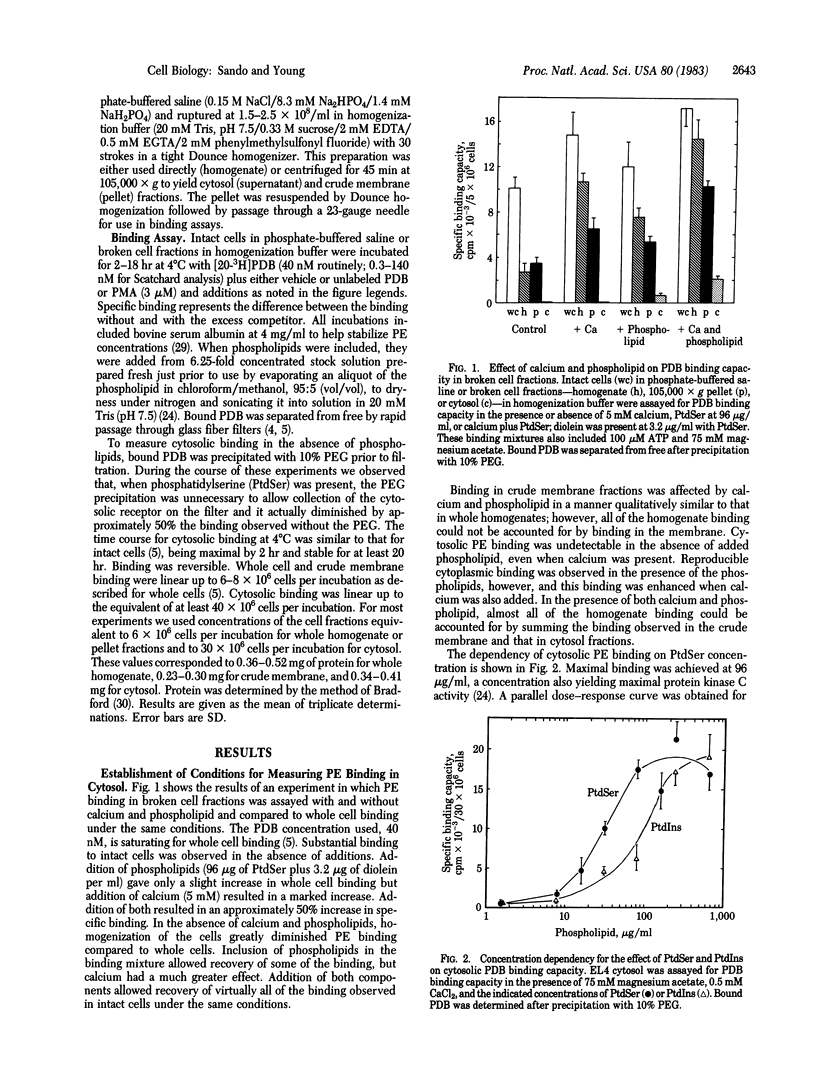
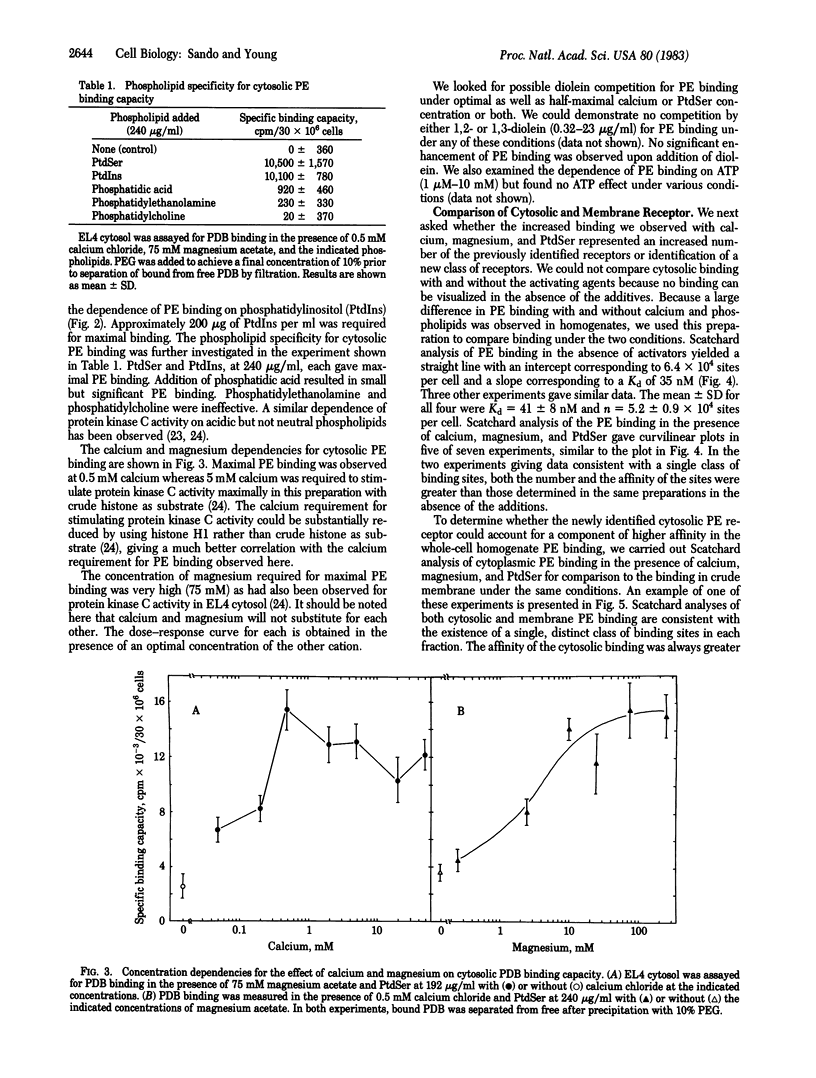
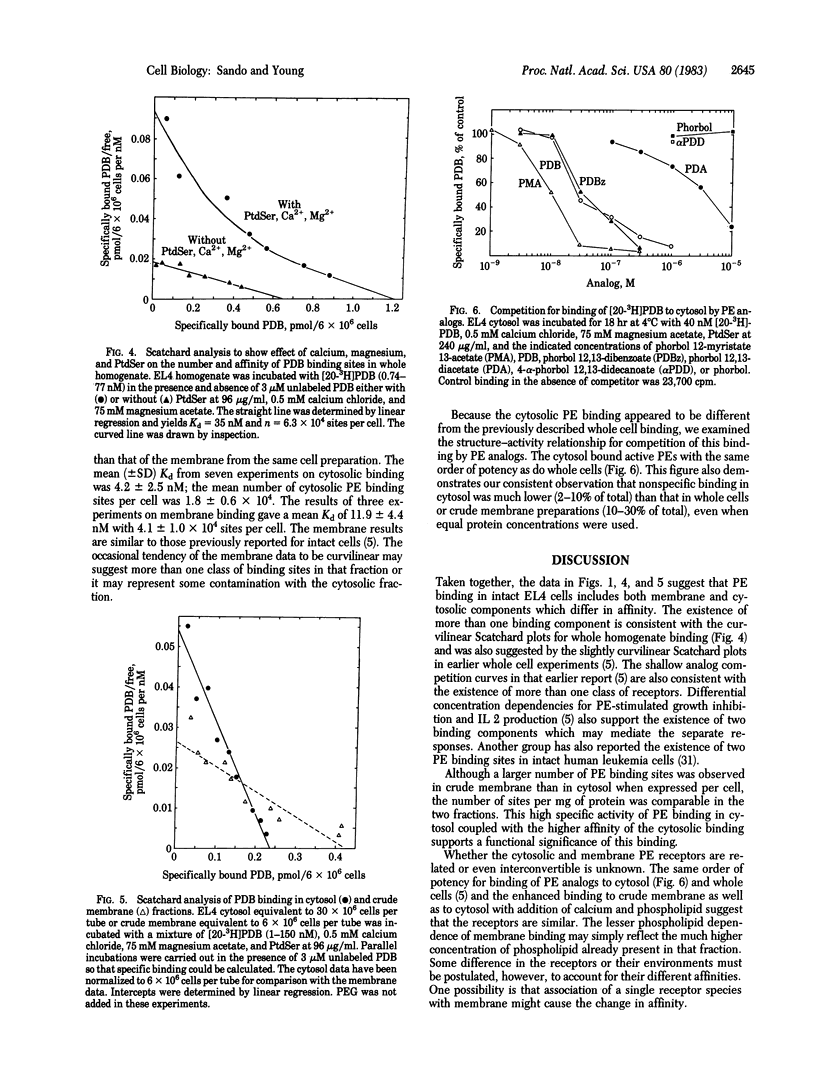
Abstract
A specific high-affinity phorbol ester binding component has been identified in the cytosol of an EL4 mouse thymoma line by using conditions similar to those for demonstrating activity of a calcium/phospholipid-dependent protein kinase. Specific binding is absolutely dependent on acidic phospholipids (maximal binding at 96 micrograms of phosphatidylserine per ml or 200 micrograms of phosphatidylinositol per ml) and is greatly enhanced by addition of calcium (0.5 mM) and magnesium (75 mM). Scatchard analysis of the cytosolic binding component indicated a Kd of 4.2 +/- 2.5 nM with 1.8 +/- 0.6 x 10(4) sites per cell compared to a Kd of 11.9 +/- 4.4 nM and 4.1 +/- 1.0 x 10(4) sites per cell for the membrane receptor. Consistent with the existence of at least two phorbol ester binding sites in EL4 cells was the observation of curvilinear Scatchard plots for binding to whole homogenates. The cytosolic binding showed the same order of potency for binding phorbol ester analogs as has been observed for intact cells. These results further support the similarity between phorbol ester receptors and the calcium/phospholipid-dependent protein kinase and suggest that the cytosolic receptor may be involved in subsequent phorbol ester effects in EL4 cells including loss of the kinase activity from the cytosol and production of T-cell growth factor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleackley R. C., Caplan B., Havele C., Ritzel R. G., Mosmann T. R., Farrar J. J., Paetkau V. Translation of lymphocyte mRNA into biologically-active Interleukin 2 in oocytes. J Immunol. 1981 Dec;127(6):2432–2435. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Delclos K. B., Nagle D. S., Blumberg P. M. Specific binding of phorbol ester tumor promoters to mouse skin. Cell. 1980 Apr;19(4):1025–1032. doi: 10.1016/0092-8674(80)90093-8. [DOI] [PubMed] [Google Scholar]
- Deleers M., Malaisse W. J. Binding of tumor-promoting and biologically inactive phorbol esters to artificial membranes. Cancer Lett. 1982 Nov-Dec;17(2):135–140. doi: 10.1016/0304-3835(82)90025-8. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):567–571. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Delclos K. B., Blumberg P. M. Characterization of specific binding of [3H]phorbol 12,13-dibutyrate and [3H]phorbol 12-myristate 13-acetate to mouse brain. Cancer Res. 1980 Oct;40(10):3635–3641. [PubMed] [Google Scholar]
- Dunphy W. G., Delclos K. B., Blumberg P. M. Characterization of specific binding of [3H]phorbol 12,13-dibutyrate and [3H]phorbol 12-myristate 13-acetate to mouse brain. Cancer Res. 1980 Oct;40(10):3635–3641. [PubMed] [Google Scholar]
- Efrat S., Pilo S., Kaempfer R. Kinetics of induction and molecular size of mRNAs encoding human interleukin-2 and gamma-interferon. Nature. 1982 May 20;297(5863):236–239. doi: 10.1038/297236a0. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Weinstein I. B. Enhancement of cell proliferation in low calcium medium by tumor promoters. Carcinogenesis. 1981;2(2):89–95. doi: 10.1093/carcin/2.2.89. [DOI] [PubMed] [Google Scholar]
- Froscio M., Guy G. R., Murray A. W. Calmodulin inhibitors modify cell surface changes triggered by a tumor promoter. Biochem Biophys Res Commun. 1981 Feb 12;98(3):829–835. doi: 10.1016/0006-291x(81)91186-4. [DOI] [PubMed] [Google Scholar]
- Grove R. I., Schimmel S. D. Generation of 1,2-diacylglycerol in plasma membranes of phorbol ester-treated myoblasts. Biochem Biophys Res Commun. 1981 Sep 16;102(1):158–164. doi: 10.1016/0006-291x(81)91502-3. [DOI] [PubMed] [Google Scholar]
- Helmes C. T., Hillesund T., Boutwell R. K. The binding of tritium-labeled phorbol esters to the macromolecular constituents of mouse epidermis. Cancer Res. 1974 Jun;34(6):1360–1365. [PubMed] [Google Scholar]
- Hirata F., Toyoshima S., Axelrod J., Waxdal M. J. Phospholipid methylation: a biochemical signal modulating lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1980 Feb;77(2):862–865. doi: 10.1073/pnas.77.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Stadler B. M., Rabin H. Synthesis of biologically active interleukin 2 by Xenopus oocytes in response to poly(A)-RNA from a gibbon T-cell line. J Biol Chem. 1982 Feb 25;257(4):1587–1590. [PubMed] [Google Scholar]
- Nagle D. S., Jaken S., Castagna M., Blumberg P. M. Variation with embryonic development and regional localization of specific [3H]phorbol 12,13-dibutyrate binding to brain. Cancer Res. 1981 Jan;41(1):89–93. [PubMed] [Google Scholar]
- Perrella F. W., Ashendel C. L., Boutwell R. K. Specific high-affinity binding of the phorbol ester tumor promoter 12-O-tetradecanoylphorbol-13-acetate to isolated nuclei and nuclear macromolecules in mouse epidermis. Cancer Res. 1982 Sep;42(9):3496–3501. [PubMed] [Google Scholar]
- Sando J. J., Hilfiker M. L., Piacentini M. J., Laufer T. M. Identification of phorbol ester receptors in T-cell growth factor-producing and -nonproducing EL4 mouse thymoma cells. Cancer Res. 1982 May;42(5):1676–1680. [PubMed] [Google Scholar]
- Sando J. J., Hilfiker M. L., Salomon D. S., Farrar J. J. Specific receptors for phorbol esters in lymphoid cell populations: role in enhanced production of T-cell growth factor. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1189–1193. doi: 10.1073/pnas.78.2.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Todaro G. J. Specific high affinity cell membrane receptors for biologically active phorbol and ingenol esters. Nature. 1980 Dec 4;288(5790):451–455. doi: 10.1038/288451a0. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Scribner J. D., Rice J. M., Das S. B., Thompson S. Inhibition by dexamethasone of intracellular binding of phorbol esters to mouse skin. J Natl Cancer Inst. 1974 May;52(5):1611–1618. doi: 10.1093/jnci/52.5.1611. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Phorbol myristate acetate-induced release of granule enzymes from human neutrophils: inhibition by the calcium antagonist, 8-(N,N-diethylamino)-octyl 3,4,5-trimethoxybenzoate hydrochloride. Biochem Biophys Res Commun. 1979 Nov 14;91(1):263–271. doi: 10.1016/0006-291x(79)90612-0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y., Tamura A., Fujii T. A role of membranes in the activation of a new multifunctional protein kinase system. J Biochem. 1979 Aug;86(2):575–578. doi: 10.1093/oxfordjournals.jbchem.a132557. [DOI] [PubMed] [Google Scholar]
- Takai Y., Yamamoto M., Inoue M., Kishimoto A., Nishizuka Y. A proenzyme of cyclic nucleotide-independent protein kinase and its activation by calcium-dependent neutral protease from rat liver. Biochem Biophys Res Commun. 1977 Jul 25;77(2):542–550. doi: 10.1016/s0006-291x(77)80013-2. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Mueller G. C. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-accelerated phospholipid metabolism by 5,8,11,14-eicosatetraynoic acid. Cancer Res. 1980 Mar;40(3):776–781. [PubMed] [Google Scholar]
- Wertz P. W., Mueller G. C. Rapid stimulation of phospholipid metabolism in bovine lymphocytes by tumor-promoting phorbol esters. Cancer Res. 1978 Sep;38(9):2900–2904. [PubMed] [Google Scholar]
- Yamamoto M., Takai Y., Inoue M., Kishimoto A., Nishizuka Y. Characterization of cyclic nucleotide-independent protein kinase produced enzymatically from its proenzyme by calcium-dependent neutral protease from rat liver. J Biochem. 1978 Jan;83(1):207–212. doi: 10.1093/oxfordjournals.jbchem.a131893. [DOI] [PubMed] [Google Scholar]


