotidectomy—conventional antegrade dissection of the facial nerve, and retrograde dissection. Numerous soft tissue and bony landmarks have been proposed to assist the surgeon in the early identification of this nerve. Most commonly used anatomical landmarks to identify facial nerve trunk are stylomastoid foramen, tympanomastoid suture (TMS), posterior belly of digastric (PBD), tragal pointer (TP), mastoid process and peripheral branches of the facial nerve. Use of so many landmarks to identify the facial nerve trunk points to the fact that there is lack of consensus regarding the safety and reliability of each of these landmarks.
Aims and Objectives
The aim of the cadaveric dissection was to dissect all the landmarks of facial nerve that has been described in the literature and study their respective anatomical relationship with the facial nerve. The three most easily identifiable and anatomically constant landmarks were selected for demonstration in live surgery and the findings were corroborated with that of the anatomical dissections.
Materials and Methods
Ten fresh cadaver dissections and ten live parotid surgeries for different types of parotid tumours were done in the present study. Standard incision (Modified Blair’s) for parotidectomy was used in both cadaver and live surgery. We tried to identify and demonstrate all the landmarks for the facial nerve and study their respective relations to the facial nerve trunk in cadaver. The distance of the individual landmark from the facial nerve trunk was measured with the help of slide callipers. In case of the bony landmarks the measurement was taken from the bone itself; in case of the posterior belly of digastric muscle the distance was measured from the insertion of the muscle into the mastoid process. In live surgery for different pathological conditions of parotid gland, the three most easily identifiable and anatomically constant landmarks were selected and their respective distance from the facial nerve trunk was measured. Live surgical data was compared with that of the anatomical dissection.
Results
In the cadaver dissection the mean distance of the facial nerve from the tympanomastoid suture line was 3.5 mm (range 2.5–4.5 mm), in case of tragal pointer the mean was 16.61 mm (14–21 mm) and posterior belly of digastric was found to be at a mean distance of 7.5 mm from the trunk of the facial nerve (range 6–9.5 mm) (Table 1]; Figs. 1, 2). In the live surgical patient group, the mean distance of the tympanomastoid suture from the facial nerve was 3.87 mm (2–6 mm)—a little more than that in the cadaveric study. The tragal pointer was at an average distance of 16.36 mm the range being from 13.6 to 19 mm. The mean separation of the posterior belly of digastrics muscle from the nerve trunk during surgery was 8.03 mm (range 6–11.5 mm) (Table 2; Figs. 3, 4).
Table 1.
Cadaver study: distance of facial nerve trunk from different anatomic landmarks
| Cadaver no | Tympanomastoid suture (in mm) | Tragal pointer (in mm) | Posterior belly of digastric (in mm) |
|---|---|---|---|
| 1 | 2.5 | 14 | 6.3 |
| 2 | 3 | 18 | 7 |
| 3 | 3 | 15.5 | 8 |
| 4 | 2.5 | 18 | 6 |
| 5 | 3.7 | 16 | 7.5 |
| 6 | 2.8 | 14.6 | 6.8 |
| 7 | 3 | 16 | 8 |
| 8 | 4.5 | 21 | 7 |
| 9 | 3 | 17 | 9.5 |
| 10 | 3.5 | 16 | 8 |
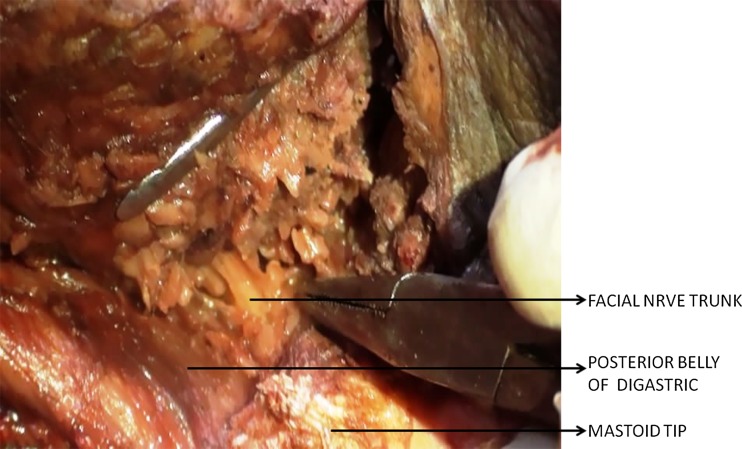
Fig. 1.
Cadaveric dissection showing relationship of the facial nerve with the posterior belly of digastrics muscle
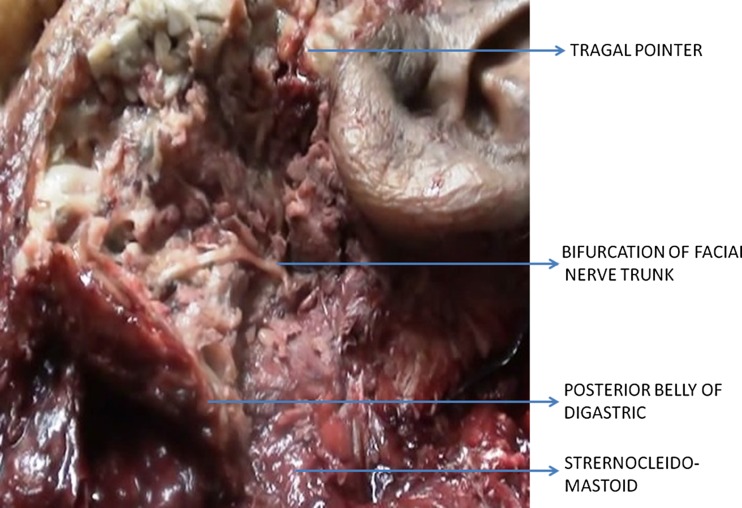
Fig. 2.
Cadaver dissection specimen showing the facial nerve, tragal pointer and the posterior belly of digastric
Table 2.
Surgical study: distance of facial nerve trunk from different anatomic landmarks
| Surgical patient no | Tympanomastoid suture (in mm) | Tragal pointer (in mm) | Posterior belly of digastric (in mm) |
|---|---|---|---|
| 1 | 3.5 | 17.5 | 7.5 |
| 2 | 4 | 19 | 8 |
| 3 | 2.7 | 13.6 | 7 |
| 4 | 2 | 17 | 8.8 |
| 5 | 4.9 | 15 | 6 |
| 6 | 5.3 | 18.5 | 7.5 |
| 7 | 4 | 16 | 6.5 |
| 8 | 2.5 | 15 | 9.5 |
| 9 | 3.8 | 18 | 8 |
| 10 | 6 | 14 | 11.5 |
Fig. 3.
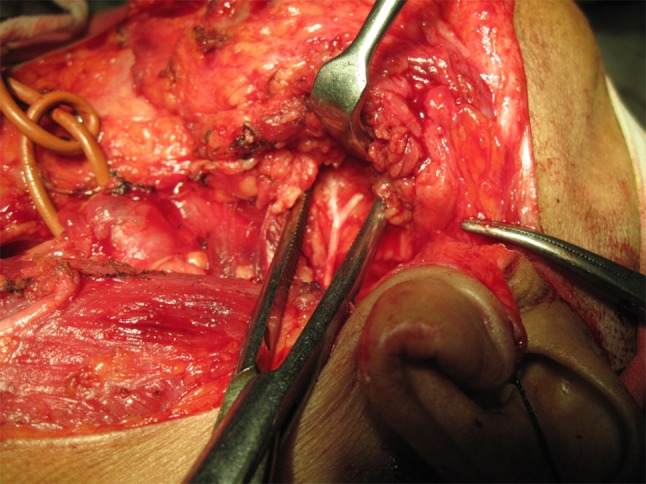
Superficial parotidectomy in progress with display of the facial nerve
Fig. 4.
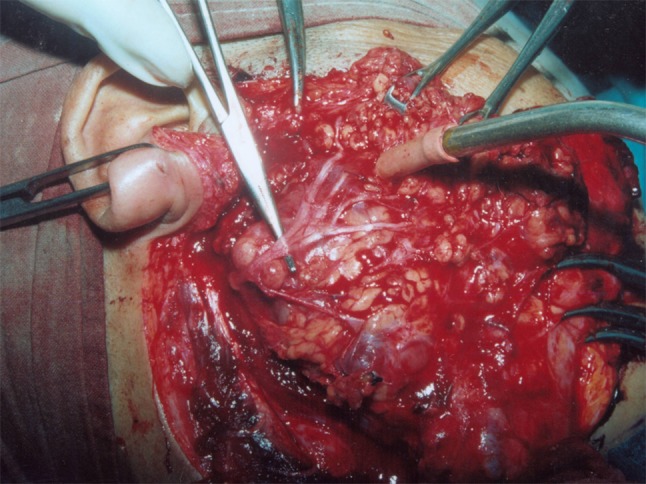
A deep lobe parotid tumour lying below the dissected facial nerve
Discussion
Facial nerve injury is the most common complication of parotid surgery as the two structures are intimately related to each other. This is because of the fact that during embryogenesis, the parotid gland entraps mesenchymal structures which later develops into the facial nerve. The facial nerve however is said to divide the gland into a deep and superficial lobe but this concept is not anatomically based. The facial nerve along with the accompanying vessels creates a potential plane which lies in between the deep and superficial lobes of the parotid gland. Dissection in this plane is never possible until and unless the surgeon identifies the nerve and proceeds along the nerve and its branches. This clearly indicates to the fact that parotid gland surgery is purely an anatomical dissection and sound anatomical knowledge sharpened further by cadaveric dissection goes a long way in improving the surgical skill of a surgeon.
Postoperative facial nerve weakness can be temporary or permanent. Temporary weakness is much more common and incidence is between 10 and 50 % of parotidectomies [1, 2]. The cause of temporary weakness is neurapraxia, which results from a combination of trauma while dissecting right on the nerve, traction injury to the nerve, heat injury secondary to use of electro-cautery, and prolonged operating time. The incidence of permanent facial nerve injury is generally reported as 0.5 % [1, 2]. The cause of such weakness is due to transection of, or cautery injury to the main trunk. In a large series from France comprising 131 patients of parotid tumour, Gaillard et al. reported that there is a high percentage of facial nerve dysfunction (42.7 % on the first postoperative day) immediately after parotidectomy which gradually improves over time to the tune of 30.7 % at 1 month post op and 0 % at 6 months after the surgery. The marginal mandibular branch was reported as the single most affected nerve branch following parotidectomy (48.2 %). Facial nerve dysfunction after total parotidectomy was found to be significantly higher (P < 0.001) than that in superficial parotidectomy (18.2 % at day 1 and 10.9 % at month 1) [3].
In another study from Australia the authors reported that the incidence of initial postoperative facial weakness varied with the type of pathology and the extent of surgery. In cases of limited superficial parotidectomy for superficial lobe neoplasia the rates of initial facial nerve dysfunction were 16.5 % for benign lesions and 13 % for malignant tumours. But when near total parotidectomy was done for deep lobe tumours the percentage increased to 31 % for benign and 100 % for malignant lesions in the early post operative period. Surgery for inflammatory lesions like chronic sialadenitis was associated with relatively higher facial palsy rates of 30 % for complete superficial parotidectomy and 34 % for near-total parotidectomy, respectively. In cases of parotidectomy associated with a neck dissection 83 % of patients had facial paresis and 33 % of cases had initial facial nerve dysfunction while undergoing revision parotid surgery. In their series, permanent paralysis occurred in 13 (5.6 %) of 230 patients, but 10 of these 13 had simultaneous neck dissection and facial nerve dysfunction involved only the marginal mandibular branch. In 46 of 67 of those with initial weakness (68 %), normal facial movements recovered within 6 months [4].
In another series involving paediatric patients, the incidence of immediate facial nerve paresis was 21 % (9/43). In this series also the most common branch involved was the marginal mandibular nerve (n = 7). Facial nerve function recovered fully in all the cases within 6 months [5].
In absence of facial nerve monitor, facial nerve is generally located by means of anterograde or retrograde dissection methods. Retrograde dissection is the less commonly used technique with the surgeons preferring this method mostly during revision parotidectomy. Anterograde dissection or proximal surgical identification technique is aimed at identifying the facial nerve at its point of exit from the stylomastoid foramen and is the preferred method of dissecting the facial nerve.
Over the years, various authors have performed cadaveric dissection as well as live surgery to compare the different landmarks. Different available studies showing the measurement of various landmarks from the facial nerve trunk have been presented in Table 3.
Table 3.
Comparison of present study with similar studies published in literature
| Clinical studies | Distance from PBDM | Distance from tragal pointer | Distance from TMS |
|---|---|---|---|
| Annals of Anatomy (Vol. 192, Feb’ 2010) Rea et al. [8] | 5.5 ± 2.1 mm | 6.9 ± 1.8 mm | 2.5 ± 0.4 mm |
| Surgical and Radiologic Anatomy (Vol. 28, Nov’ 2005) Pather and Osman [9] | 9.7–24.3 mm | 24.3–49.2 mm | 4.9–18.6 mm |
| The Laryngoscope (Vol. 115, April’ 2005) Witt et al. [6] | 12.4 mm (cad) | – | 1.8 mm (cad) |
| 10.7 mm (live) | 2.0 mm (live) | ||
| Present study | 6–9.5 mm (cad) | 14–21 mm (cad) | 2.5–4.5 mm (cad) |
| 6–11.5 mm (live) | 13.5–19 mm (live) | 2–6 mm (live) |
Though stylomastoid foramen is anatomically a very constant landmark for facial nerve, but in live surgical situation it is very difficult to find this foramen as it is mainly a palpatory landmark and most importantly because it remains surrounded by thick fascia which is continuous with the periosteum of skull base. Excessive dissection in this area very often leads to permanent paralysis of the nerve.
The tympanomastoid suture line is palpable as a hard ridge deep to the cartilaginous portion of the external auditory canal. The facial nerve emerges a few millimeters deep to its outer edge. Tympanomastoid suture can be identified in the cadavers without much difficulty but in live surgery it is basically a palpatory landmark and direct visualization of the suture is practically not possible. In the present study we found that the TMS lies about 2.5–4.5 mm (cad) and 2–6 mm (live) respectively from the facial nerve trunk (Table 1). Witt et al. [6] stated that TMS is a significantly closer and less variable anatomic landmark compared with the PBD both in cadaver dissection and in live patients. In their prospective study of 14 cadaver specimens and 22 live patients, the mean closest distances from the TMS and PBD to the facial nerve were 1.8 (range 0–4) mm and 12.4 (range 7–17) mm, respectively (P < .05) for cadavers. The mean closest distances in live patients from the TMS and PBD to the facial nerve were 2.0 (range 0–4) mm and 10.7 (range 5–14) mm, respectively (P < .05). Bushey et al. [7] also showed in his cadaveric study as tympanomastoid suture is a close and predictable anatomic landmark that can be used to identify the facial nerve trunk. In his study on 30 cadavers, the distance from TMS to the facial nerve trunk ranged from 3.3 to 9.2 mm with a mean of 4.9 mm. In the study on 26 cadavers by Rea et al. [8] the main trunk of the facial nerve was found 2.5 ± .4 mm from the TMS. Pather and Osman [9] in their study on 40 cadavers found that the facial nerve trunk was about 4.9–18.6 mm away from TMS.
Tragal pointer is a very popular landmark and facial nerve usually lies around 1 cm deep and inferior to the pointer. The only drawback of the pointer is that it is a cartilaginous structure which is mobile, asymmetrical and has a blunt and irregular tip. In the present study we found that it lies at a distance of about 14-21 mm (cad) and 13.5-19 mm (live) from the facial nerve trunk respectively. In the study on 26 cadavers by Rea et al. [8] the main trunk of the facial nerve was found 6.9 ± 1.8 mm from the TP. In the study on 40 cadavers by Pather and Osman [9] the facial nerve trunk was found 24.3–49.2 mm from the TP.
Mastoid process is also described as one of the landmarks but the process lies deep to the insertion of the sternocleidomastoid muscle and hence it is mainly a palpatory landmark. Greyling et al. [10] demonstrated that the mean distance between the mastoid process and facial nerve for the left was 9.18 ± 2.05 mm and for the right, 9.35 ± 1.67 mm on cadaver dissection.
During parotidectomy lateral retraction of the sternocleidomastoid muscle exposes the posterior belly of digastric muscle. This muscle is very easy to identify by the position (just deep to sternomastoid) and also by the direction of the muscle fibres that run towards the mastoid tip. The facial nerve trunk lies approximately 1 cm above and parallel to the upper border of the digastric muscle near its insertion at the mastoid tip. In the present study, we found that the facial nerve trunk lies at a distance of 6–9.5 mm (cad) and 6–11.5 mm (live) respectively from PBD and it has the minimum anatomical variation. Rea et al. [8] demonstrated that the main trunk of the facial nerve was found to lie 5.5 ± 2.1 mm from the PBD. Pather and Osman [9] demonstrated that the facial nerve trunk was found 9.7–24.3 mm from the PBD. Their results demonstrated that the posterior belly of digastric, tragal pointer and transverse process of the axis are consistent landmarks to the facial nerve trunk and they advocated use of transverse process of axis as a palpatory landmarks as it does not require complex dissection and ensures safety of the nerve [9].
However in cases of large tumours of the parotid gland especially in malignant ones, the posterior belly of digastric may become adherent to the tumour itself or to the surrounding structures. In those cases this landmark may be difficult to dissect or may not be in proper anatomical location which calls for the use of another landmark. In this situation the tragal pointer significantly helps in locating the facial nerve trunk. In extreme cases even the tragal pointer may be displaced and there the tympanomastoid suture needs to be dissected which being a bony landmark is rarely involved by the disease.
Facial nerve trunk can also be identified by performing retrograde dissection whereby peripheral branches are traced back to reach the main trunk. This technique is sometimes needed on account of difficulty in locating the main trunk, due to the presence of a post-inflammatory fibrosis or overlying neoplasm. So peripheral branches can also be considered to be one of the landmarks for the facial nerve trunk, but in the absence of facial nerve stimulator it is difficult to identify the peripheral branches which are thin with wide anatomical variations. Sharma et al. recommended the use of buccal branch of facial nerve to locate the main trunk. The reason behind was due to the regular course and adequate size of this branch of facial nerve in its peripheral area co-located with stenson’s duct, which enabled it to be easily identified during surgery [11]. Pia et al. [12] used orbicular branch, on account of difficulty in locating the main trunk of the nerve in 5 cases of retrograde exploration of the nerve. Keefe et al. [13] used postauricular branch to identify the main facial nerve trunk in his study. Kanatas et al. [14] demonstrated the use of digastric branch to identify the main trunk. In the present study, peripheral branches were identified in the cadaveric study and followed back to the main trunk. However in the live surgery retrograde dissection was not required in any of the cases.
Conclusion
Parotidectomy is a technically demanding operation. This surgery becomes significantly less complicated for the surgeon once he/she identifies the facial nerve trunk. For easy and prompt identification of the nerve trunk, the surgeon needs to systematically look for the anatomical landmarks. The present study showed that the posterior belly of digastric is the best landmark to start with and in case of difficulties in dissection or identification it may be supplemented with the tragal pointer and the tympanomastoid suture line subsequently in that order.
Contributor Information
Somnath Saha, Phone: +919830642186, Email: sreekar_saha@hotmail.com.
Sudipta Pal, Email: drsudiptapal@gmail.com.
Moushumi Sengupta, Email: goodugala@gmail.com.
Kanishka Chowdhury, Email: drkanishka@gmail.com.
Vedula Padmini Saha, Email: sahasomnath11@yahoo.in.
Lopamudra Mondal, Email: lopamudradhali@yahoo.com.
References
- 1.O’Brien CJ. Current management of benign parotid tumors—the role of limited superficial parotidectomy. Head Neck. 2003;25(11):946–952. doi: 10.1002/hed.10312. [DOI] [PubMed] [Google Scholar]
- 2.Leverstein H, van der Wal JE, Tiwari RM, et al. Surgical management of 246 previously untreated pleomorphic adenomas of the parotid gland. Br J Surg. 1997;84(3):399–403. doi: 10.1002/bjs.1800840341. [DOI] [PubMed] [Google Scholar]
- 3.Gaillard C, Périé S, Susini B, St Guily JL. Facial nerve dysfunction after parotidectomy: the role of local factors. Laryngoscope. 2005;115(2):287–291. doi: 10.1097/01.mlg.0000154735.61775.cd. [DOI] [PubMed] [Google Scholar]
- 4.Bron LP, O’Brien CJ. Facial nerve function after parotidectomy. Arch Otolaryngol Head Neck Surg. 1997;123(10):1091–1096. doi: 10.1001/archotol.1997.01900100065009. [DOI] [PubMed] [Google Scholar]
- 5.Owusu JA, Parker NP, Rimell FL. Postoperative facial nerve function in pediatric parotidectomy: a 12-year review. Otolaryngol Head Neck Surg. 2013;148(2):249–252. doi: 10.1177/0194599812467299. [DOI] [PubMed] [Google Scholar]
- 6.Witt RL, Weinstein GS, Rejto LK. Tympanomastoid suture and digastric muscle in cadaver and live parotidectomy. Laryngoscope. 2005;115(4):574–577. doi: 10.1097/01.mlg.0000161343.85009.4c. [DOI] [PubMed] [Google Scholar]
- 7.Bushey A, Quereshy F, Boice JG, Landers MA, Baur DA. Utilization of the tympanomastoid fissure for intraoperative identification of the facial nerve: a cadaver study. J Oral Maxillofac Surg. 2011;69(9):2473–2476. doi: 10.1016/j.joms.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Rea PM, McGarry G, Shaw-Dunn J. The precision of four commonly used surgical landmarks for locating the facial nerve in anterograde parotidectomy in humans. Ann Anat. 2010;192(1):27–32. doi: 10.1016/j.aanat.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Pather N, Osman M. Landmarks of the facial nerve: implications for parotidectomy. Surg Radiol Anat. 2006;28(2):170–175. doi: 10.1007/s00276-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 10.Greyling LM, Glanvill R, et al. Bony landmarks as an aid for intraoperative facial nerve identification. Clin Anat. 2007;20(7):739–744. doi: 10.1002/ca.20508. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Sirohi D. Proximal and distal facial nerve exploration during superficial parotidectomy. J Maxillofac Oral Surg. 2010;9(2):150–154. doi: 10.1007/s12663-010-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pia F, Policarpo M, Dosdegani R, Olina M, Brovelli F, Aluffi P. Centripetal approach to the facial nerve in parotid surgery: personal experience. Acta Otorhinolaryngol Ital. 2003;23(2):111–115. [PubMed] [Google Scholar]
- 13.Keefe MA, Castro JR, Keefe MS. Identification of the facial nerve main trunk by retrograde dissection of the postauricular branch. Otolaryngol Head Neck Surg. 2009;140(1):126–127. doi: 10.1016/j.otohns.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Kanatas AN, McCaul JA. Use of digastric branch of the facial nerve for identification of the facial nerve itself in parotidectomy: technical note. Br J Oral Maxillofac Surg. 2011;49(6):493–494. doi: 10.1016/j.bjoms.2010.10.019. [DOI] [PubMed] [Google Scholar]