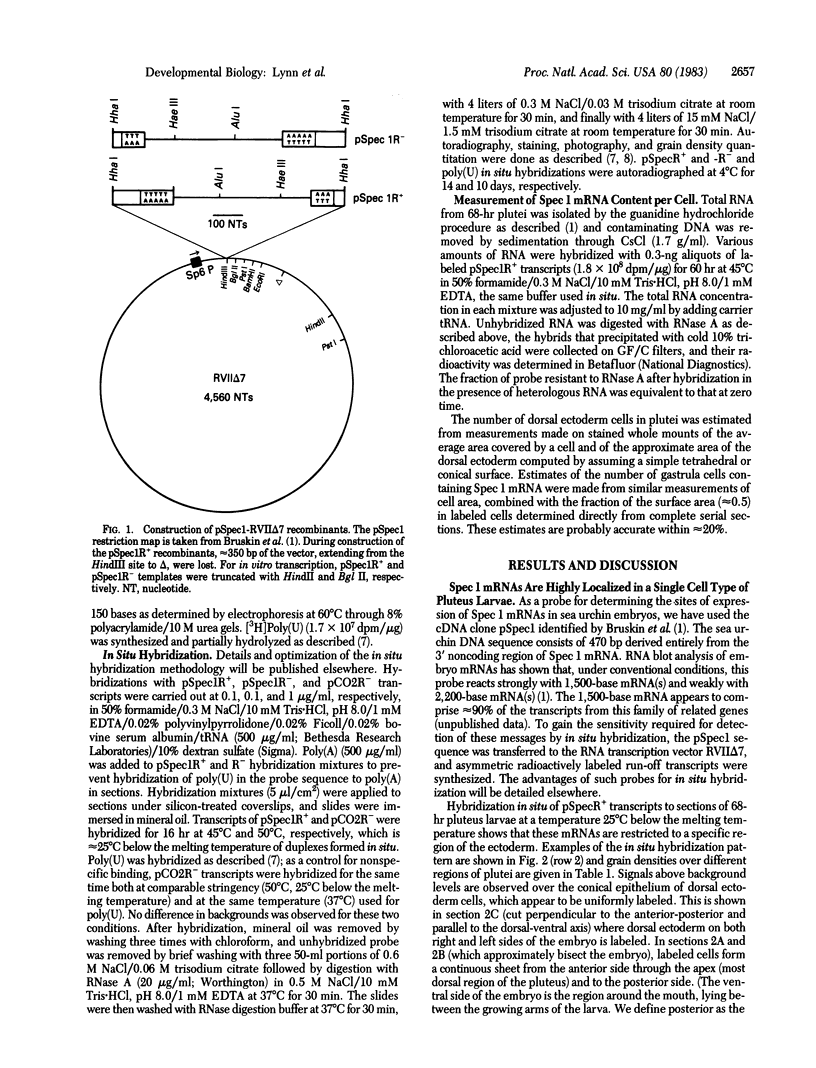
Abstract
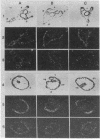
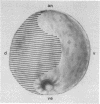
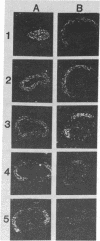
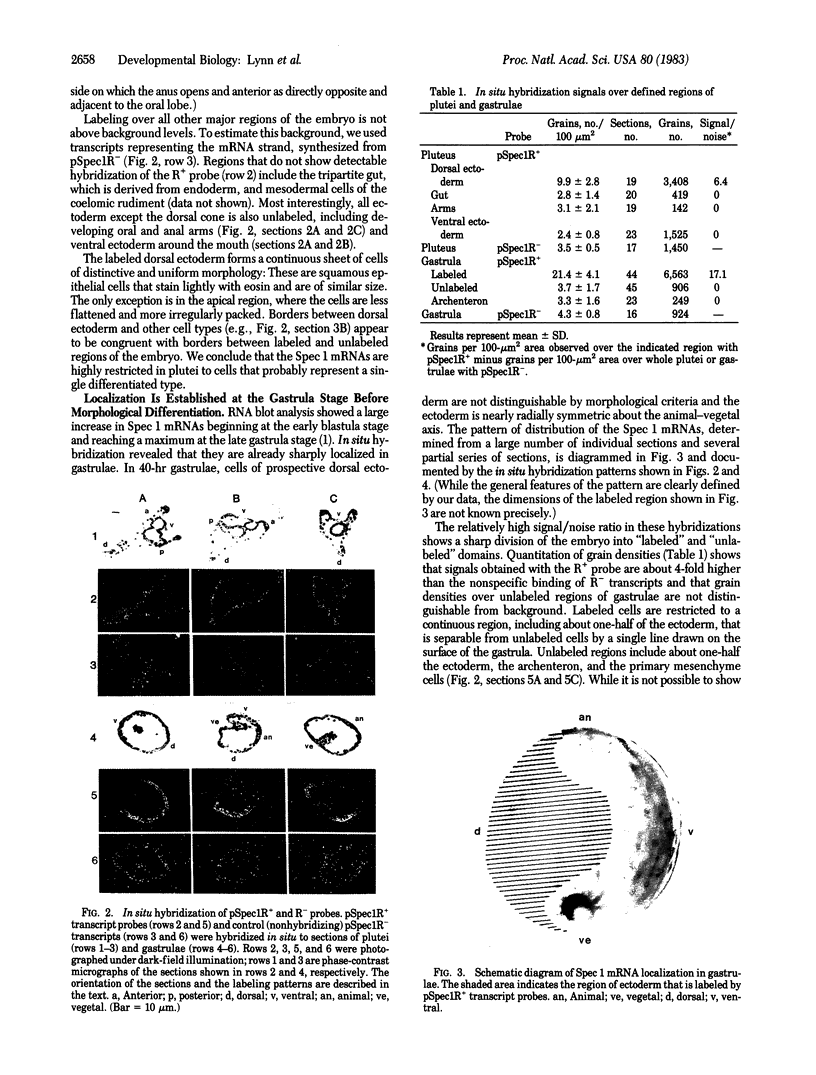
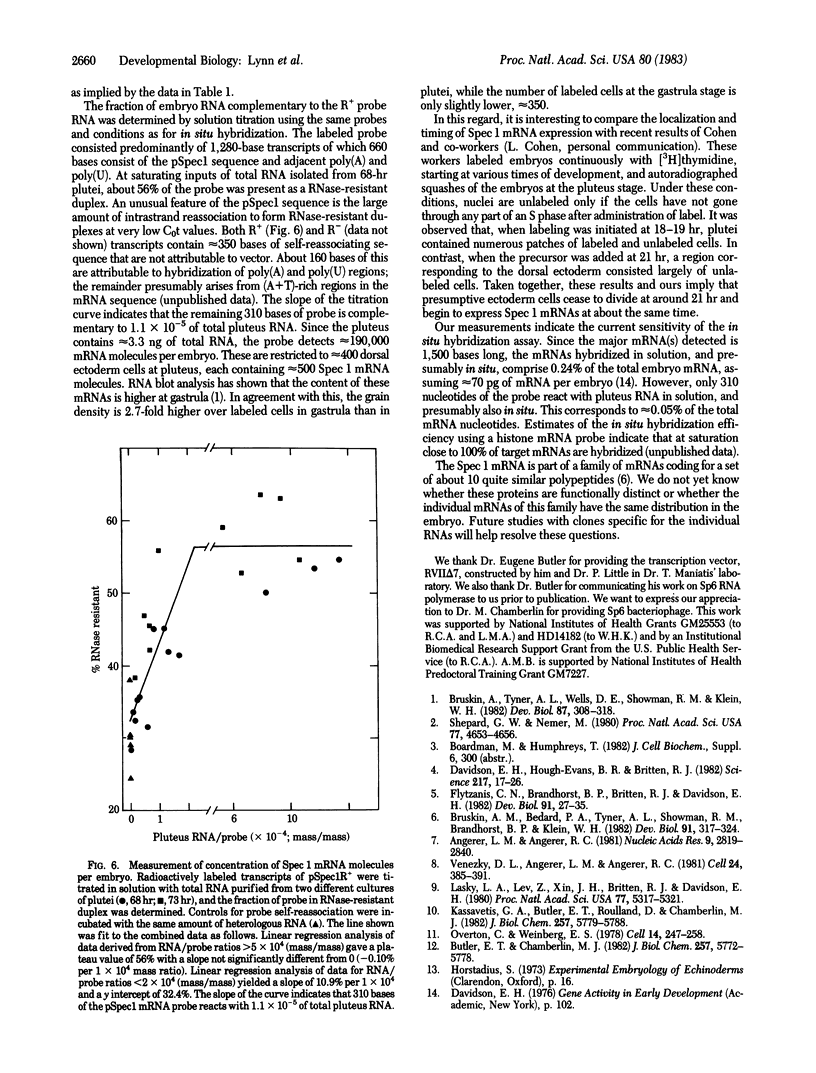
Spec 1 mRNAs increase 100-fold in abundance per embryo during early sea urchin development. Previous studies indicated an enrichment of this mRNA in ectoderm fractions of gastrulae and plutei. We have determined the precise localization of this mRNA by in situ hybridization techniques. In pluteus larvae, the mRNA is highly restricted to a set of morphologically uniform ectoderm cells in the dorsal part of the embryo. The mRNA is not detectable in other regions of ectoderm or in endoderm and mesoderm. The pattern of localization is already established at the gastrula stage, before these cells are distinguishable by morphological criteria. This pattern of distribution of Spec 1 mRNA is distinct from that of bulk poly(A)+ mRNA. Measurements of the amount of Spec 1 mRNA per embryo and the number of cells containing this RNA indicate that there are about 500 Spec 1 mRNA molecules per cell at the pluteus stage and probably twice as many at the gastrula stage. These results indicate that the sensitivity of the in situ hybridization method allows detection of sequences that comprise approximately equal to 0.05% of the embryo mRNA nucleotides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruskin A. M., Bedard P. A., Tyner A. L., Showman R. M., Brandhorst B. P., Klein W. H. A family of proteins accumulating in ectoderm of sea urchin embryos specified by two related cDNA clones. Dev Biol. 1982 Jun;91(2):317–324. doi: 10.1016/0012-1606(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Bruskin A. M., Tyner A. L., Wells D. E., Showman R. M., Klein W. H. Accumulation in embryogenesis of five mRNAs enriched in the ectoderm of the sea urchin pluteus. Dev Biol. 1981 Oct 30;87(2):308–318. doi: 10.1016/0012-1606(81)90154-8. [DOI] [PubMed] [Google Scholar]
- Butler E. T., Chamberlin M. J. Bacteriophage SP6-specific RNA polymerase. I. Isolation and characterization of the enzyme. J Biol Chem. 1982 May 25;257(10):5772–5778. [PubMed] [Google Scholar]
- Davidson E. H., Hough-Evans B. R., Britten R. J. Molecular biology of the sea urchin embryo. Science. 1982 Jul 2;217(4554):17–26. doi: 10.1126/science.6178156. [DOI] [PubMed] [Google Scholar]
- Flytzanis C. N., Brandhorst B. P., Britten R. J., Davidson E. H. Developmental patterns of cytoplasmic transcript prevalence in sea urchin embryos. Dev Biol. 1982 May;91(1):27–35. doi: 10.1016/0012-1606(82)90004-5. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Butler E. T., Roulland D., Chamberlin M. J. Bacteriophage SP6-specific RNA polymerase. II. Mapping of SP6 DNA and selective in vitro transcription. J Biol Chem. 1982 May 25;257(10):5779–5788. [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton G. C., Weinberg E. S. Length and sequence heterogeneity of the histone gene repeat unit of the sea urchin, S. purpuratus. Cell. 1978 Jun;14(2):247–257. doi: 10.1016/0092-8674(78)90111-3. [DOI] [PubMed] [Google Scholar]
- Shepherd G. W., Nemer M. Developmental shifts in frequency distribution of polysomal mRNA and their posttranscriptional regulation in the sea urchin embryo. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4653–4656. doi: 10.1073/pnas.77.8.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezky D. L., Angerer L. M., Angerer R. C. Accumulation of histone repeat transcripts in the sea urchin egg pronucleus. Cell. 1981 May;24(2):385–391. doi: 10.1016/0092-8674(81)90328-7. [DOI] [PubMed] [Google Scholar]