Abstract
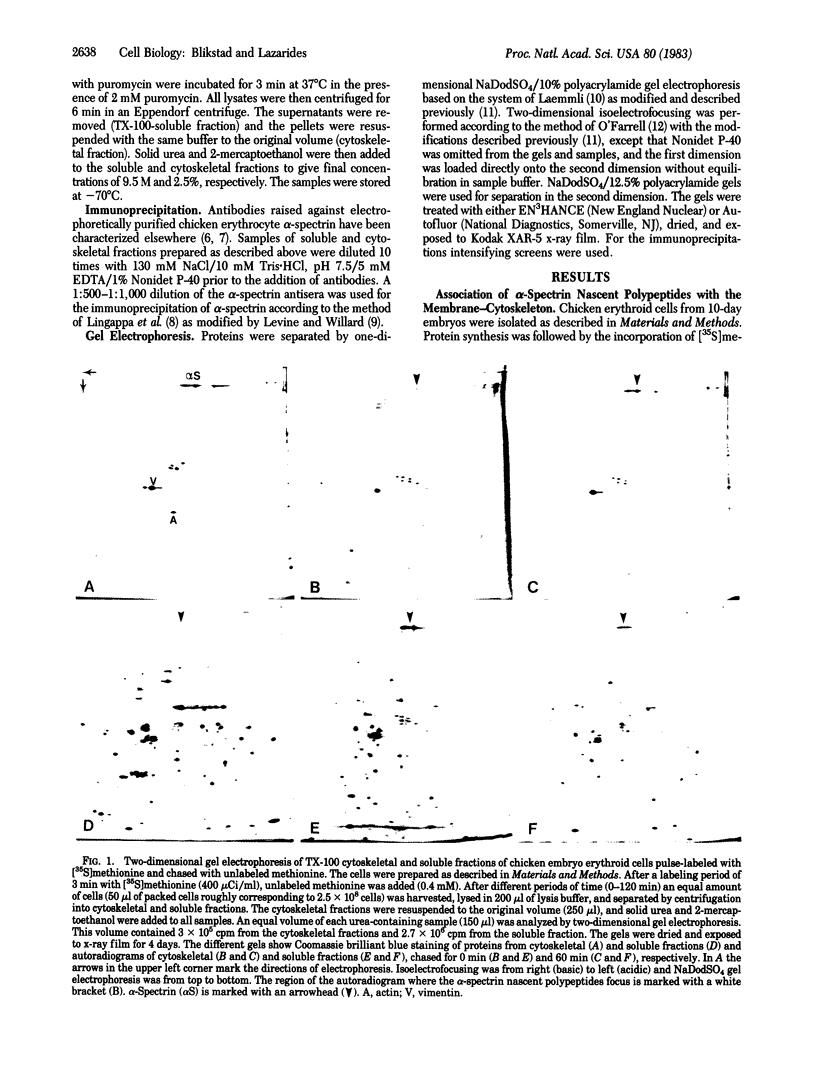
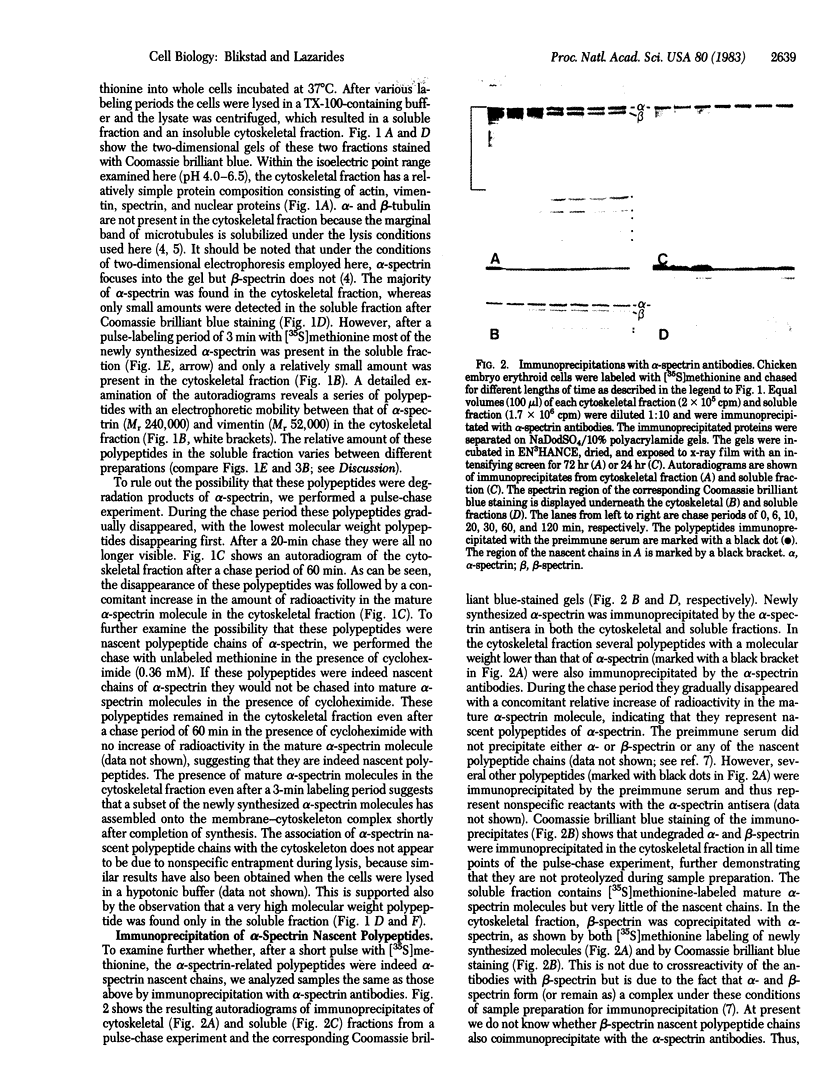
The site of synthesis of spectrin was investigated in erythroid cells from 10-day chicken embryos. After various periods of [35S]methionine incorporation the cells were lysed in a Triton X-100 (TX-100)-containing buffer and were separated into a TX-100-soluble and -insoluble (cytoskeletal) fraction. Analysis of these two fractions by two-dimensional gel electrophoresis after a short pulse-labeling period reveals that alpha-spectrin nascent polypeptides are present predominantly in the TX-100-insoluble fraction. These polypeptides can be immunoprecipitated with alpha-spectrin antisera and the [35S]methionine incorporated into them during a short pulse can be chased into mature alpha-spectrin molecules. The alpha-spectrin nascent polypeptide chains are released quantitatively from the TX-100 cytoskeleton by treatment of lysed cells with puromycin, suggesting that they themselves are not associated with the cytoskeleton. A small fraction of the newly synthesized mature alpha-spectrin molecules is rapidly incorporated into the cytoskeleton, as shown by the fact that they are not released by the puromycin treatment; the rest are recovered in the soluble fraction. These results suggest that alpha-spectrin is synthesized in association with the cytoskeleton during chicken erythropoiesis and assembles onto the membrane-cytoskeleton posttranslationally.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Beam K. G., Greengard P. Hormonal control of Na+-K+ co-transport in turkey erythrocytes. Multiple site phosphorylation of goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 May 25;255(10):4864–4871. [PubMed] [Google Scholar]
- Beam K. G., Alper S. L., Palade G. E., Greengard P. Hormonally regulated phosphoprotein of turkey erythrocytes: localization to plasma membrane. J Cell Biol. 1979 Oct;83(1):1–15. doi: 10.1083/jcb.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Biosynthesis of the erythrocyte anion transport protein. J Biol Chem. 1981 Nov 10;256(21):11337–11344. [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. The erythrocyte anion transport protein is contranslationally inserted into microsomes. Cell. 1982 Jan;28(1):23–31. doi: 10.1016/0092-8674(82)90371-3. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Chan L. L. Changes in the composition of plasma membrane proteins during differentiation of embryonic chick erythroid cell. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1062–1066. doi: 10.1073/pnas.74.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Structural associations of synemin and vimentin filaments in avian erythrocytes revealed by immunoelectron microscopy. Cell. 1982 Aug;30(1):263–275. doi: 10.1016/0092-8674(82)90032-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Repasky E. A., Lazarides E. Synemin and vimentin are components of intermediate filaments in avian erythrocytes. J Cell Biol. 1982 Feb;92(2):299–312. doi: 10.1083/jcb.92.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard B. D., Lazarides E. Copurification of actin and desmin from chicken smooth muscle and their copolymerization in vitro to intermediate filaments. J Cell Biol. 1979 Jan;80(1):166–182. doi: 10.1083/jcb.80.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery W. R. Messenger RNA in the cytoskeletal framework: analysis by in situ hybridization. J Cell Biol. 1982 Oct;95(1):1–7. doi: 10.1083/jcb.95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Attachment of ribosomes to membranes during polysome formation in mouse sarcoma 180 cells. J Cell Biol. 1971 Jun;49(3):683–691. doi: 10.1083/jcb.49.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Jacobsen M. Regulation of hemoglobin synthesis. Equal rates of translation and termination of - and -globin chains. J Biol Chem. 1972 Jun 10;247(11):3622–3629. [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Expression of the beta subunit of spectrin in nonerythroid cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):363–367. doi: 10.1073/pnas.80.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Weise M. J., Chan L. N. Membrane protein synthesis in embryonic chick erythroid cells. J Biol Chem. 1978 Mar 25;253(6):1892–1897. [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Sillekens P. T., van Eekelen C. A., Reinders R. J. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981 Sep;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]