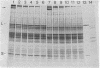
Abstract
We have detected a proteolytic mechanism in chloroplasts that selectively and rapidly degrades the imported small subunit of ribulose 1,5-bisphosphate carboxylase when pools of the chloroplast-synthesized large subunit are depleted. This degradation system is constitutively present and appears to be responsible for precise stoichiometric accumulation of the two subunits of the enzyme. We believe similar proteolytic mechanisms participate in regulating the accumulation of other photosynthetic proteins during chloroplast biogenesis.
Keywords: Chlamydomonas reinhardtii, pulse-chase labeling, chloroplast ribosome mutant, chloramphenicol
Full text
PDF
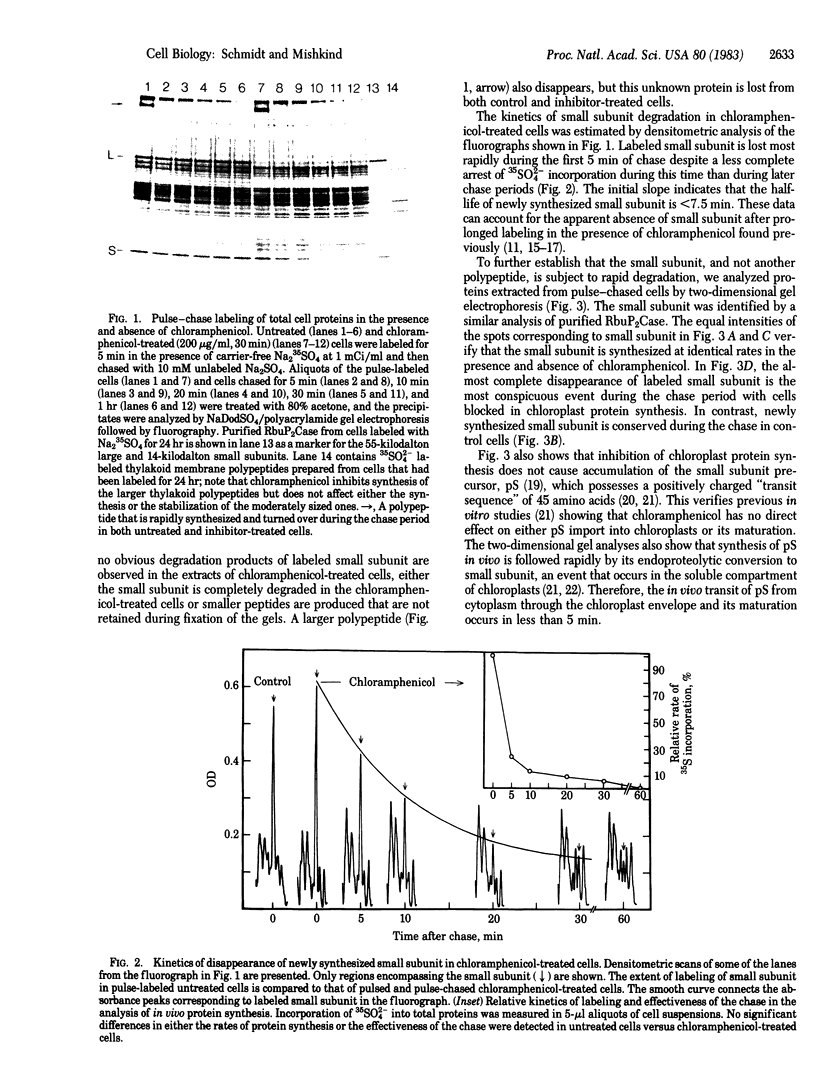
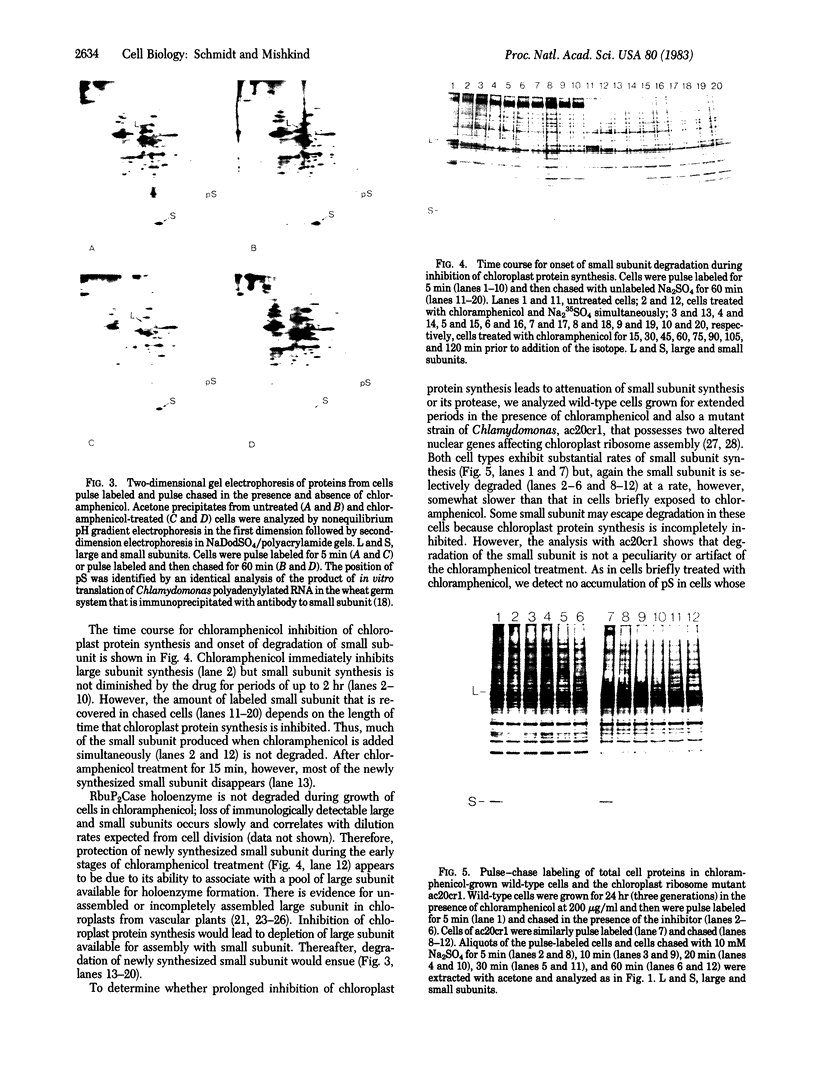


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Barraclough R., Ellis R. J. Protein synthesis in chloroplasts. IX. Assembly of newly-synthesized large subunits into ribulose bisphosphate carboxylase in isolated intact pea chloroplasts. Biochim Biophys Acta. 1980 Jun 27;608(1):19–31. doi: 10.1016/0005-2787(80)90129-x. [DOI] [PubMed] [Google Scholar]
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Chabot J. F. Chloroplast ribosome deficient mutants in the green alga Chlamydomonas reinhardi and the question of chloroplast ribosome function. J Cell Sci. 1972 Mar;10(2):267–305. doi: 10.1242/jcs.10.2.267. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Steinback K. E., Arntzen C. J. Analysis of the Light-harvesting Pigment-Protein Complex of Wild Type and a Chlorophyll-b-less Mutant of Barley. Plant Physiol. 1979 Feb;63(2):237–243. doi: 10.1104/pp.63.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Matlin K., Bennoun P. A chlorophyll-protein complex lacking in photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol. 1975 Nov;67(2PT1):361–377. doi: 10.1083/jcb.67.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuming A. C., Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Control of messenger RNA activity by light. Eur J Biochem. 1981 Aug;118(1):71–80. doi: 10.1111/j.1432-1033.1981.tb05487.x. [DOI] [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J Biol Chem. 1982 Oct 10;257(19):11673–11679. [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Wildner G. Formation of the small subunit in the absence of the large subunit of ribulose 1,5-bisphosphate carboxylase in 70 S ribosome-deficient rye leaves. Arch Biochem Biophys. 1978 Mar;186(2):283–291. doi: 10.1016/0003-9861(78)90437-x. [DOI] [PubMed] [Google Scholar]
- Givan A. L., Criddle R. S. Ribulosediphosphate carboxylase from Chlamydomonas reinhardi: purification, properties and its mode of synthesis in the cell. Arch Biochem Biophys. 1972 Mar;149(1):153–163. doi: 10.1016/0003-9861(72)90309-8. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. H., Boynton J. E., Gillham N. W. Chloroplast ribosome biogenesis in Chlamydomonas. Selection and characterization of mutants blocked in ribosome formation. J Cell Biol. 1974 Oct;63(1):160–179. doi: 10.1083/jcb.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Marks D. B., Keller B. J., Margulies M. M. Regulation of accumulation of the major thylakoid polypeptides in Chlamydomonas reinhardtii y-1 at 25 degrees C and 38 degrees C. J Cell Biol. 1982 Nov;95(2 Pt 1):552–558. doi: 10.1083/jcb.95.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Kirk D. L., Kirk M. M. Carrier-mediated Uptake of Arginine and Urea by Chlamydomonas reinhardtii. Plant Physiol. 1978 Apr;61(4):556–560. doi: 10.1104/pp.61.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Akazawa T. Biosynthesis of ribulose-1,5-bisphosphate carboxylase in spinach leaf protoplasts. Plant Physiol. 1978 Jul;62(1):97–100. doi: 10.1104/pp.62.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Roy H., Bloom M., Milos P., Monroe M. Studies on the assembly of large subunits of ribulose bisphosphate carboxylase in isolated pea chloroplasts. J Cell Biol. 1982 Jul;94(1):20–27. doi: 10.1083/jcb.94.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Devillers-Thiery A., Desruisseaux H., Blobel G., Chua N. H. NH2-terminal amino acid sequences of precursor and mature forms of the ribulose-1,5-bisphosphate carboxylase small subunit from Chlamydomonas reinhardtii. J Cell Biol. 1979 Dec;83(3):615–622. doi: 10.1083/jcb.83.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S. A., Gressel J., Reisfeld A., Edelman M. Characterization of the 32,000 Dalton Chloroplast Membrane Protein: III. Probing Its Biological Function in Spirodela. Plant Physiol. 1979 Nov;64(5):828–832. doi: 10.1104/pp.64.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]