TRAF6 mediates specific effects of LMP1 in vivo
Keywords: antibody, B cell, CD40, EBV, mouse
Abstract
EBV-encoded latent membrane protein 1 (LMP1) is critical for EBV-driven B-cell transformation and most EBV-associated malignancies and is also implicated in exacerbation of autoimmunity. LMP1 functionally mimics the TNFR superfamily member CD40, but LMP1-induced signals and downstream B-cell functions are amplified and sustained compared with those mediated by CD40. CD40 and LMP1 both depend upon TNFR-associated factor (TRAF) adaptor molecules to mediate signaling but use them differently. LMP1 is dependent upon TRAFs 3 and 5 to deliver B-cell activation signals, while CD40 predominantly uses TRAFs 2 and 6 for this purpose. Both LMP1 and CD40 functions in B cells require TRAF6, which physically associates with both receptors but via different binding sites. In B-cell CD40 signaling, TRAF6 is required for a particular subset of CD40-dependent immune functions in vivo. Inasmuch as CD40 and LMP1 use other TRAFs differentially, we predicted that TRAF6 is critical for a specific subset of LMP1 functions in vivo and that this subset will be overlapping but distinct from the TRAF6-requiring functions of CD40. This study tests this prediction using a B-cell-specific TRAF6-deficient mouse model. We found that B-cell TRAF6 is important for LMP1-mediated antibody and autoantibody production in mice, as well as germinal center formation, but not the secondary lymphoid organ enlargement that results from LMP1 transgenic expression. Results highlight differential TRAF6 requirements for specific B-cell functions by LMP1 versus CD40. These differences may make important contributions to the contrasts between normally regulated CD40 versus pathogenic LMP1-mediated signals.
Introduction
EBV, the causative agent of infectious mononucleosis, latently infects memory B cells in >90% of humans (1, 2). The EBV-encoded latent membrane protein 1 (LMP1) is not expressed in infected cells unless EBV emerges from latency, usually in the context of immunosuppression or autoimmunity (1, 3, 4). Aberrant EBV reactivation in immunocompromised individuals is associated with B-cell malignancies. LMP1 is required for EBV-mediated B-cell transformation and is expressed in the majority of EBV-associated malignancies (2, 4–12). EBV reactivation is also observed in systemic lupus erythematosus and rheumatoid arthritis (13, 14), and LMP1 has been implicated in the exacerbation of autoimmunity (15, 16).
LMP1 contains a short cytoplasmic (CY) N-terminal domain that anchors the protein to the plasma membrane and regulates its rapid processing. The protein has six transmembrane (TM) domains that promote ligand-independent, constitutive signaling via spontaneous self-aggregation and oligomerization within the plasma membrane, and a long CY C-terminal domain that mediates signal transduction (17–20). Two subdomains within the C-terminus, C-terminal activating region (CTAR) 1 and CTAR2, are the sites of formation of signaling complexes (17, 20, 21). It has been demonstrated, in both cell lines and primary cells, that the CY C-terminal domain is necessary and sufficient to mediate most LMP1 functions in B lymphocytes, including early pathway activation and downstream effector functions (2, 18, 19, 22–25).
LMP1 is a functional mimic in B cells of the TNFR superfamily member CD40, with both molecules inducing activation of MAP kinases, NF-κB pathways, cytokine and antibody production, and up-regulation of costimulatory and adhesion molecules (26). Additionally, LMP1 substitutes for CD40 in vivo to generate a humoral immune response in mice, including immunoglobulin isotype switching, germinal center (GC) formation and antibody affinity maturation (22). However, LMP1 delivers amplified and sustained signals to B cells compared with CD40 (24–27). As LMP1 and CD40 lack enzymatic activity, both use TNFR-associated factors (TRAFs) to induce signaling (26), but they use TRAFs differently. This distinct TRAF usage and regulation contributes to the differential effects of CD40 versus LMP1 on B cells (10, 17, 19, 23, 28–30). Specifically, TRAF3 is a negative regulator of CD40 signaling to B cells but a positive mediator of LMP1 effects, while CD40, but not LMP1, requires both TRAFs 1 and 2 for optimal B-cell activation (19, 30). Additionally, LMP1 is much more dependent upon TRAF5 to activate B cells than is CD40 (23, 29).
Both CD40 and LMP1 also employ TRAF6 for B-cell activation. Both in vitro and in vivo functions of CD40 require TRAF6 (26, 31–34). In vitro, TRAF6 is also required for LMP1-mediated activation of the MAP kinases JNK, p38, TGF-β-activated kinase 1 and the canonical NF-κB pathway, as well as up-regulation of the costimulatory molecule CD80 (10). However, LMP1 requires the TRAF6 TRAF-C receptor-binding domain to activate these TRAF6-dependent pathways, while CD40 can utilize a TRAF6 molecule that lacks the receptor-binding domain (10, 31, 34). TRAF6 binds to a unique membrane-proximal site on CD40 but interacts with the shared TRAF1/2/3/5 binding site (TBS) of LMP1 (10, 26, 31). The LMP1 TBS is critical for inducing production of IgG1 and autoantibodies, as well as GC formation, both spontaneous and in response to immunization. Expression of LMP1 as a transgene in mice induces secondary lymphoid organ enlargement, and this effect does not require the TBS (35). However, mutations in the shared TBS affect the binding of multiple TRAFs to LMP1, so studies of LMP1 mutants cannot delineate the specific roles played by TRAF6 in LMP1 functions in vivo, the knowledge gap that this study was designed to fill.
Given the significant differences in the requirements of TRAF6 in vitro by LMP1 and CD40 (10), we predicted that TRAF6 is critical for a unique subset of LMP1 versus CD40 functions in vivo, and this study addressed this prediction, utilizing a mouse model in which B cells express a transgenic (Tg) LMP1 molecule in the presence or absence of TRAF6.
Methods
Reagents and antibodies
FCS was obtained from Atlanta Biologicals (Atlanta, GA, USA). ELISA alkaline phosphatase substrate tablets (5mg) were purchased from Sigma–Aldrich (St Louis, MO, USA). Proteinase K was purchased from Roche Diagnostics (Indianapolis, IN, USA). Rat serum was purchased from Pel-Freez Biologicals (Rogers, AR, USA). FITC-labeled peanut agglutinin (PNA) was purchased from Vector Laboratories (Burlingame, CA, USA). PageRuler prestained protein electrophoresis size markers were purchased from Fermentas, Inc. (Glen Burnie, MD, USA). Percoll density gradient solution was purchased from GE Healthcare (Uppsala, Sweden). Biolase DNA polymerase was purchased from Bioline (Tauton, MA, USA). HBSS was purchased from Invitrogen (Grand Island, NY, USA).
The following primary antibodies were used for western blotting: mouse anti-actin (Millipore, Billerica, MA, USA or Sigma–Aldrich), chicken anti-TRAF6 (Medical and Biological Laboratories, Nagoya, Japan) and S12 (mouse anti-LMP1 IgG mAb; produced from a hydridoma that was a gift from Dr Fred Wang, Harvard University, Cambridge, MA, USA). The secondary antibodies used included goat anti-mouse IgG and goat anti-chicken IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The following antibodies were used for immunofluorescence flow cytometry: rat anti-mouse CD16/32 (to block FcR binding), FITC-labeled anti-mouse B220 (clone RA3-6B2), FITC-labeled rat anti-IgG, FITC-labeled anti-mouse CD21/35, FITC-labeled anti-mouse CD11b, PE-labeled rat anti-IgG, PE-labeled anti-mouse B220 (clone RA3-6B2), PE-labeled anti-mouse CD3, PE-labeled anti-mouse CD23, allophycocyanin (APC)-labeled anti-mouse B220 (clone RA3-6B2) and APC-labeled rat anti-IgG (eBioscience, San Diego, CA, USA). The following antibodies were used for ELISAs: purified mouse IgA, IgG1, IgG2b, IgG3 and IgM antibodies for ELISA standards, goat anti-mouse IgA, goat anti-mouse IgG1, goat anti-mouse IgG2b, goat anti-mouse IgG3, goat anti-mouse IgM, AP-conjugated goat anti-mouse IgA, AP-conjugated goat anti-mouse IgG1, AP-conjugated goat anti-mouse IgG2b, AP-conjugated goat anti-mouse IgG3 and AP-conjugated goat anti-mouse IgM (Southern Biotech, Birmingham, AL, USA).
Mice
The mouse (m)CD40−/− (36) and mCD40LMP1 Tg mice (22), TRAF6flox/flox mice (a gift from Dr Yongwon Choi, University of Pennsylvania, Philadelphia, PA, USA) (32, 37), and CD19Cre mice (Jackson Laboratories, Bar Harbor, ME, USA) (38) were described previously. Briefly, the mCD40LMP1 transgene consists of the extracellular and TM domains of mCD40 and the CY domain of LMP1 (22). Expression of mCD40LMP1 is driven by the MHC class II Eα promoter on a CD40−/− background. Thus, B lymphocytes and myeloid cells normally expressing CD40 instead express mCD40LMP1 (22), and the endogenous CD40 ligand, CD154, induces signaling through mCD40LMP1 in these mice (22). EBV can also infect myeloid cells (26), so it is reasonable to express LMP1 on these cell types and B cells. Wild-type (WT) LMP1 signals constitutively in a ligand-independent manner (17–20), so we created the mCD40LMP1 chimera to allow deliberate initiation of LMP1 signaling, and direct comparison of the functions of the signaling domains of CD40 versus LMP1 (22). Another LMP1 Tg mouse model has been described in which mCD40LMP1 is expressed only in B cells on a CD40-deficient background (39). However, loss of CD40-dependent functions in other cell types could negatively impact immune responses in these mice, and we wanted to directly compare CD40- versus LMP1-mediated immune functions in vivo. For example, expression of WT LMP1 restricted to B cells on a CD40−/− background mimics many CD40-mediated immune functions, but these mice do not produce high-affinity antibodies or form GCs, both of which are restored in our mCD40LMP1 Tg mice (22, 40). This difference is likely due to expression of mCD40LMP1 on dendritic cells, as CD40 signals to these cells are important for optimal T-cell activation and B-cell help, and the CD40−/− WT LMP1 mice do not express CD40 on myeloid cells. Thus, our model allows us to investigate the role of TRAF6 in B-cell functions, where the only change is in B-cell TRAF6 expression, without the complication of uncompensated CD40 deficiency in non-B cells. Using mCD40LMP1 Tg mice, we demonstrated that LMP1 substitutes for CD40-mediated humoral immune responses in vivo (22). Mice of all strains used here develop and breed normally, and they were genotyped by PCR using tail snip DNA as previously described (22). Primers used to screen the TRAF6flox/flox mice were 5′, 5′-GGTGTAGCGTCCATGTACTTGTG-3′, and 3′, 5′-GATCGCCATATTTGGTGTCCCC-3′ (Integrated DNA Technologies, Inc., Coralville, IA, USA). All mice were housed in a pathogen-free barrier facility with restricted access, and all procedures were performed as approved by the University of Iowa Animal Care and Use Committee, Iowa City, IA.
Mouse splenic B-cell isolation
Splenic B cells were first isolated by centrifugation through a Percoll density gradient as previously described (35), and B cells were further purified by negative selection using MACS mouse CD43 (Ly-48) MicroBeads, MACS LD separation columns, and a MidiMACS separator (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s protocols. The purity of isolated B cells was monitored by FACS analysis using FITC-conjugated antibodies. B-cell purity was >95%. Flow cytometry was performed on a Guava EasyCyte cytometer using CytoSoft software (Guava Technologies, Inc., Hayward, CA, USA), and data were analyzed using FlowJo Software (Tree Star, Ashland, OR, USA).
Western blots
Whole cell lysates from 2.5×106 splenic B cells were prepared by centrifuging the cells at 4°C for 1min at ~3200 × g, removing the supernatant, and adding 75 µl of 2× SDS–PAGE loading buffer to the cell pellet. Lysates were sonicated and denatured as previously described (10). A sample of 10–20 µl was resolved by 10% SDS–PAGE, and proteins were transferred to Immobilon-P polyvinylidenefluoride membranes (Millipore). Membranes were blocked with 10% dry milk in Tris-buffered saline with Tween 20(TBST) (3% 5M NaCl, 1% 1M Tris and 0.1% Tween 20, in H2O) for 1h, washed in TBST, and incubated overnight at 4°C with one of the above primary antibodies. Blots were washed in 10% dry milk in TBST and incubated with secondary antibodies for 1–2h or overnight and developed using SuperSignal West Pico with SuperSignal West Femto Maximum Sensitivity Substrate as needed (Thermo Scientific, Rockford, IL, USA). Western blot chemiluminescence of protein bands was read with an LAS-4000 low-light camera and analyzed using Multi Gauge software (Fujifilm Life Science, Edison, NJ, USA).
Flow cytometric analysis of B cells
Spleens and cervical lymph nodes (LNs) were harvested from 4- to 6-month-old mice. Whole splenocytes and lymphocytes were collected, and 5×105 cells were added to wells of a 96-well round-bottom plate in 200 µl HBSS containing 5% FCS. Anti-mouse CD16/32 mAb (0.5mg ml–1) and rat serum (25%) were added 10min prior to staining with FITC-, PE-, and APC-labeled antibodies. Flow cytometry was performed on a FACScalibur (BD Biosciences, San Jose, CA, USA) flow cytometer, and forward/side scatter gating was used to analyze flow cytometric data via FlowJo Software. GC B cells were identified by a distinct population of B220+ PNAhi cells.
Autoantibody production
Sera from 4- to 6-month-old mice were tested for anti-dsDNA antibodies by ELISA (Alpha Diagnostic International, San Antonio, TX, USA) using the manufacturer’s protocols. Sera were measured at a 1:100 dilution, and the HRP-conjugated secondary antibody was goat anti-mouse IgG. Thus, all IgG isotypes were measured. Mouse anti-DNA IgG samples provided by the manufacturer were run concurrently with the unknown samples. ELISA plates were read as previously described (22).
Serum antibodies
Sera from 4- to 6-month-old mice were tested for the presence of antibody isotypes using ELISA as previously described (22, 41, 42). Sera were diluted 1:2000 (IgA), 1:4000 (IgG1 and IgG3), 1:1000 (IgG2b) or 1:8000 (IgM). Detection antibodies were labeled with alkaline phosphatase (1:250 dilution), and plates were developed using alkaline phosphatase substrate.
Statistics
Analyses were performed with GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Statistical comparisons were made using a two-sided unpaired Student’s t-test. P values <0.05 were considered statistically significant.
Results
Generation of mCD40LMP1 Tg B-cell TRAF6−/− mice
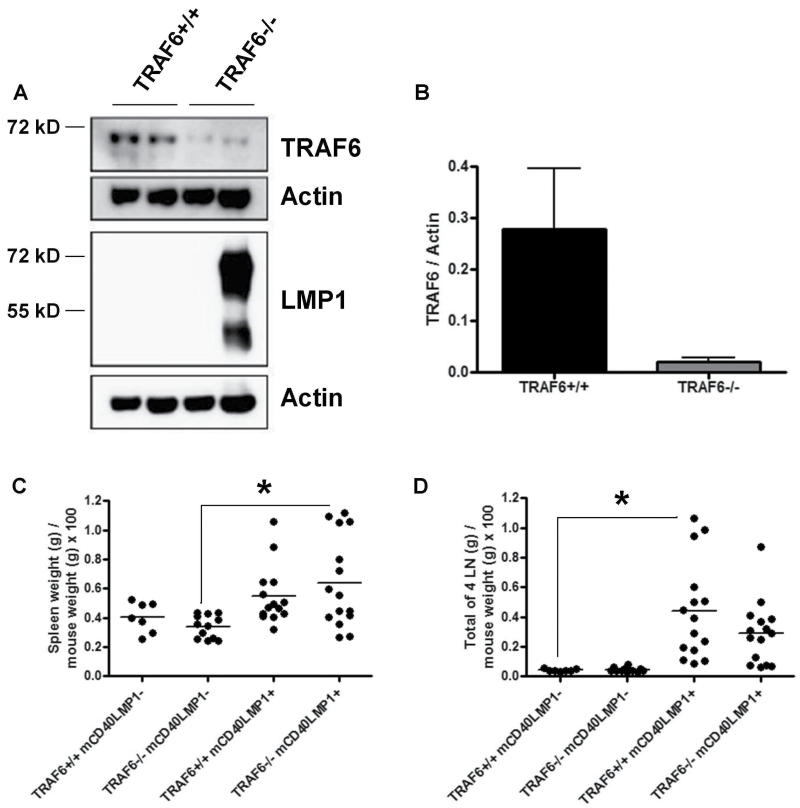
The TRAF6flox/flox, mCD40LMP1 Tg, and CD19Cre mice (used to deplete TRAF6 in B cells specifically) were previously described (22, 37, 38). Conditional B-cell TRAF6−/− mice expressing the mCD40LMP1 transgene were generated by intercrossing, which resulted in four groups of mice: TRAF6flox/flox CD19+/+ CD40−/− mCD40LMP1− (TRAF6+/+ mCD40LMP1–), TRAF6flox/flox CD19Cre/+ CD40−/− mCD40LMP1– (TRAF6−/− mCD40LMP1–), TRAF6flox/flox CD19+/+ CD40−/− mCD40LMP1+ (TRAF6+/+ mCD40LMP1+) and TRAF6flox/flox CD19Cre/+ CD40−/− mCD40LMP1+ (TRAF6−/− mCD40LMP1+). The mCD40LMP1– mice served as controls for non-LMP1-mediated effects of TRAF6 depletion in B cells. Splenic B cells were purified from TRAF6+/+ and TRAF6−/− mice to determine the level of B-cell TRAF6 expression (Fig. 1A and B). TRAF6 levels were reduced by ≥90% compared with WT levels. Thus, CD19Cre-mediated depletion efficiently reduced TRAF6 expression in B cells. As stated in Methods, B-cell purity was >95%. Although a remote possibility, it is unlikely that contaminating cells are the source of residual TRAF6 levels. Previous work showed that CD19Cre-mediated deletion efficiency in B cells is ~90% (38). Thus, this likely accounts for the small amount of residual TRAF6 observed. It is important to note that CD19Cre does not alter TRAF6 levels in non-B-cell splenocytes (32), and that TRAF6 deficiency does not affect mCD40LMP1 expression on B cells (data not shown).
Fig. 1.
Characterization of mCD40LMP1 Tg B-cell-specific TRAF6-deficient mice. (A) Splenic B cells from TRAF6-sufficient or TRAF6-deficient mCD40LMP1-negative (first three lanes) or mCD40LMP1 Tg (right lane) mice were lysed and examined for TRAF6 expression via western blot. Shown are the levels of TRAF6 and LMP1, with actin as a loading control (mCD40LMP1, ~72 kD). (B) Quantification of the samples shown in A, with protein levels of TRAF6 normalized to actin. (C) TRAF6+/+ mCD40LMP1-negative, TRAF6−/− mCD40LMP1-negative, TRAF6+/+ mCD40LMP1 Tg and TRAF6−/− mCD40LMP1 Tg mouse spleen weight expressed as a percentage of mouse weight. Each point represents one mouse. (D) Similar to C, except the combined weight of four LNs normalized to mouse weight is shown. *Statistical differences as determined by the Student’s unpaired t-test, P < 0.05.
Characterization of secondary lymphoid organs
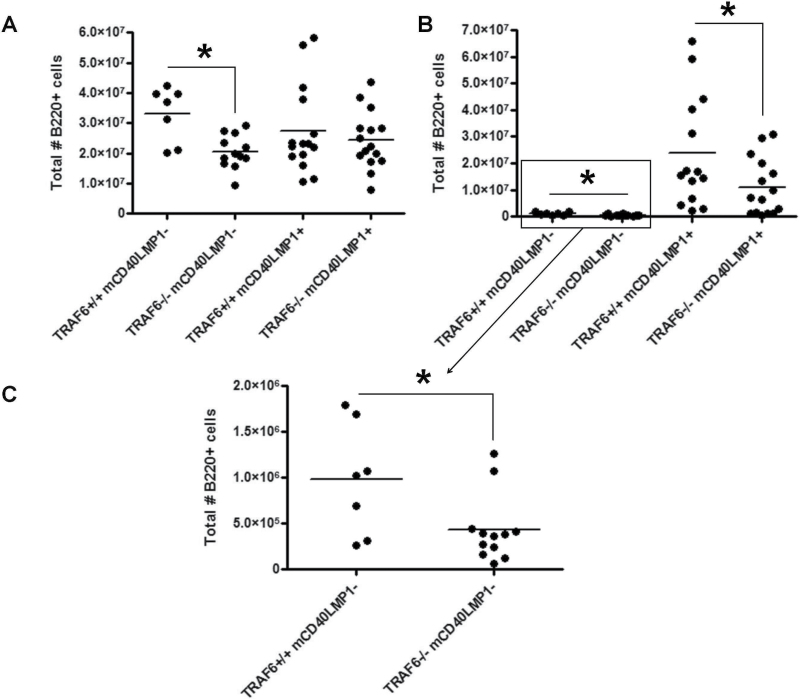
The mCD40LMP1 Tg mice have noticeably enlarged spleens and LNs compared with littermate control or WT mice (22). The LMP1 TBS is not required for this LMP1-specific phenotype (35), indicating that TRAF6 binding to LMP1 is not required, as TRAF6 binds to this site in LMP1 (10). However, TRAF6 mediates certain CD40-induced signals to B cells without binding to CD40 (31, 34). We thus tested the possibility that B-cell TRAF6 contributes to LMP1-induced secondary lymphoid organ enlargement in a manner independent of interaction with the LMP1 TBS. We observed strain-independent mouse-to-mouse variation in body weight (data not shown), so spleen and LN weights were normalized to mouse weight for more accurate comparisons. B-cell TRAF6−/− mCD40LMP1 Tg mice displayed the splenomegaly and lymphadenopathy characteristic of TRAF6+/+ mCD40LMP1 Tg mice (Fig. 1C and D). Although spleen size was not significantly altered by the absence of B-cell TRAF6, loss of TRAF6 in mCD40LMP1– mice resulted in a reduction in total splenic B-cell numbers (Fig. 2A). B-cell TRAF6 depletion led to a decrease in the total numbers of LN B cells in both mCD40LMP1-negative and mCD40LMP1 Tg mice (Fig. 2B and C). No significant differences in splenic or LN T cell or macrophage numbers were observed (data not shown).
Fig. 2.
Characterization of secondary lymphoid organs. (A) Splenocytes from TRAF6+/+ mCD40LMP1-negative, TRAF6−/− mCD40LMP1-negative, TRAF6+/+ mCD40LMP1 Tg and TRAF6−/− mCD40LMP1 Tg mice were stained with anti-B220 antibody to determine the total number of B cells in the spleen. Each point represents one mouse. (B) Similar to A, except the graph depicts the total number of LN B cells. (C) Zoomed-in view of B cells in TRAF6+/+ and TRAF6−/− mCD40LMP1-negative mice in B. *Statistical difference as determined by Student’s unpaired t-test, P < 0.05.
Taken together, these results demonstrate that B-cell TRAF6 deficiency has no significant effect on LMP1-specific secondary lymphoid organ enlargement or B-cell populations in the spleen but does affect LN B-cell numbers. This defect was observed in both mCD40LMP1-negative and mCD40LMP1 Tg mice, indicating that this effect of TRAF6 deficiency is not specific to LMP1 and may be due to altered signaling through other TRAF6-dependent receptors, such as TLRs (32, 43). B cells from B-cell-specific TRAF6-deficient mice exhibit impaired TLR-induced p38, ERK, JNK, Akt and NF-κB activation, as well as IL-6 production (32).
Role of TRAF6 in LMP1-mediated autoreactivity
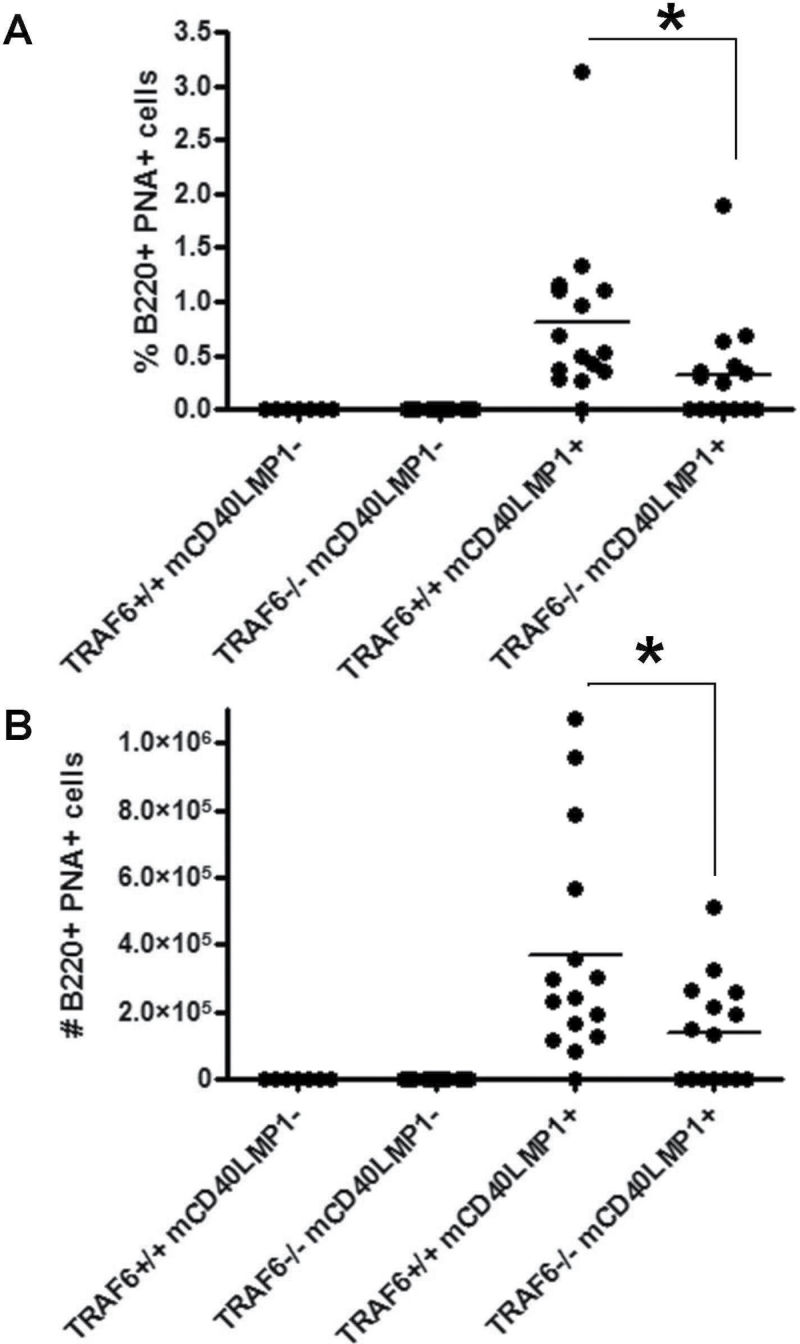
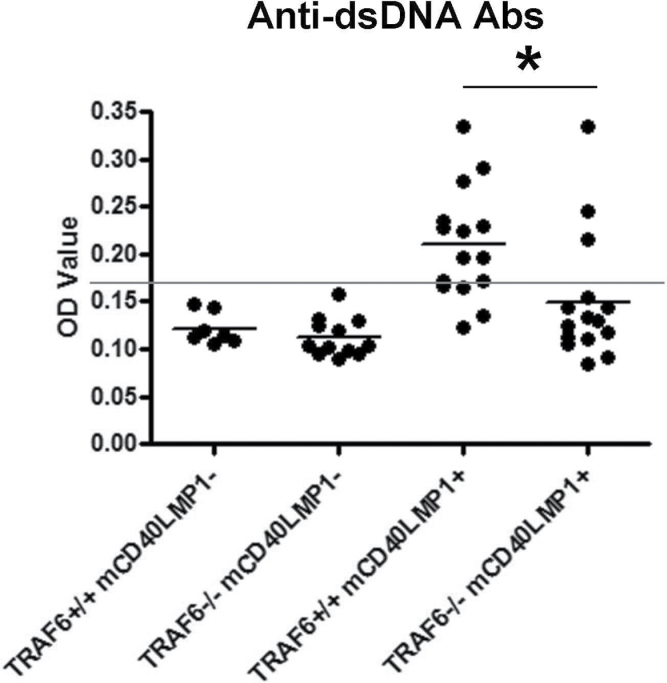
mCD40LMP1 Tg mice have an autoreactive phenotype, developing spontaneous GCs in the absence of immunization and displaying high levels of serum autoantibodies (22). The LMP1 TBS is critical for these in vivo functions (44). B-cell TRAF6 deficiency led to a significant reduction in the incidence of spontaneous GC B cells (14/15 or 93% of TRAF6+/+ mCD40LMP1 Tg mice versus 8/15 or 53% of TRAF6−/− mCD40LMP1 Tg mice), as well as both the percentage and number of B cells in the spleen binding to PNA, a marker of GC B cells (Fig. 3A and B). This difference in spontaneous GC B cells was not due to a difference in B-cell numbers between TRAF6-sufficient and TRAF6-deficient mCD40LMP1 Tg mice, as we observed no change in splenic B-cell number in the absence of TRAF6 (Fig. 2A). These data were of particular interest as, in contrast, TRAF6 is dispensable for CD40-mediated GC formation (32). IL-6 plays a role in GC formation (45). However, only B cells in our mouse model lack TRAF6, while mCD40LMP1 is also expressed on dendritic cells, monocytes and macrophages in these mice. All these cell types are TRAF6-sufficient and produce more IL-6 on a per-cell basis than do B cells, so we would not expect systemic IL-6 levels to change in this mouse, and in fact they do not (data not shown). B-cell TRAF6-deficient mCD40LMP1 Tg mice also had significantly decreased levels of anti-dsDNA antibodies compared with TRAF6+/+ mCD40LMP1 Tg mice (Fig. 4). Again, the modest changes observed in B-cell numbers in the LN did not account for the lack of anti-dsDNA antibody production in the majority of TRAF6-deficient mice. Furthermore, these mice were capable of producing immunoglobulin of switched isotypes (see below). Thus, B-cell TRAF6 is important for LMP1-mediated autoreactivity.
Fig. 3.
Impact of TRAF6 on spontaneous GC formation in mCD40LMP1 Tg mice. (A) Splenocytes from TRAF6+/+ mCD40LMP1-negative, TRAF6−/− mCD40LMP1-negative, TRAF6+/+ mCD40LMP1 Tg and TRAF6−/− mCD40LMP1 Tg mice were stained with anti-B220 antibody and the GC marker PNA. The percentage of B220+ PNA+ GC B cells is shown, where each point on the graph represents one mouse. (B) Similar to A, except the total number of B220+ PNA+ GC B cells is shown. *Statistical difference between TRAF6+/+ and TRAF6−/− mCD40LMP1 Tg mice as determined by the Student’s unpaired t-test, P < 0.05.
Fig. 4.
Requirement of TRAF6 in LMP1-induced production of auto-antibodies. Sera from TRAF6+/+ mCD40LMP1-negative, TRAF6−/− mCD40LMP1-negative, TRAF6+/+ mCD40LMP1 Tg and TRAF6−/− mCD40LMP1 Tg mice were tested for levels of antibodies binding to dsDNA via ELISA. The horizontal line represents the baseline as determined by negative controls, where every sample above the line is considered positive. Each point represents one mouse. *Statistical difference between TRAF6+/+ and TRAF6−/− mCD40LMP1 Tg mice as determined by the Student’s unpaired t-test, P < 0.05.
Immunoglobulin isotype production
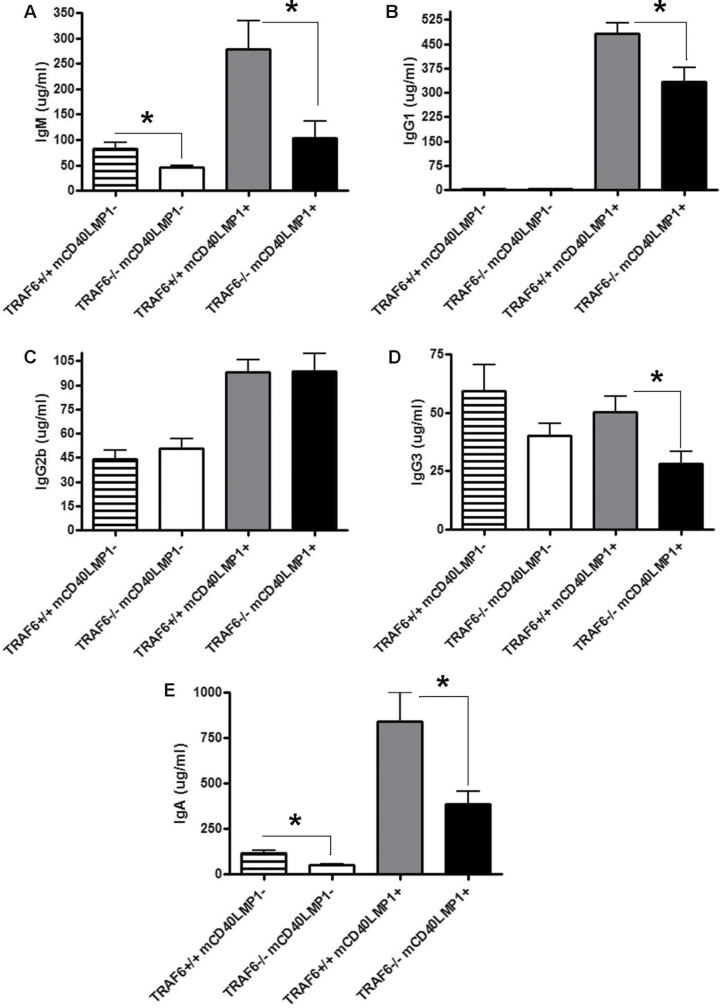
mCD40LMP1 can effectively substitute for CD40 in mediating antibody production in vivo, including immunoglobulin isotype switching and affinity maturation (22). TRAF6 contributes to CD40-induced IgM and IgG2b production but not other serum immunoglobulin isotypes (32), so we investigated the requirement of TRAF6 in LMP1-mediated immunoglobulin isotype production. In mCD40LMP1 Tg mice, depletion of TRAF6 resulted in a significant decrease in basal serum IgM, IgG1, IgG3 and IgA levels but had no effect on IgG2b levels (Fig. 5). TRAF6 depletion in the absence of mCD40LMP1 led to a significant decrease in IgM and IgA levels, as well as a modest reduction in IgG3 levels (Fig. 5). These differences are not due to the modest changes in B-cell numbers observed in Fig. 2, as IgG2b levels were normal in the absence of TRAF6 (Fig. 5). Thus, TRAF6 plays an important role in LMP1-mediated production of various immunoglobulin isotypes. It is important to note that the mCD40LMP1-negative mice are CD40-deficient and thus do not exhibit normal immunoglobulin isotype switching due to the critical roles of CD40 in antibody production and isotype switching (36). Thus, the serum immunoglobulin values obtained in mCD40LMP1– mice are low as they reflect this role for CD40 in antibody production. Although CD40−/− mice exhibit defective T-cell-dependent (TD) immune responses predominantly mediated by IgG1 and IgG2, they display normal T-cell-independent (TI) immune responses and thus produce IgM and other isotypes normally associated with TI responses (36). Therefore, TRAF6-dependent reduction of IgM, IgA and IgG3 in mCD40LMP1– mice reflects a role for TRAF6 in CD40-independent pathways in B cells, such as those mediated by TLRs, where TRAF6 plays a critical role in receptor-mediated signaling pathways (32). However, the differences in immunoglobulin production that we observed in mCD40LMP1 Tg mice are not entirely due to LMP1-independent effects as mCD40LMP1-negative mice do not make IgG1, and changes observed in IgG3 and especially IgM were more dramatic in the mCD40LMP1 Tg mice. Thus, TRAF6 is important in LMP1-mediated antibody production.
Fig. 5.
Role of TRAF6 in Ig production. (A–E) Sera collected from TRAF6+/+ mCD40LMP1-negative (n = 7), TRAF6−/− mCD40LMP1-negative (n = 12), TRAF6+/+ mCD40LMP1 Tg (n = 15) and TRAF6−/− mCD40LMP1 Tg (n = 13) mice were tested for levels of IgM (A), IgG1 (B), IgG2b (C), IgG3 (D) and IgA (E). *Statistical difference between TRAF6+/+ and TRAF6−/− mCD40LMP1 Tg mice as determined by the Student’s unpaired t-test, P < 0.05.
Discussion
TRAF6 plays a key role in early LMP1-mediated B-cell signaling events in vitro (10). Here, we investigated the role of TRAF6 in important LMP1 functions in vivo using CD19Cre to deplete TRAF6 in the B cells of mCD40LMP1 Tg mice. Although B-cell TRAF6 deficiency did not significantly affect secondary lymphoid organ size, it did lead to a modest decrease in LN B-cell numbers and immunoglobulin production. However, B-cell TRAF6 was important for LMP1-mediated development of spontaneous GCs and anti-dsDNA antibody production, as well as basal immunoglobulin production and isotype switching, where effects of TRAF6-deficiency were much greater in mCD40LMP1 Tg compared with mCD40LMP1-negative mice. Thus, TRAF6 is a key mediator of a subset of LMP1-mediated functions unique to B cells.
TRAF6 in CD40-sufficient mice is critical for normal basal IgM and IgG2b levels, and plays a modest role in antigen-specific antibody production in response to TD or TI antigens but has no apparent role in GC formation or antibody affinity maturation following immunization (32). These results reveal that LMP1 in vivo functions in B cells are more highly dependent upon TRAF6 than are CD40 functions. This is consistent with the partially redundant roles of TRAF2 and TRAF6 in CD40-mediated signaling events, whereas TRAF2 has no detectable role in early LMP1-mediated signaling pathways (30, 31). The stronger reliance of LMP1 versus CD40 on TRAF6 may contribute to the dysregulation of LMP1 effects compared with CD40, by making LMP1 less susceptible to TRAF2-mediated regulation.
The TRAF6 TRAF-C receptor-binding domain is required for TRAF6-dependent LMP1-mediated activation of JNK, p38 and NF-κB, as well as CD80 up-regulation in vitro, implying that TRAF6–LMP1 association is important for LMP1-induced TRAF6-dependent signaling pathways (10). The LMP1 TBS, which in contrast to the similar region in CD40 is required for TRAF6 interaction with LMP1 (10), is important for LMP1-mediated production of spontaneous GC B cells and autoantibodies, but not secondary lymphoid organ enlargement (35). The phenotype of LMP1 TBS mutant mice is strikingly similar to that of the B-cell TRAF6-deficient mice presented in this study (35). Taken together, these results support a model in which LMP1 must associate with TRAF6 in its signaling complex for TRAF6-dependent LMP1 functions. This is in direct contrast to CD40, which can use a form of TRAF6 that cannot bind to the receptor to induce key TRAF6-dependent signals (31, 34), and TLRs, which are well known to utilize TRAF6 in this manner (46). For example, CD40 does not require TRAF6 binding for early signaling pathways and up-regulation of costimulatory molecules (31), or antibody production and isotype switching in vivo (33, 34). TRAF5 is also important for LMP1-mediated autoantibody production and development of spontaneous GC B cells (23), suggesting that TRAF6 and TRAF5 may cooperate in these LMP1-mediated functions or show partial redundancy, a possibility that will be explored in future studies.
CD40 or, as a substitute, LMP1 signals are required for GC formation and effective antibody isotype switching (22, 26). TRAF6 plays an important role in CD40-induced basal production of IgM and IgG2b but not in production of other immunoglobulin isotypes (32). Present results show that TRAF6 was critical for LMP1-driven basal production of all immunoglobulin isotypes except IgG2b, illustrating a more prominent role for TRAF6 in LMP1- versus CD40-mediated antibody production involving isotype switching. Furthermore, TRAF6 is dispensable for CD40-mediated GC formation in response to TD immunization (32). Interestingly, our results here demonstrate that TRAF6 plays an important role in LMP1-induced GC B-cell production, further highlighting the unique requirements for TRAF6 in CD40 versus LMP1 functions.
Together with previous studies, our results support a model in which LMP1 requires TRAF6 association with the TBS for early signaling and downstream B-cell functions (10, 35). This is in direct contrast to CD40, which binds TRAF6 at a unique membrane-proximal site distinct from the TBS (31). Additionally, this association is not required for all CD40-mediated TRAF6-dependent signaling pathways and downstream B-cell functions (31, 33, 34). Furthermore, TRAF2 shares partially overlapping roles with TRAF6 in a subset of CD40-mediated signaling pathways (31). Delineating the unique roles of TRAF6 in CD40 and LMP1 functions is critical to our basic understanding of how CD40 normally functions and how LMP1 uses distinct mechanisms to mimic CD40 in a way that drives pathogenesis. Here, we provide evidence that TRAF6 is an important mediator of in vivo LMP1 B-cell functions and that LMP1 relies substantially more on TRAF6 to promote B-cell effector functions than does CD40. As TRAF6 also interacts with LMP1 via a distinct mechanism from CD40, it may be possible to target downstream LMP1 signaling while leaving normal CD40-dependent functions intact. This possibility will be the subject of future investigation.
Funding
American Heart Association Midwest Affiliate Predoctoral Fellowship (to K.M.A.); National Institutes of Health grant (R01-CA099997 to G.A.B.); Iowa City Veterans Affairs Medical Center; Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
References
- 1. Küppers R. 2003. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 3:801. [DOI] [PubMed] [Google Scholar]
- 2. Busch L. K., Bishop G. A. 1999. The EBV transforming protein, latent membrane protein 1, mimics and cooperates with CD40 signaling in B lymphocytes. J. Immunol. 162:2555. [PubMed] [Google Scholar]
- 3. Bishop G. A., Busch L. K. 2002. Molecular mechanisms of B-lymphocyte transformation by Epstein-Barr virus. Microbes Infect. 4:853. [DOI] [PubMed] [Google Scholar]
- 4. Thorley-Lawson D. A. 2001. Epstein-Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1:75. [DOI] [PubMed] [Google Scholar]
- 5. Pai S., Khanna R. 2001. Role of LMP1 in immune control of EBV infection. Semin. Cancer Biol. 11:455. [DOI] [PubMed] [Google Scholar]
- 6. Eliopoulos A. G., Young L. S. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435. [DOI] [PubMed] [Google Scholar]
- 7. Schubert S., Abdul-Khaliq H., Lehmkuhl H. B., et al. 2009. Diagnosis and treatment of post-transplantation lymphoproliferative disorder in pediatric heart transplant patients. Pediatr. Transplant. 13:54. [DOI] [PubMed] [Google Scholar]
- 8. Yin Q., Wang X., McBride J., Fewell C., Flemington E. 2008. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J. Biol. Chem. 283:2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adam P., Bonzheim I., Fend F., Quintanilla-Martínez L. 2011. Epstein-Barr virus-positive diffuse large B-cell lymphomas of the elderly. Adv. Anat. Pathol. 18:349. [DOI] [PubMed] [Google Scholar]
- 10. Arcipowski K. M., Stunz L. L., Graham J. P., Kraus Z. J., Vanden Bush T. J., Bishop G. A. 2011. Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1). J. Biol. Chem. 286:9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scheinfeld A. G., Nador R. G., Cesarman E., Chadburn A., Knowles D. M. 1997. Epstein-Barr virus latent membrane protein-1 oncogene deletion in post-transplantation lymphoproliferative disorders. Am. J. Pathol. 151:805. [PMC free article] [PubMed] [Google Scholar]
- 12. Kaye K. M., Izumi K. M., Kieff E. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl Acad. Sci. U. S. A. 90:9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abou-Raya A., Abou-Raya S. 2006. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun. Rev. 5:331. [DOI] [PubMed] [Google Scholar]
- 14. Gross A. J., Hochberg D., Rand W. M., Thorley-Lawson D. A. 2005. EBV and systemic lupus erythematosus: a new perspective. J. Immunol. 174:6599. [DOI] [PubMed] [Google Scholar]
- 15. Peters A. L., Stunz L. L., Meyerholz D. K., Mohan C., Bishop G. A. 2010. Latent membrane protein 1, the EBV-encoded oncogenic mimic of CD40, accelerates autoimmunity in B6.Sle1 mice. J. Immunol. 185:4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poole B. D., Templeton A. K., Guthridge J. M., Brown E. J., Harley J. B., James J. A. 2009. Aberrant Epstein-Barr viral infection in systemic lupus erythematosus. Autoimmun. Rev. 8:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu S., Xie P., Welsh K., et al. 2005. LMP1 protein from the Epstein-Barr virus is a structural CD40 decoy in B lymphocytes for binding to TRAF3. J. Biol. Chem. 280:33620. [DOI] [PubMed] [Google Scholar]
- 18. Xie P., Bishop G. A. 2004. Roles of TNF receptor-associated factor 3 in signaling to B lymphocytes by carboxyl-terminal activating regions 1 and 2 of the EBV-encoded oncoprotein latent membrane protein 1. J. Immunol. 173:5546. [DOI] [PubMed] [Google Scholar]
- 19. Xie P., Hostager B. S., Bishop G. A. 2004. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 199:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schultheiss U., Püschner S., Kremmer E., et al. 2001. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 20:5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu L., Nakano H., Wu Z. 2006. The C-terminal activating region 2 of the Epstein-Barr virus-encoded latent membrane protein 1 activates NF-kappaB through TRAF6 and TAK1. J. Biol. Chem. 281:2162. [DOI] [PubMed] [Google Scholar]
- 22. Stunz L. L., Busch L. K., Munroe M. E., et al. 2004. Expression of the cytoplasmic tail of LMP1 in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity 21:255. [DOI] [PubMed] [Google Scholar]
- 23. Kraus Z. J., Nakano H., Bishop G. A. 2009. TRAF5 is a critical mediator of in vitro signals and in vivo functions of LMP1, the viral oncogenic mimic of CD40. Proc. Natl Acad. Sci. U. S. A. 106:17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham J. P., Moore C. R., Bishop G. A. 2009. Roles of the TRAF2/3 binding site in differential B cell signaling by CD40 and its viral oncogenic mimic, LMP1. J. Immunol. 183:2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown K. D., Hostager B. S., Bishop G. A. 2001. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1). J. Exp. Med. 193:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graham J. P., Arcipowski K. M., Bishop G. A. 2010. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol. Rev. 237:226. [DOI] [PubMed] [Google Scholar]
- 27. Busch L. K., Bishop G. A. 2001. Multiple carboxyl-terminal regions of the EBV oncoprotein, latent membrane protein 1, cooperatively regulate signaling to B lymphocytes via TNF receptor-associated factor (TRAF)-dependent and TRAF-independent mechanisms. J. Immunol. 167:5805. [DOI] [PubMed] [Google Scholar]
- 28. Bradley J. R., Pober J. S. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482. [DOI] [PubMed] [Google Scholar]
- 29. Nakano H., Sakon S., Koseki H., et al. 1999. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc. Natl Acad Sci. U. S. A. 96:9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie P., Hostager B. S., Munroe M. E., Moore C. R., Bishop G. A. 2006. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J. Immunol. 176:5388. [DOI] [PubMed] [Google Scholar]
- 31. Rowland S. R., Tremblay M. L., Ellison J. M., Stunz L. L., Bishop G. A., Hostager B. S. 2007. A novel mechanism for TRAF6-dependent CD40 signaling. J. Immunol. 179:4645. [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi T., Kim T. S., Jacob A., et al. 2009. TRAF6 is required for generation of the B-1a B cell compartment as well as T cell-dependent and -independent humoral immune responses. PLoS One 4:e4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahonen C., Manning E., Erickson L. D., et al. 2002. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat. Immunol. 3:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jabara H., Laouini D., Tsitsikov E., et al. 2002. The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity 17:265. [DOI] [PubMed] [Google Scholar]
- 35. Arcipowski K. M., Bishop G. A. 2012. TRAF binding is required for a distinct subset of in vivo B cell functions of the oncoprotein LMP1. J. Immunol. 189:5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawabe T., Naka T., Yoshida K., et al. 1994. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1:167. [DOI] [PubMed] [Google Scholar]
- 37. King C. G., Kobayashi T., Cejas P. J., et al. 2006. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat. Med. 12:1088. [DOI] [PubMed] [Google Scholar]
- 38. Rickert R. C., Roes J., Rajewsky K. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rastelli J., Hömig-Hölzel C., Seagal J., et al. 2008. LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood 111:1448. [DOI] [PubMed] [Google Scholar]
- 40. Uchida J., Yasui T., Takaoka-Shichijo Y., et al. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286:300. [DOI] [PubMed] [Google Scholar]
- 41. Xie P., Stunz L. L., Larison K. D., Yang B., Bishop G. A. 2007. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity 27:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waldschmidt T. J., Panoskaltsis-Mortari A., McElmurry R. T., Tygrett L. T., Taylor P. A., Blazar B. R. 2002. Abnormal T cell-dependent B-cell responses in SCID mice receiving allogeneic bone marrow in utero. Severe combined immune deficiency. Blood 100:4557. [DOI] [PubMed] [Google Scholar]
- 43. Lomaga M. A., Yeh W. C., Sarosi I., et al. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arcipowski K. M., Bishop G. A. 2012. Roles of the kinase TAK1 in TRAF6-dependent signaling by CD40 and its oncogenic viral mimic, LMP1. PLoS One 7:e42478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kopf M., Herren S., Wiles M. V., Pepys M. B., Kosco-Vilbois M. H. 1998. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J. Exp. Med. 188:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kawai T., Akira S. 2007. TLR signaling. Semin. Immunol. 19:24. [DOI] [PubMed] [Google Scholar]