Abstract
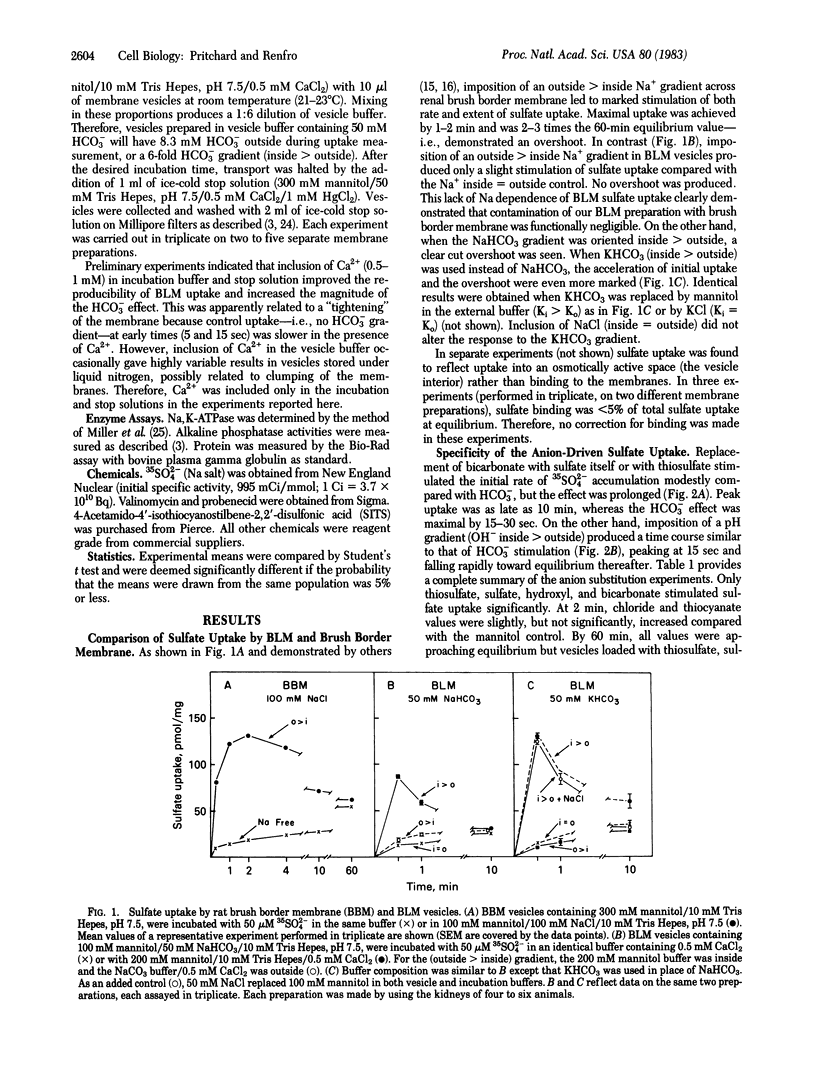
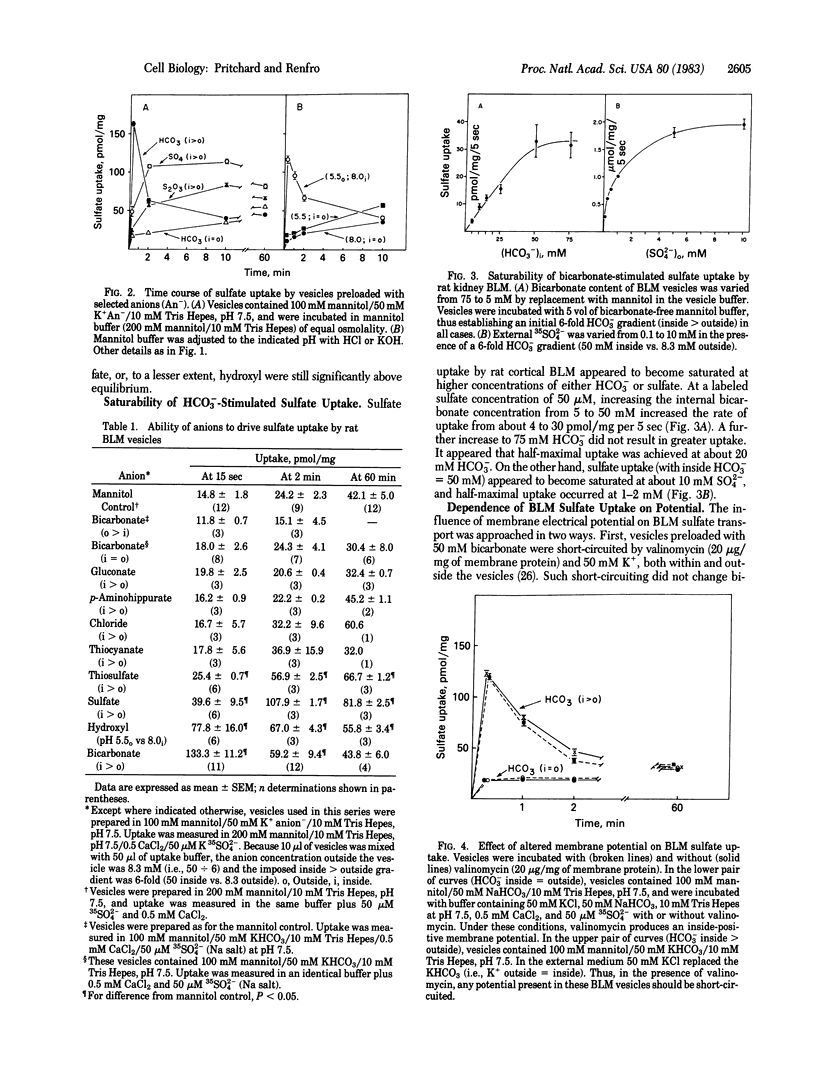
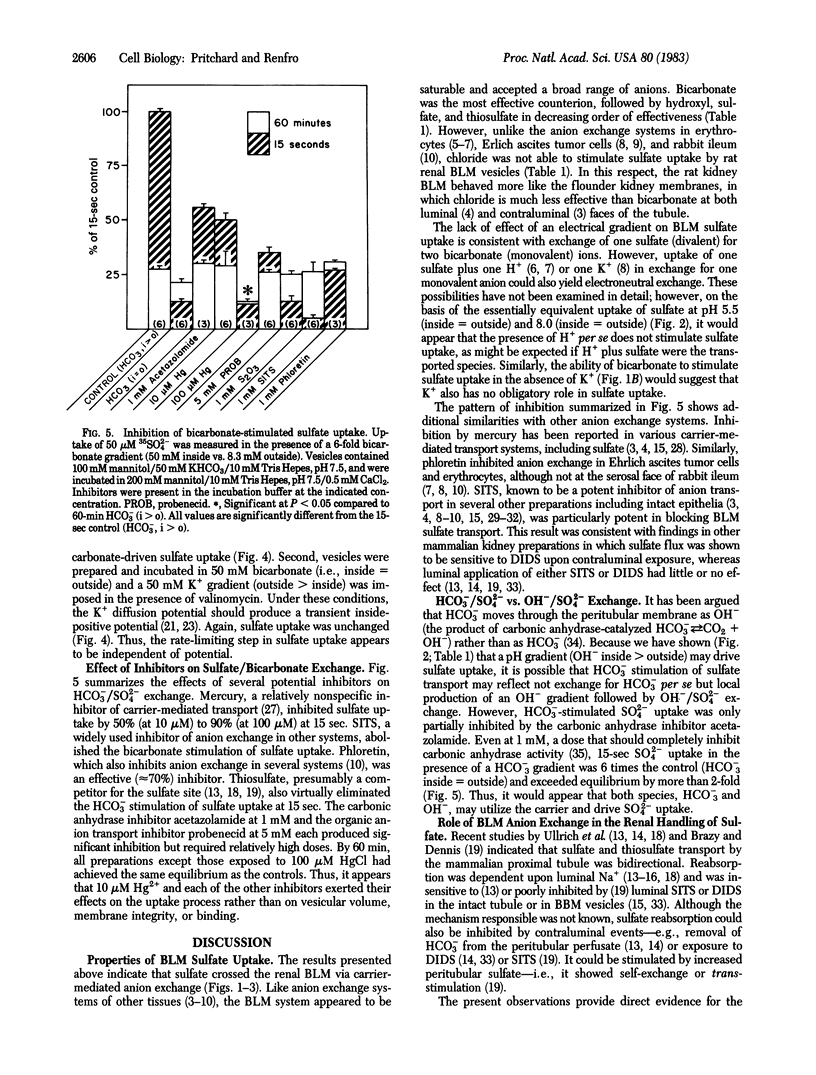
The transport of sulfate was studied in basolateral membrane (BLM) vesicles isolated from rat kidney cortex by centrifugation on a Percoll self-generating gradient. In contrast to sulfate transport at the luminal membrane, sulfate uptake by BLM vesicles was not sodium dependent. However, imposition of an inside greater than outside bicarbonate gradient stimulated BLM sulfate uptake nearly 10-fold and produced a transient overshoot of about 4-fold. This process appeared to become saturated at high concentrations of either bicarbonate or sulfate. Sulfate itself, thiosulfate, and hydroxyl, but not chloride or thiocyanate, were able to substitute for bicarbonate. None of these anions was as effective as bicarbonate. The sulfate/bicarbonate exchange was unaltered by manipulation of membrane potential, suggesting that it was electroneutral. Both bicarbonate stimulation and overshoot could be prevented by known inhibitors of anion transport, including mercuric chloride, 4-acetamido-4'-isothiocyanostilbene-2,2'-disulfonic acid, and phloretin. Bicarbonate-stimulated sulfate uptake also was inhibited by thiosulfate, probenecid, and acetazolamide. Thus, rat kidney BLM vesicles showed carrier-mediated anion exchange. Its properties indicate that this carrier may participate in both reabsorptive and secretory sulfate transport.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGLUND F., FORSTER R. P. Renal tubular transport of inorganic divalent ions by the aglomerular marine teleost, Lophius americanus. J Gen Physiol. 1958 Jan 20;41(3):429–440. doi: 10.1085/jgp.41.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. The sodium electrochemical potential-mediated uphill transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1978 Aug 10;253(15):5531–5535. [PubMed] [Google Scholar]
- Brazy P. C., Dennis V. W. Sulfate transport in rabbit proximal convoluted tubules: presence of anion exchange. Am J Physiol. 1981 Sep;241(3):F300–F307. doi: 10.1152/ajprenal.1981.241.3.F300. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Dantzler W. H., Bentley S. K. Bath and lumen effects of SITS on PAH transport by isolated perfused renal tubules. Am J Physiol. 1980 Jan;238(1):F16–F25. doi: 10.1152/ajprenal.1980.238.1.F16. [DOI] [PubMed] [Google Scholar]
- Dantzler W. H., Bentley S. K. Bath and lumen effects of SITS on PAH transport by isolated perfused renal tubules. Am J Physiol. 1980 Jan;238(1):F16–F25. doi: 10.1152/ajprenal.1980.238.1.F16. [DOI] [PubMed] [Google Scholar]
- Ehrenspeck G., Brodsky W. A. Effects of 4-acetamido-4'-isothiocyano-2,2-disulfonic stilbene on ion transport in turtle bladders. Biochim Biophys Acta. 1976 Feb 6;419(3):555–558. doi: 10.1016/0005-2736(76)90268-6. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Turner R. J., Silverman M., Rothstein A. Inorganic anion transport in kidney and intestinal brush border and basolateral membranes. Am J Physiol. 1980 Jun;238(6):F452–F460. doi: 10.1152/ajprenal.1980.238.6.F452. [DOI] [PubMed] [Google Scholar]
- HIERHOLZER K., CADE R., GURD R., KESSLER R., PITTS R. Stop-flow analysis of renal reabsorption and excretion of sulfate in the dog. Am J Physiol. 1960 Apr;198:833–837. doi: 10.1152/ajplegacy.1960.198.4.833. [DOI] [PubMed] [Google Scholar]
- Hickman C. P., Jr Urine composition and kidney tubular function in southern flounder, Paralichthys lethostigma, in seawater. Can J Zool. 1968 May;46(3):439–455. doi: 10.1139/z68-062. [DOI] [PubMed] [Google Scholar]
- Hong S. K., Goldinger J. M., Song Y. K., Koschier F. J., Lee S. H. Effect of SITS on organic anion transport in the rabbit kidney cortical slice. Am J Physiol. 1978 Apr;234(4):F302–F307. doi: 10.1152/ajprenal.1978.234.4.F302. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Inui K., Okano T., Takano M., Kitazawa S., Hori R. A simple method for the isolation of basolateral plasma membrane vesicles from rat kidney cortex. Enzyme activities and some properties of glucose transport. Biochim Biophys Acta. 1981 Sep 21;647(1):150–154. doi: 10.1016/0005-2736(81)90303-5. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Proton fluxes associated with erythrocyte membrane anion exchange. J Membr Biol. 1976 Aug 26;28(2-3):187–205. doi: 10.1007/BF01869697. [DOI] [PubMed] [Google Scholar]
- Langridge-Smith J. E., Field M. Sulfate transport in rabbit ileum: characterization of the serosal border anion exchange process. J Membr Biol. 1981;63(3):207–214. doi: 10.1007/BF01870982. [DOI] [PubMed] [Google Scholar]
- Lepke S., Passow H. Asymmetric inhibition by phlorizin of sulfate movements across the red blood cell membrane. Biochim Biophys Acta. 1973 Mar 16;298(2):529–533. doi: 10.1016/0005-2736(73)90379-9. [DOI] [PubMed] [Google Scholar]
- Levinson C. Chloride and sulfate transport in Ehrlich ascites tumor cells: evidence for a common mechanism. J Cell Physiol. 1978 Apr;95(1):23–32. doi: 10.1002/jcp.1040950104. [DOI] [PubMed] [Google Scholar]
- Lücke H., Stange G., Murer H. Sulphate-ion/sodium-ion co-transport by brush-border membrane vesicles isolated from rat kidney cortex. Biochem J. 1979 Jul 15;182(1):223–229. doi: 10.1042/bj1820223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. S. Heavy metal inhibition of p-aminohippurate transport in flounder renal tissue: sites of HgCl2 action. J Pharmacol Exp Ther. 1981 Nov;219(2):428–434. [PubMed] [Google Scholar]
- Miller D. S., Kinter W. B., Peakall D. B. Enzymatic basic for DDE-induced eggshell thinning in a sensitive bird. Nature. 1976 Jan 15;259(5539):122–124. doi: 10.1038/259122a0. [DOI] [PubMed] [Google Scholar]
- Renfro J. L., Pritchard J. B. H+-dependent sulfate secretion in the marine teleost renal tubule. Am J Physiol. 1982 Aug;243(2):F150–F159. doi: 10.1152/ajprenal.1982.243.2.F150. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Rosenbloom I. L., Liang C. T., Cheng L. Sodium gradient- and sodium plus potassium gradient-dependent L-glutamate uptake in renal basolateral membrane vesicles. J Membr Biol. 1981 May 15;60(1):63–71. doi: 10.1007/BF01870833. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Pessah N. I., Maren T. H. Kinetics and inhibition of membrane-bound carbonic anhydrase from canine renal cortex. Biochim Biophys Acta. 1981 Jan 15;657(1):128–137. doi: 10.1016/0005-2744(81)90136-4. [DOI] [PubMed] [Google Scholar]
- Scalera V., Huang Y. K., Hildmann B., Murer H. A simple isolation method for basal-lateral plasma membranes from rat kidney cortex. Membr Biochem. 1981;4(1):49–61. doi: 10.3109/09687688109065422. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Capasso G., Rumrich G., Papavassiliou F., Klöss S. Coupling between proximal tubular transport processes. Studies with ouabain, SITS and HCO3-free solutions. Pflugers Arch. 1977 Apr 25;368(3):245–252. doi: 10.1007/BF00585203. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Murer H. Sulphate and phosphate transport in the renal proximal tubule. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):549–558. doi: 10.1098/rstb.1982.0151. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Active sulfate reabsorption in the proximal convolution of the rat kidney: specificity, Na+ and HCO3- dependence. Pflugers Arch. 1980 Jan;383(2):159–163. doi: 10.1007/BF00581877. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Bidirectional active transport of thiosulfate in the proximal convolution of the rat kidney. Pflugers Arch. 1980 Sep;387(2):127–132. doi: 10.1007/BF00584263. [DOI] [PubMed] [Google Scholar]
- Villereal M. L., Levinson C. Chloride-stimulated sulfate efflux in Ehrlich ascites tumor cells: evidence for 1:1 coupling. J Cell Physiol. 1977 Mar;90(3):553–563. doi: 10.1002/jcp.1040900317. [DOI] [PubMed] [Google Scholar]


