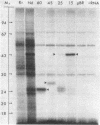
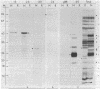
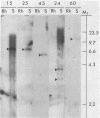
Abstract
Plant gene products that could play a role in the process of symbiotic nitrogen fixation in leguminous plants were detected by screening a cDNA library prepared from soybean nodule poly(A)+ RNA. About 13% of the 5,700 clones screened contained sequences detectable with a root cDNA probe while about 2,100 clones (37% of the library) contained sequences that were detectable only with nodule cDNA. Five unique sequence species, accounting for more than half of the 2,100 nodule-specific clones, were identified by cross-hybridization experiments. The most abundant species, represented by 860 clones, encodes the well-characterized protein, leghemoglobin (Lb). The other four species, designated NodA, NodB, NodC, and NodD, are represented by 350, 55, 61, and 6 clones, respectively. Each of these four species was found to be encoded by the plant nuclear genome at low copy number. The transcripts corresponding to the nodule-specific clones represented 12-15% (Lb), 6% (NodA), and 0.5-1.1% (NodB, NodC, and NodD) mole fraction of nodule polysomal mRNAs but could not be detected in root polysomal RNA. Hybrid-selection of nodule mRNAs by representative clones and in vitro translation indicated that polypeptides of Mr 44,000, 27,000, 24,000, and 100,000-120,000 are encoded by NodA, NodB, NodC, and NodD sequences, respectively. These polypeptides reacted with antiserum prepared against total soluble nodule proteins suggesting that the cloned sequences encode nodule-specific proteins, nodulins.
Keywords: symbiotic nitrogen fixation, cDNA library, hybrid-selection of mRNAs, in vitro translation, immunoprecipitation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger S., Baulcombe D., Verma D. P. Sequence complexities of the poly(A)-containing mRNA in uninfected soybean root and the nodule tissue developed due to the infection by Rhizobium. Biochim Biophys Acta. 1979 Jul 26;563(2):496–507. doi: 10.1016/0005-2787(79)90068-6. [DOI] [PubMed] [Google Scholar]
- Auger S., Verma D. P. Induction and expression of nodule-specific host genes in effective and ineffective root nodules of soybean. Biochemistry. 1981 Mar 3;20(5):1300–1306. doi: 10.1021/bi00508a040. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R. M., Bancroft F. C. Construction and identification by positive hybridization-translation of a bacterial plasmid containing a rat growth hormone structural gene sequence. Nucleic Acids Res. 1978 Jun;5(6):2039–2053. doi: 10.1093/nar/5.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Hooykaas P. J., Snijdewint F. G., Schilperoort R. A. Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid. 1982 Jul;8(1):73–82. doi: 10.1016/0147-619x(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. A nodule-specific plant protein (nodulin-35) from soybean. Science. 1979 Jul 13;205(4402):190–193. doi: 10.1126/science.205.4402.190. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Noyes B. E., Agarwal K. L. Detection of gastrin-specific mRNA using oligodeoxynucleotide probes of defined sequence. J Biol Chem. 1979 Aug 25;254(16):7472–7475. [PubMed] [Google Scholar]
- Noel K. D., Stacey G., Tandon S. R., Silver L. E., Brill W. J. Rhizobium japonicum mutants defective in symbiotic nitrogen fixation. J Bacteriol. 1982 Oct;152(1):485–494. doi: 10.1128/jb.152.1.485-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Sundaresan V., Ausubel F. M. Directed transposon Tn5 mutagenesis and complementation analysis of Rhizobium meliloti symbiotic nitrogen fixation genes. Cell. 1982 Jun;29(2):551–559. doi: 10.1016/0092-8674(82)90171-4. [DOI] [PubMed] [Google Scholar]
- Scott K. F., Hughes J. E., Gresshoff P. M., Beringer J. E., Rolfe B. G., Shine J. Molecular cloning of Rhizobium trifolii genes involved in symbiotic nitrogen fixation. J Mol Appl Genet. 1982;1(4):315–326. [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsanyi-Breiner A., Gusella J. F., Keys C., Housman D. E., Sullivan D., Brisson N., Verma D. P. The organization of a nuclear DNA sequence from a higher plant: molecular cloning and characterization of soybean ribosomal DNA. Gene. 1979 Nov;7(3-4):317–334. doi: 10.1016/0378-1119(79)90051-9. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Bal A. K. Intracellular site of synthesis and localization of leghemoglobin in root nodules. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3843–3847. doi: 10.1073/pnas.73.11.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Ball S., Guérin C., Wanamaker L. Leghemoglobin biosynthesis in soybean root nodules. Characterization of the nascent and released peptides and the relative rate of synthesis of the major leghemoglobins. Biochemistry. 1979 Feb 6;18(3):476–483. doi: 10.1021/bi00570a016. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]