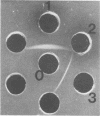
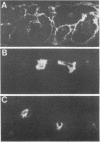
Abstract
Differentiation of the metanephric mesenchyme, which is triggered by an inductive tissue interaction, has been shown to proceed in a chemically defined medium containing transferrin. Here, we report that neither transferrin-depleted serum nor a chemically defined medium devoid of transferrin promote differentiation and that activity can be restored by the addition of transferrin. It thus appears that we have identified the serum factor required for kidney differentiation. Transferrin seems to affect differentiation by stimulating cell proliferation. We show by using an organ-culture model system that only mesenchymes induced to differentiate by the 24-hr tissue interaction respond to transferrin by proliferation and differentiation, whereas uninduced mesenchymes remain unresponsive. The inductor tissue used is not responsive to transferrin. Thus, the data suggest that the short-range cell-mediated tissue interaction acts by making the nephrogenic mesenchyme responsive to the long-range mediator, which is transferrin. Transferrin is suggested to be an important circulating growth factor required for proliferation during embryogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978 Aug;205(2):181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Applewhite G. T., Underwood L. E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980 Mar 15;75(2):315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Drews U., Drews U. Regression of mouse mammary gland anlagen in recombinants of Tfm and wild-type tissues: testosterone acts via the mesenchyme. Cell. 1977 Mar;10(3):401–404. doi: 10.1016/0092-8674(77)90027-7. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Alitalo K., Vaheri A., Timpl R., Saxén L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):485–489. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Lehtonen E., Saxén L., Timpl R. Shift in collagen type as an early response to induction of the metanephric mesenchyme. J Cell Biol. 1981 May;89(2):276–283. doi: 10.1083/jcb.89.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Miettinen A., Virtanen I., Wahlström T., Dawnay A., Saxén L. In vitro segregation of the metanephric nephron. Dev Biol. 1981 May;84(1):88–95. doi: 10.1016/0012-1606(81)90373-0. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Thesleff I., Miettinen A., Saxén L. Organogenesis in a defined medium supplemented with transferrin. Cell Differ. 1981 Nov;10(5):281–288. doi: 10.1016/0045-6039(81)90010-5. [DOI] [PubMed] [Google Scholar]
- Ewton D. Z., Florini J. R. Effects of the somatomedins and insulin on myoblast differentiation in vitro. Dev Biol. 1981 Aug;86(1):31–39. doi: 10.1016/0012-1606(81)90312-2. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- GROBSTEIN C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp Cell Res. 1956 Apr;10(2):424–440. doi: 10.1016/0014-4827(56)90016-7. [DOI] [PubMed] [Google Scholar]
- Goldin G. V., Opperman L. A. Induction of supernumerary tracheal buds and the stimulation of DNA synthesis in the embryonic chick lung and trachea by epidermal growth factor. J Embryol Exp Morphol. 1980 Dec;60:235–243. [PubMed] [Google Scholar]
- Gospodarowicz D., Delgado D., Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4094–4098. doi: 10.1073/pnas.77.7.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haba G. de L., Cooper G. W., Elting V. Hormonal requirements for myogenesis of striated muscle in vitro: insulin and somatotropin. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1719–1723. doi: 10.1073/pnas.56.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R. The development of rat palatal shelves in vitro. An ultrastructural analysis of the inhibition of epithelial cell death and palate fusion by the epidermal growth factor. Dev Biol. 1975 Jul;45(1):90–102. doi: 10.1016/0012-1606(75)90244-4. [DOI] [PubMed] [Google Scholar]
- Heuberger B., Fitzka I., Wasner G., Kratochwil K. Induction of androgen receptor formation by epithelium-mesenchyme interaction in embryonic mouse mammary gland. Proc Natl Acad Sci U S A. 1982 May;79(9):2957–2961. doi: 10.1073/pnas.79.9.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. E. Effect of insulin on fetal growth. Semin Perinatol. 1978 Oct;2(4):319–328. [PubMed] [Google Scholar]
- Jost A. Fetal hormones and fetal growth. Contrib Gynecol Obstet. 1979;5:1–20. [PubMed] [Google Scholar]
- Kratochwil K., Schwartz P. Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4041–4044. doi: 10.1073/pnas.73.11.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Lehtonen E. Tranmission of signals in embryonic induction. Med Biol. 1976 Apr;54(2):108–128. [PubMed] [Google Scholar]
- Miettinen A., Linder E. Membrane antigens shared by renal proximal tubules and other epithelia associated with absorption and excretion. Clin Exp Immunol. 1976 Mar;23(3):568–577. [PMC free article] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., White R. M., Knight A. B., Higa O. Z. Increased levels of multiplication-stimulating activity, an insulin-like growth factor, in fetal rat serum. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3649–3653. doi: 10.1073/pnas.77.6.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nexø E., Hollenberg M. D., Figueroa A., Pratt R. M. Detection of epidermal growth factor-urogastrone and its receptor during fetal mouse development. Proc Natl Acad Sci U S A. 1980 May;77(5):2782–2785. doi: 10.1073/pnas.77.5.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordling S., Aho S. Fluorimetric microassay of DNA using a modified thiobarbituric acid assay. Anal Biochem. 1981 Aug;115(2):260–266. doi: 10.1016/0003-2697(81)90004-x. [DOI] [PubMed] [Google Scholar]
- Oh T. H., Markelonis G. J. Dependence of in vitro myogenesis on a trophic protein present in chicken embryo extract. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6922–6925. doi: 10.1073/pnas.77.11.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Sanford K. K., Evans V. J. Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J Natl Cancer Inst. 1972 Dec;49(6):1705–1712. doi: 10.1093/jnci/49.6.1705. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Liotta L. A., Kidwell W. R. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981 Jan;78(1):382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara V. R., Hall K., Rodeck C. H., Wetterberg L. Human embryonic somatomedin. Proc Natl Acad Sci U S A. 1981 May;78(5):3175–3179. doi: 10.1073/pnas.78.5.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén L., Lehtonen E. Transfilter induction of kidney tubules as a function of the extent and duration of intercellular contacts. J Embryol Exp Morphol. 1978 Oct;47:97–109. [PubMed] [Google Scholar]
- Shindelman J. E., Ortmeyer A. E., Sussman H. H. Demonstration of the transferrin receptor in human breast cancer tissue. Potential marker for identifying dividing cells. Int J Cancer. 1981 Mar 15;27(3):329–334. doi: 10.1002/ijc.2910270311. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R. Antibodies to collagens and procollagens. Methods Enzymol. 1982;82(Pt A):472–498. doi: 10.1016/0076-6879(82)82079-x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio T., Jainchill J., Clement K., Saxén L. Studies on kidney tubulogenesis. VI. Survival and nucleic acid metabolism of differentiating mouse metanephrogenic mesenchyme in vitro. J Cell Physiol. 1965 Dec;66(3):311–317. doi: 10.1002/jcp.1030660308. [DOI] [PubMed] [Google Scholar]
- WESSELLS N. K. SUBSTRATE AND NUTRIENT EFFECTS UPON EPIDERMAL BASAL CELL ORIENTATION AND PROLIFERATION. Proc Natl Acad Sci U S A. 1964 Aug;52:252–259. doi: 10.1073/pnas.52.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Wartiovaara J., Nordling S., Lehtonen E., Saxén L. Transfilter induction of kidney tubles: correlation with cytoplasmic penetration into nucleopore filters. J Embryol Exp Morphol. 1974 Jun;31(3):667–682. [PubMed] [Google Scholar]