Abstract
Aims
Because low-grade inflammation may play a role in the pathogenesis of coronary heart disease (CHD), and pro-inflammatory cytokines govern inflammatory cascades, this study aimed to assess the associations of several pro-inflammatory cytokines and CHD risk in a new prospective study, including meta-analysis of prospective studies.
Methods and results
Interleukin-6 (IL-6), IL-18, matrix metalloproteinase-9 (MMP-9), soluble CD40 ligand (sCD40L), and tumour necrosis factor-α (TNF-α) were measured at baseline in a case-cohort study of 1514 participants and 833 incident CHD events within population-based prospective cohorts at the Danish Research Centre for Prevention and Health. Age- and sex-adjusted hazard ratios (HRs) for CHD per 1-SD higher log-transformed baseline levels were: 1.37 (95% CI: 1.21–1.54) for IL-6, 1.26 (1.11–1.44) for IL-18, 1.30 (1.16–1.46) for MMP-9, 1.01 (0.89–1.15) for sCD40L, and 1.13 (1.01–1.27) for TNF-α. Multivariable adjustment for conventional vascular risk factors attenuated the HRs to: 1.26 (1.08–1.46) for IL-6, 1.12 (0.95–1.31) for IL-18, 1.21 (1.05–1.39) for MMP-9, 0.93 (0.78–1.11) for sCD40L, and 1.14 (1.00–1.31) for TNF-α. In meta-analysis of up to 29 population-based prospective studies, adjusted relative risks for non-fatal MI or CHD death per 1-SD higher levels were: 1.25 (1.19–1.32) for IL-6; 1.13 (1.05–1.20) for IL-18; 1.07 (0.97–1.19) for MMP-9; 1.07 (0.95–1.21) for sCD40L; and 1.17 (1.09–1.25) for TNF-α.
Conclusions
Several different pro-inflammatory cytokines are each associated with CHD risk independent of conventional risk factors and in an approximately log-linear manner. The findings lend support to the inflammation hypothesis in vascular disease, but further studies are needed to assess causality.
Keywords: Inflammation, CHD, Cytokines, Risk factors, Meta-analysis
See page 540 for the editorial comment on this article (doi:10.1093/eurheartj/eht398)
Introduction
As inflammatory processes may play an important role in the pathogenesis of vascular disease,1,2 there is interest in the relevance of circulating markers of inflammation to coronary heart disease (CHD). Previous epidemiological studies have mainly reported on associations between ‘downstream’ markers of inflammation [e.g. C-reactive protein (CRP) and fibrinogen] with the risk of incident CHD.3,4 However, human genetic evidence has reduced the likelihood that these liver-derived factors are causally relevant.5–7 In contrast, ‘upstream’ markers of inflammation, such as pro-inflammatory cytokines, may be more likely to be directly aetiologically relevant to CHD because they govern inflammation cascades.8 Most epidemiological evidence on the relevance of such markers has been obtained for interleukin-6 (IL-6).9 Previous work has suggested that long-term soluble IL-6 levels are associated with CHD risk about as strongly as some major established risk factors,9 and a causal role for IL-6 signalling in CHD has been supported by human genetic evidence.10,11 These findings have intensified interest in recently launched large-scale phase 3 clinical trials of various anti-inflammatory agents in the secondary prevention of cardiovascular disease (CVD).12,13
However, in contrast with IL-6, other further upstream markers of the inflammatory response have been less well studied (Supplementary material online, Table S1). These include factors which are involved in vascular inflammation and which are produced mainly by cells of the innate immune system, such as IL-1814 and matrix metalloproteinase-9 (MMP-9),15 and factors that are also produced by cells of the adaptive immune system, such as soluble CD40 ligand (sCD40L),16 and tumour necrosis factor-α (TNF-α).17 So far, direct comparison between the markers has been difficult, because the individual markers have not been measured in the same studies or participants. Furthermore, there is little information about the extent to which these analytes fluctuate within individuals over time, as such data are essential to the interpretation of epidemiological studies with an aetiological motivation.
We report new findings on the association of the above five inflammatory markers and incident CHD outcomes based on data from population-based prospective cohorts at the Danish Research Centre for Prevention and Health (RCPH),18 comprising a total of 1514 participants aged 30–70 years at baseline and an average of follow-up of 12 years. Furthermore, we used data from serial measurements of these cytokines from two other prospective studies [Reykjavik9 and British Regional Heart Study (BRHS)16,17,19,20] to assess long-term within person variability. Finally, to contextualize our findings, we also report an updated systematic review and meta-analyses of the association of these cytokines and non-fatal myocardial infarction (MI) or CHD death in population-based prospective studies.
Methods
Participants
We measured circulating levels of IL-6, IL-18, MMP-9, sCD40L, and TNF-α in a case-cohort study nested within population-based prospective cohorts at the Danish RCPH. Details of the RCPH cohorts have been described previously.18 By 2001 the study composed of 8314 participants from five cohorts in the south-western part of Copenhagen county (Supplementary material online, Table S2): (i) 70 year olds from the 1914 birth-cohort (70yr1914, n = 804), (ii) 45 year olds from the 1936 birth-cohort (45yr1935, n = 992), (iii) 5-year re-examination of the first Dan-MONICA cohort (GenMon, n = 2987), (iv) the second Dan-MONICA cohort (MONICAII, n = 1504), and (v) the third Dan-MONICA cohort (MONICAIII, n = 2027). Standardized methods for follow-up and risk factor assessment have been maintained in these five cohorts.18 Our case-cohort study of inflammatory cytokines included all incident CHD cases (defined using ICD8 codes 410-414 or ICD10 codes I20-I25) recorded as of 31 December 2006, plus subcohorts of randomly sampled participants within each cohort (7% in each of 45yr1936, MONICAII, and MONICAIII; 9% in GenMon; and 28% in 70yr1914; reflecting the proportion of CHD cases within each cohort) for a total of 1514 participants aged 30–70 years (Supplementary material online, Table S2). In total there were 833 incident CHD cases (comprising 320 non-fatal MI, 195 CHD deaths, and 318 angina; Supplementary material online, Table S2). The study complies with the Declaration of Helsinki and was approved by the ethical committee of the county of Copenhagen.
Biochemical measurements
Blood samples were drawn after 8–12 h of fasting and stored at −20°C before measurements of pro-inflammatory cytokines in November 2008 (mean storage duration = 22 years). Assays of IL-6,9 IL-18,17 MMP-9,15 sCD40L,16 and TNF-α17 were performed centrally at the University of Glasgow laboratory using high-sensitivity ELISAs (R&D systems, Abingdon, UK) as previously reported.9,15–17 Intra- and inter-assay coefficients of variation were 7.0 and 8.0% (IL-6); 5.6 and 10.4% (IL-18); 4.4 and 10.4% (MMP-9); 2.9 and 14.9% (sCD40L); and 8.4 and 12.5% (TNF-α), respectively.9,15,17 Participants from the MONICA II cohort were excluded from the analyses of sCD40L due to technical pre-analytical issues related to sample handling (previous thawing and freezing) that led to erroneous low values of the sCD40L assay. CRP measurements were later done in another laboratory (MORGAM) using serum samples that remained available in three of the RCPH cohorts (55% of participants in the GenMon cohort and all participants in the MONICAII and MONICAIII cohorts). Measurements of other biochemical factors involved standard methods as described previously.21
Outcomes
Assessment of the cardiovascular endpoints was based on data from the Danish National Patient Registry (DNPR)22 and the Danish Register of Causes of Death (DRCD).23 Individuals who had died or emigrated were identified through the Central Population Registry of Statistics Denmark. Linkage between population surveys and national registries was made possible due to a unique individual 10 digit code. The primary endpoint in the design of our study was pre-specified as the composite of all CHD outcomes (including non-fatal MI, fatal CHD, and angina) in order to enhance statistical power. The composite of non-fatal MI and fatal CHD was analysed as a secondary endpoint. Participants with pre-existing CHD at baseline were excluded from all analyses to avoid reverse association bias. While specific validation of endpoints against coding in the DNPR and DRCD national registers was not conducted in the current study, previous validation studies of the same registers have found excellent completeness and validity of recorded diagnosis of MI [positive predictive value (PPV) = 82% overall, and 92% if associated with a ward diagnosis] although less so for the diagnosis of unstable angina pectoris (PPV = 28% overall).24,25
Statistical analyses
As none of the inflammatory cytokines studied was normally distributed, natural logarithm (loge) transformed values of each cytokine were used for all analyses. Partial correlation coefficients (adjusted for cohort, age, and sex) were calculated to assess the correlation between inflammatory markers and continuous vascular risk factors, whereas mean differences between groups were calculated for categorical factors. Analyses of the associations between inflammatory cytokines and CHD outcomes involved weighted Cox-regression modelling, using the Barlow weighting method to account for the case-cohort study design.26,27 Shapes of association were assessed by plotting hazard ratios (HRs) estimated within quintiles of each cytokine relative to the bottom quintile against the mean cytokine level in each quintile. 95% confidence intervals (CIs) were estimated from variances attributed to the groups to reflect the amount of information within each group (including the reference category).28 To assess the independence of association between each cytokine and CHD, we calculated HRs per 1-SD higher baseline levels with progressive adjustment for baseline levels of potential confounders. The use of 1-SD higher HRs also facilitated direct comparison of the strength of association across the five cytokines. All analyses were stratified by cohort and a two-sided P-value < 0.05 was considered statistically significant.
Literature review and meta-analysis
Prospective studies of the association between any of the five cytokines and risk of non-fatal MI or CHD death that were published before 31 December 2012 were sought using computer-based databases (MEDLINE, EMBASE, and Science Citation Index), by scanning the reference lists of articles identified for all relevant studies and review articles (including meta-analyses) and hand searching of relevant journals, without language restriction. The computer-based searches combined free and MeSH search terms and combinations of keywords related to the cytokines (e.g. ‘interleukin-6’, ‘IL-6’, ‘interleukin-18’, ‘IL-18’, ‘matrix metalloproteinase-9’, ‘MMP-9’, ‘soluble CD40 ligand’, ‘sCD40L’, ‘tumour necrosis factor-α’, ‘TNF-α’) and coronary disease (e.g.‘coronary heart disease’, ‘myocardial ischemia’, ‘myocardial infarction’, ‘CHD’, ‘heart attack’, ‘MI’) without restricting language or publication date. Studies were eligible for inclusion if they had at least 1 year of follow-up; had recruited participants from approximately general populations (i.e. did not select participants on the basis of pre-existing disease at baseline); and had assessed non-fatal MI or CHD death as outcome. Study level characteristics and participant characteristics were extracted using a standardized data extraction form, including information on: geographic location, publication date, sample population, sampling methods (i.e. complete, random, etc.), years of baseline survey, number of participants, number of non-fatal MI or CHD death cases, age range or mean age, percentage of males, duration of follow-up, and assay/sample characteristics (including source, type, sample storage, sample type, and fasting status). Also extracted were the reported measures of association [i.e. HRs, odds ratios, or other measure of relative risk (RR)] and their confidence intervals or standard errors, units of comparison, and degree of adjustment for confounders. Information from multivariable models adjusted for conventional risk factors for CHD was prioritized for inclusion in the meta-analysis (as opposed to models further adjusted for inflammatory markers or socio-economic variables, as such information was more limited within studies). The reported RRs were standardized to correspond to comparison of RR per 1-SD higher values of each cytokine,9,29 and were pooled across studies using random-effects meta-analysis. Heterogeneity between studies was quantified using the I2 statistic.30 Potential publication and small-study bias was visually assessed from funnel plots, complemented by formal statistical tests of funnel plot asymmetry and use of trim and fill procedure to assess impact.31 For IL-6, we updated our previous meta-analysis of 17 studies9 with new information from recently published studies. Two authors (P.G. and S.R.K.S.) abstracted the details sought for each marker and any discrepancies were resolved through discussion with a third author (S.K.).
Within-person variability
The extent of long-term within person variability in log-transformed cytokine levels was assessed based on regression dilution ratios (RDRs) previously reported in the BRHS study16,17,19,20 or calculated from serial measurements available from 300 participants in the Reykjavik study an average of 12 years apart.9 The RDR quantifies the extent to which a single measurement of a biomarker reflects the long-term average (i.e. ‘usual’) level of the biomarker, and correction for long-term within person variability in log-transformed cytokine levels was achieved dividing the regression coefficients (i.e. log RRs) for association with baseline levels by the estimated RDR.32
All analyses were conducted using the Stata version 11 software (Stata Corp, College Station, TX, USA).
Results
Results from the Research Centre for Prevention and Health cohorts
Overall, 1514 participants contributed to the analyses comprising 807 participants in the randomly sampled subcohort and a further 707 incident CHD cases included as part of the case-sample enrichment in the case-cohort design.26,27 Within the random subcohort, there were 126 incident CHD cases recorded over 12 570 person-years of follow-up (median 9 years), giving a crude incidence rate of 10 (95% CI: 7–12) per 1000 person-years. Hence, in total, the analyses involved 833 CHD cases and 681 non-cases. Table 1 summarizes the participant characteristics by the incident CHD status. Compared with non-cases, participants with incident CHD were more likely to be older, male, current smokers, and to have diabetes at baseline. They also had higher BMI, higher systolic and diastolic blood pressure, and higher levels of pro-atherogenic lipids. Loge levels of cytokines were generally higher among individuals with CHD than those without. CRP measurements were available in half of the participants. There were generally weak positive pair-wise correlations between individual cytokines (Table 2), with the exception of modest correlations between loge IL-6 and loge TNF-α (r = 0.22, 95% CI: 0.17–0.24), and between loge MMP-9 and loge sCD40L (r = 0.31, 95% CI: 0.26–0.37) and stronger correlations between loge CRP and each of loge IL-6 (r = 0.48, 95% CI: 0.42–0.54) and loge MMP-9 (r = 0.31, 95% CI: 0.23–0.38). Weak correlations were also observed between cytokines and other continuous variables, with the exception of statistically significant correlations between loge IL-6 and loge IL-18 with age, BMI, HDL-cholesterol and loge triglycerides; and between loge CRP and the preceding factors plus blood pressure (Table 2). Concentrations of most cytokines were generally lower in women than men (sCD40L higher), higher among current than never/ex-smokers, lower in current vs. never/ex-drinkers, higher in people with vs. without diabetes, and lower in people of higher socio-economic position adjusted for cohort, age, and sex (Table 2).
Table 1.
Summary of baseline characteristics of participants by incident coronary heart disease status
| Cases |
Non-cases |
Difference* | |||
|---|---|---|---|---|---|
| Variable | n | Mean (SD) or % | n | Mean (SD) or % | P |
| Demographics and lifestyle | |||||
| Age at survey (years) | 833 | 57.4 (7.2) | 681 | 48.2 (9.8) | <0.0001 |
| Sex | <0.0001 | ||||
| Male | 556 | 67% | 334 | 49% | |
| Female | 277 | 33% | 347 | 51% | |
| Smoking status | <0.0001 | ||||
| Never/former | 369 | 44% | 373 | 55% | |
| Current | 462 | 56% | 306 | 45% | |
| Alcohol consumption | 0.002 | ||||
| Never/former | 207 | 25% | 132 | 19% | |
| Current | 624 | 75% | 547 | 81% | |
| History of diabetes | <0.0001 | ||||
| No | 699 | 92% | 600 | 98% | |
| Yes | 58 | 8% | 13 | 2% | |
| Socio-economic position | 0.032 | ||||
| Low | 167 | 26% | 232 | 29% | |
| Low/medium | 189 | 30% | 235 | 30% | |
| Medium | 150 | 23% | 189 | 24% | |
| Medium/high | 92 | 14% | 102 | 13% | |
| High | 42 | 7% | 36 | 5% | |
| Physical measurements | |||||
| Systolic blood pressure (mmHg) | 832 | 138 (20) | 680 | 128 (18) | <0.001 |
| Diastolic blood pressure (mm Hg) | 832 | 84 (11) | 681 | 81 (10) | 0.022 |
| Pulse rate (count/min) | 833 | 69 (11) | 680 | 67 (10) | 0.002 |
| Body mass index (kg/m2) | 827 | 26 (4.2) | 678 | 25 (4.4) | <0.0001 |
| Lipids | |||||
| Total cholesterol (mmol/L) | 829 | 6.63 (1.21) | 680 | 6.14 (1.14) | <0.0001 |
| LDL cholesterol (mmol/L) | 809 | 4.52 (1.09) | 670 | 4.00 (1.02) | <0.0001 |
| Non-HDL cholesterol (mmol/L) | 827 | 5.33 (1.23) | 677 | 4.63 (1.16) | <0.0001 |
| HDL cholesterol (mmol/L) | 827 | 1.30 (0.37) | 677 | 1.49 (0.42) | <0.0001 |
| Loge triglycerides (loge mmol/L) | 829 | 0.44 (0.49) | 680 | 0.19 (0.48) | <0.0001 |
| Inflammatory cytokines | |||||
| Loge IL-6 (loge ng/L) | 732 | 0.86 (0.67) | 594 | 0.58 (0.68) | <0.0001 |
| Loge IL-18 (loge ng/L) | 740 | 5.71 (0.42) | 596 | 5.55 (0.40) | <0.0001 |
| Loge MMP-9 (loge µg/L) | 748 | 5.97 (0.57) | 612 | 5.87 (0.56) | <0.0001 |
| Loge sCD40L (loge ng/L) | 652 | 8.79 (0.75) | 529 | 8.80 (0.75) | 0.938 |
| Loge TNF-α (loge ng/L) | 738 | 0.43 (0.39) | 595 | 0.40 (0.45) | 0.244 |
| Loge CRP (loge mg/L) | 389 | 0.94 (1.02) | 369 | 0.22 (1.15) | <0.0001 |
*P-value for difference in risk marker distribution between cases and non-cases adjusted for cohort, age, and sex.
Table 2.
Associations between inflammatory cytokines and coronary heart disease risk markers
| Partial correlation (95% CI) with row variable or per cent difference (95% CI) compared with reference categorya |
||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean (SD) | IL-6 (ng/L) | IL-18 (ng/L) | MMP-9 (µg/L) | sCD40L (ng/L) | TNF-α (ng/L) | CRP (mg/L) |
| Demographics and lifestyle | ||||||||
| Age at survey (years) | 1514 | 52.9 (9.3) | 0.28 (0.22, 0.34) | 0.15 (0.08, 0.21) | 0.01 (−0.05, 0.08) | 0.00 (−0.07, 0.08) | −0.08 (−0.14, −0.01) | 0.24 (0.17, 0.30) |
| Sex | ||||||||
| Male | 1514 | 890 (59%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Female | 1514 | 624 (41%) | −10% (−16%, −3%) | −16% (−19%, −12%) | −5% (−11%, 1%) | 30% (20%, 42%) | −3% (−7%, 2%) | −8% (−22%, 8%) |
| Smoking status | ||||||||
| Never/former | 1510 | 742 (49%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Current | 1510 | 768 (51%) | 18% (9%, 26%) | 9% (5%, 14%) | 32% (25%, 40%) | 13% (4%, 23%) | 0% (−4%, 5%) | 58% (36%, 85%) |
| Alcohol consumption | ||||||||
| Never/former | 1510 | 339 (22%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Current | 1510 | 1171 (78%) | −11% (−19%, −2%) | −2% (−8%, 3%) | 4% (−4%, 12%) | 7% (−4%, 19%) | −1% (−7%, 5%) | −12% (−28%, 8%) |
| History of diabetes | ||||||||
| No | 1370 | 1299 (95%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 1370 | 71 (5%) | 23% (3%, 46%) | 3% (−7%, 14%) | −9% (−21%, 5%) | −8% (−23%, 11%) | −3% (−13%, 8%) | 57% (6%, 133%) |
| Socio-economic position | ||||||||
| Low | 1434 | 399 (28%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Low/medium | 1434 | 424 (30%) | −12% (−20%, −3%) | −7% (−12%, −1%) | −3% (−11%, 5%) | −5% (−16%, 7%) | −4% (−10%, 2%) | −2% (−21%, 20%) |
| Medium | 1434 | 339 (24%) | −17% (−25%, −8%) | −8% (−14%, −2%) | −4% (−12%, 5%) | 2% (−10%, 15%) | 2% (−5%, 9%) | 2% (−20%, 29%) |
| Medium/high | 1434 | 194 (14%) | −12% (−22%, 0%) | −14% (−20%, −7%) | −11% (−20%, −1%) | −4% (−17%, 12%) | −7% (−14%, 0%) | 3% (−22%, 36%) |
| High | 1434 | 78 (5%) | −27% (−38%, −13%) | −14% (−22%, −5%) | −11% (−23%, 3%) | 4% (−16%, 29%) | −6% (−16%, 5%) | −14% (−42%, 28%) |
| Physical measurements | ||||||||
| Systolic blood pressure (mmHg) | 1512 | 134 (20) | 0.01 (−0.04, 0.06) | 0.04 (−0.01, 0.09) | −0.04 (−0.09, 0.02) | −0.03 (−0.09, 0.02) | 0.00 (−0.05, 0.06) | 0.15 (0.08, 0.22) |
| Diastolic blood pressure (mmHg) | 1513 | 83 (11) | 0.01 (−0.05, 0.06) | 0.07 (0.02, 0.12) | −0.05 (−0.11, −0.00) | −0.04 (−0.10, 0.02) | 0.03 (−0.03, 0.08) | 0.12 (0.05, 0.19) |
| Pulse rate (count/min) | 1513 | 68 (11) | 0.12 (0.07, 0.17) | 0.06 (0.01, 0.12) | 0.04 (−0.01, 0.10) | 0.01 (−0.05, 0.07) | 0.04 (−0.01, 0.10) | 0.20 (0.13, 0.27) |
| Body mass index (kg/m2) | 1505 | 25.8 (4.3) | 0.12 (0.07, 0.18) | 0.04 (−0.01, 0.10) | −0.02 (−0.08, 0.03) | −0.00 (−0.06, 0.06) | 0.08 (0.02, 0.13) | 0.30 (0.24, 0.37) |
| Lipids | ||||||||
| Total cholesterol (mmol/L) | 1509 | 6.41 (1.20) | −0.02 (−0.08, 0.03) | 0.03 (−0.03, 0.08) | 0.02 (−0.04, 0.07) | −0.01 (−0.07, 0.04) | 0.00 (−0.05, 0.06) | 0.05 (−0.02, 0.12) |
| LDL cholesterol (mmol/L) | 1479 | 4.29 (1.09) | −0.02 (−0.08, 0.03) | 0.02 (−0.03, 0.08) | 0.03 (−0.02, 0.09) | −0.01 (−0.07, 0.04) | 0.01 (−0.04, 0.07) | 0.05 (−0.02, 0.12) |
| Non-HDL cholesterol (mmol/L) | 1504 | 5.02 (1.25) | 0.02 (−0.03, 0.08) | 0.09 (0.03, 0.14) | 0.03 (−0.03, 0.08) | −0.00 (−0.06, 0.05) | 0.04 (−0.02, 0.09) | 0.13 (0.06, 0.20) |
| HDL cholesterol (mmol/L) | 1504 | 1.39 (0.41) | −0.14 (−0.19, −0.09) | −0.21 (−0.26, −0.16) | −0.03 (−0.09, 0.02) | −0.03 (−0.09, 0.02) | −0.10 (−0.16, −0.05) | −0.23 (−0.30, −0.16) |
| Loge triglycerides (loge mmol/L) | 1509 | 0.33 (0.50) | 0.11 (0.05, 0.16) | 0.20 (0.14, 0.25) | 0.03 (−0.02, 0.08) | 0.02 (−0.03, 0.08) | 0.08 (0.02, 0.13) | 0.28 (0.21, 0.34) |
| Inflammatory cytokines | ||||||||
| Loge IL-6 (loge ng/L) | 1326 | 0.73 (0.69) | — | 0.11 (0.06, 0.17) | 0.16 (0.11, 0.21) | 0.04 (−0.02, 0.09) | 0.22 (0.17, 0.27) | 0.48 (0.42, 0.54) |
| Loge IL-18 (loge ng/L) | 1336 | 5.64 (0.42) | 0.11 (0.06, 0.17) | — | 0.07 (0.02, 0.13) | 0.03 (−0.03, 0.09) | 0.17 (0.12, 0.23) | 0.18 (0.10, 0.26) |
| Loge MMP-9 (loge µg/L) | 1360 | 5.93 (0.57) | 0.16 (0.11, 0.21) | 0.07 (0.02, 0.13) | — | 0.31 (0.26, 0.37) | 0.09 (0.03, 0.14) | 0.31 (0.23, 0.38) |
| Loge sCD40L (loge ng/L) | 1181 | 8.79 (0.75) | 0.04 (−0.02, 0.09) | 0.03 (−0.03, 0.09) | 0.31 (0.26, 0.37) | — | 0.10 (0.04, 0.16) | 0.10 (0.01, 0.19) |
| Loge TNF-α (loge ng/L) | 1333 | 0.42 (0.42) | 0.22 (0.17, 0.27) | 0.17 (0.12, 0.23) | 0.09 (0.03, 0.14) | 0.10 (0.04, 0.16) | — | 0.10 (0.02, 0.17) |
| Loge CRP (loge mg/L) | 758 | 0.59 (1.14) | 0.48 (0.42, 0.54) | 0.18 (0.10, 0.26) | 0.31 (0.23, 0.38) | 0.10 (0.01, 0.19) | 0.10 (0.02, 0.17) | — |
aPartial correlation between loge levels of cytokine and continuous variables or the percentage difference in mean levels of cytokine compared with reference category for categorical variables adjusted for cohort, age, and sex.
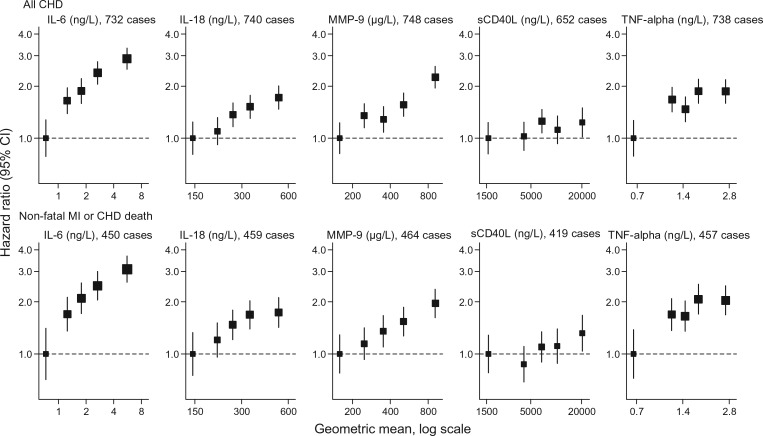
There were log-linear relationships between four of the cytokines studied and incident CHD risk (Figure 1), with the age- and sex-adjusted HRs (95% CI) for all CHD per 1-SD higher log-transformed levels being 1.37 (1.21, 1.54) for IL-6, 1.26 (1.11, 1.44) for IL-18, 1.30 (1.16, 1.46) for MMP-9, and 1.13 (1.01, 1.27) for TNF-α, but no association was seen with sCD40L [1.01 (0.89, 1.15)]. A modest attenuation in HRs was observed upon additional adjustment for several potential confounders (blood pressure, smoking, history of diabetes, BMI, loge triglycerides, non-HDL cholesterol, HDL cholesterol, alcohol consumption, and socio-economic position), with the adjusted HRs being 1.26 (1.08, 1.46) for IL-6, 1.12 (0.95, 1.31) for IL-18, 1.21 (1.05, 1.39) for MMP-9, 1.14 (1.00, 1.31) for TNF-α, and 0.93 (0.78, 1.11) for sCD40L (Table 3). The above findings were similar in analyses restricted to non-fatal MI and CHD death outcomes only (Figure 1 and Table 3). In further comparative analyses restricted to the smaller subset of participants with information on CRP, somewhat stronger dose–response associations were observed for baseline loge CRP than the preceding cytokines (Supplementary material online, Figure S1) and, as expected, further adjustment for loge CRP levels more markedly attenuated the associations between loge IL-6 and CHD (Supplementary material online, Table S3), due to the strong correlation between the two (r = 0.48). However, because CRP is downstream of IL-6 in the inflammation pathway, such attenuation may be indicative of mediation rather than confounding per se.
Figure 1.
Hazard ratios for all coronary heart disease (top panel) [The cut-points defining the fifths of each cytokine were based on the distribution of values observed in the random subcohort, so as to be representative of the expected distribution in the population (i.e. avoiding potential bias due to purposeful over-sampling of cases in the case-cohort design). Assuming a log-linear relationship the age- and sex-adjusted hazard ratio (95% CI) for any coronary heart disease per 1-SD higher values were: 1.37 (1.21, 1.54) for IL-6, 1.26 (1.11, 1.44) for IL-18, 1.30 (1.16, 1.46) for MMP-9, 1.01 (0.89, 1.15) for sCD40L, and 1.13 (1.01, 1.27) for TNF-α] and for non-fatal MI or coronary heart disease death (bottom panel) [Assuming a log-linear relationship the age- and sex-adjusted hazard ratio (95% CI) for non-fatal myocardial infarction or coronary heart disease death per 1-SD higher values were: 1.38 (1.21, 1.57) for IL-6, 1.27 (1.09, 1.47) for IL-18, 1.27 (1.12, 1.45) for MMP-9, 1.03 (0.88, 1.19) for sCD40L and 1.17 (1.03, 1.33) for TNF-α.] by fifths of inflammatory cytokine levels adjusted for age and sex and stratified by cohort.
Table 3.
Hazard ratios for coronary heart disease per 1-SD higher baseline levels of inflammatory cytokines with progressive adjustment for baseline levels of potential confounders
| Outcome\adjustmenta | Loge IL-6 (ng/L) |
Loge IL-18 (ng/L) |
Loge MMP-9 (µg/L) |
Loge sCD40L (ng/L) |
Loge TNF-α (ng/L) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | Wald 
|
HR (95% CI) | Wald 
|
HR (95% CI) | Wald 
|
HR (95% CI) | Wald 
|
HR (95% CI) | Wald 
|
|
| All CHD (including angina) | 1180 participants, 654 cases | 1190 participants, 662 cases | 1214 participants, 670 cases | 1036 participants, 575 cases | 1187 participants, 660 cases | |||||
| Adjusted for cohort, sex | 1.46 (1.30, 1.64) | 41 | 1.37 (1.20, 1.55) | 23 | 1.27 (1.14, 1.42) | 18 | 1.01 (0.88, 1.15) | 0 | 1.06 (0.95, 1.18) | 1 |
| Plus age | 1.35 (1.18, 1.53) | 21 | 1.29 (1.12, 1.49) | 13 | 1.27 (1.12, 1.44) | 14 | 0.99 (0.85, 1.14) | 0 | 1.13 (1.00, 1.27) | 4 |
| Plus systolic blood pressure | 1.35 (1.18, 1.53) | 20 | 1.29 (1.11, 1.49) | 12 | 1.26 (1.11, 1.44) | 12 | 1.00 (0.86, 1.16) | 0 | 1.14 (1.01, 1.29) | 4 |
| Plus smoking status | 1.30 (1.14, 1.49) | 15 | 1.27 (1.10, 1.47) | 10 | 1.20 (1.05, 1.37) | 8 | 0.98 (0.84, 1.14) | 0 | 1.16 (1.02, 1.31) | 5 |
| Plus history of diabetes | 1.32 (1.15, 1.51) | 16 | 1.25 (1.08, 1.46) | 9 | 1.23 (1.07, 1.41) | 9 | 0.97 (0.83, 1.14) | 0 | 1.17 (1.03, 1.32) | 6 |
| Plus BMI | 1.30 (1.14, 1.50) | 14 | 1.24 (1.07, 1.44) | 8 | 1.23 (1.07, 1.41) | 9 | 0.97 (0.83, 1.14) | 0 | 1.15 (1.01, 1.31) | 5 |
| Plus loge triglycerides | 1.27 (1.11, 1.46) | 11 | 1.16 (0.99, 1.36) | 4 | 1.22 (1.06, 1.40) | 8 | 0.96 (0.81, 1.12) | 0 | 1.15 (1.01, 1.31) | 4 |
| Plus total cholesterol | 1.28 (1.11, 1.47) | 12 | 1.17 (1.00, 1.36) | 4 | 1.22 (1.06, 1.41) | 8 | 0.97 (0.82, 1.14) | 0 | 1.18 (1.03, 1.35) | 6 |
| Plus non-HDL cholesterolb | 1.28 (1.11, 1.48) | 12 | 1.15 (0.99, 1.35) | 3 | 1.22 (1.06, 1.40) | 8 | 0.97 (0.82, 1.14) | 0 | 1.17 (1.02, 1.34) | 5 |
| Plus HDL cholesterolb | 1.27 (1.11, 1.47) | 11 | 1.12 (0.96, 1.32) | 2 | 1.22 (1.06, 1.40) | 8 | 0.95 (0.80, 1.13) | 0 | 1.14 (1.00, 1.31) | 4 |
| Plus alcoholb | 1.27 (1.10, 1.46) | 10 | 1.12 (0.96, 1.32) | 2 | 1.21 (1.06, 1.40) | 7 | 0.95 (0.80, 1.13) | 0 | 1.14 (1.00, 1.31) | 4 |
| Plus socio-economic positionb | 1.26 (1.08, 1.46) | 9 | 1.12 (0.95, 1.31) | 2 | 1.21 (1.05, 1.39) | 7 | 0.93 (0.78, 1.11) | 1 | 1.14 (1.00, 1.31) | 4 |
| Plus other cytokinesb,c | 1.24 (1.04, 1.47) | 6 | 1.03 (0.86, 1.23) | 0 | 1.25 (1.05, 1.50) | 6 | 0.80 (0.66, 0.98) | 5 | 1.09 (0.92, 1.30) | 1 |
| Non-fatal MI or CHD death | 1180 participants, 405 cases | 1190 participants, 414 cases | 1214 participants, 419 cases | 1036 participants, 375 cases | 1187 participants, 412 cases | |||||
| Adjusted for cohort, sex | 1.47 (1.29, 1.67) | 34 | 1.37 (1.18, 1.58) | 18 | 1.27 (1.12, 1.43) | 14 | 1.04 (0.89, 1.22) | 0 | 1.09 (0.96, 1.23) | 2 |
| Plus age | 1.36 (1.18, 1.56) | 19 | 1.29 (1.10, 1.52) | 10 | 1.26 (1.10, 1.45) | 11 | 1.02 (0.86, 1.21) | 0 | 1.17 (1.02, 1.34) | 5 |
| Plus systolic blood pressure | 1.36 (1.18, 1.56) | 18 | 1.29 (1.09, 1.52) | 9 | 1.26 (1.09, 1.45) | 10 | 1.03 (0.87, 1.22) | 0 | 1.18 (1.03, 1.36) | 6 |
| Plus smoking status | 1.31 (1.13, 1.51) | 14 | 1.27 (1.08, 1.50) | 8 | 1.19 (1.03, 1.37) | 6 | 1.02 (0.85, 1.21) | 0 | 1.20 (1.04, 1.38) | 7 |
| Plus history of diabetes | 1.32 (1.14, 1.52) | 14 | 1.26 (1.06, 1.49) | 7 | 1.22 (1.05, 1.41) | 7 | 1.01 (0.84, 1.21) | 0 | 1.22 (1.06, 1.40) | 8 |
| Plus BMI | 1.31 (1.13, 1.52) | 13 | 1.25 (1.05, 1.48) | 7 | 1.22 (1.05, 1.41) | 7 | 1.01 (0.84, 1.21) | 0 | 1.21 (1.04, 1.39) | 7 |
| Plus loge triglycerides | 1.29 (1.11, 1.50) | 11 | 1.17 (0.98, 1.40) | 3 | 1.22 (1.05, 1.41) | 7 | 1.00 (0.84, 1.20) | 0 | 1.19 (1.03, 1.38) | 6 |
| Plus total cholesterol | 1.30 (1.12, 1.51) | 11 | 1.18 (0.99, 1.40) | 3 | 1.22 (1.05, 1.42) | 7 | 1.02 (0.85, 1.22) | 0 | 1.22 (1.05, 1.42) | 7 |
| Plus non-HDL cholesterolb | 1.30 (1.11, 1.51) | 11 | 1.16 (0.97, 1.39) | 3 | 1.21 (1.04, 1.41) | 6 | 1.02 (0.85, 1.23) | 0 | 1.21 (1.04, 1.41) | 6 |
| Plus HDL cholesterolb | 1.29 (1.11, 1.50) | 11 | 1.13 (0.95, 1.35) | 2 | 1.21 (1.04, 1.41) | 6 | 1.01 (0.83, 1.22) | 0 | 1.18 (1.02, 1.37) | 5 |
| Plus alcoholb | 1.27 (1.09, 1.48) | 10 | 1.13 (0.95, 1.35) | 2 | 1.21 (1.04, 1.40) | 6 | 1.00 (0.83, 1.21) | 0 | 1.18 (1.02, 1.37) | 5 |
| Plus socio-economic positionb | 1.29 (1.10, 1.52) | 10 | 1.13 (0.95, 1.36) | 3 | 1.20 (1.03, 1.40) | 6 | 0.98 (0.80, 1.19) | 0 | 1.19 (1.02, 1.39) | 5 |
| Plus other cytokinesb,c | 1.27 (1.06, 1.53) | 7 | 1.06 (0.87, 1.29) | 0 | 1.19 (0.99, 1.43) | 3 | 0.87 (0.70, 1.09) | 1 | 1.12 (0.93, 1.35) | 2 |
aHazard ratios are presented per 1-SD higher observed levels at baseline for each inflammatory cytokine, progressively adjusted as shown and stratified by cohort. The sequence of adjustment generally corresponded to adjustment for established non-lipid risk factors first, then lipids (omitting one of highly correlated lipid variables) and finally other lifestyle risk factors and cytokines. Analyses were restricted to participants with complete information on the respective inflammatory cytokines and all confounding variables.
b Non-HDL cholesterol has been substituted for total cholesterol in these adjusted models.
cAdditional mutual adjustment of the four other cytokines based on data from 977 participants and 550 cases for analyses of all CHD outcome, and 355 cases for analysis of non-fatal MI or CHD death outcome.
Literature-based meta-analysis
Including the current study, we found a total of 29 studies eligible for the meta-analysis [comprising 25 studies for IL-6 (7 new), 7 studies for IL-18, 5 studies for MMP-9, 3 studies for sCD40L, and 7 studies for TNF-α; flow chart in Supplementary material online, Figure S2 and study characteristics in Supplementary material online, Table S4]. As shown in Supplementary material online, Table S4, the new prospective cohort studies involved ∼17 000 participants without known cardiovascular disease at baseline and 3000 incident non-fatal MI or CHD death cases and the previous meta-analysis of IL-6 involved 17 studies and 5730 such cases.9 The studies were mainly based in general populations in Europe or North America. Approximately half of all studies were case–control studies nested within prospective cohorts. All studies used the same definition for non-fatal MI or CHD death and ascertainment was based on standard criteria, generally involving review of medical records to assess evidence of confirmation by cardiac markers, electrocardiograms, and ICD codes among other standard diagnostic criteria (Supplementary material online, Table S5). In most studies, ELISA was used as the assay method for analysing the various inflammatory cytokines (Supplementary material online, Table S4), although further information such as duration of sample storage and assay reproducibility (coefficient of variation) were infrequently reported (Supplementary material online, Table S5). Figure 2 shows the forest plot for the updated meta-analysis of association of IL-6 and CHD and Figure 3 shows similar findings for the other cytokines. The pooled RRs (95% CI) for non-fatal MI or CHD death per 1-SD higher baseline cytokine levels adjusted for conventional CHD risk factors were: 1.25 (1.19, 1.32) for IL-6, 1.13 (1.05, 1.20) for IL-18, 1.07 (0.97, 1.19) for MMP-9, 1.09 (0.97, 1.19) for sCD40L, and 1.17 (1.09, 1.25) for TNF-α (Figure 3). There was no statistically significant between-study heterogeneity observed as evidenced by the low I2 statistic, although the power to detect heterogeneity may have been limited by the small number of studies available for each cytokine (n ≤ 7), other than IL-6 (n = 25, including studies in previous meta-analysis,9 Figure 2). Assessment of potential bias from small studies suggested some slight concern for IL-6 (P = 0.024) and MMP-9 (P = 0.039), although a visual inspection of the degree of asymmetry in the funnel plots (Supplementary material online, Figure S3) and use of a trim-and-fill procedure31 to assess potential impact suggested that the asymmetry may not be of material importance, with the pooled RR estimates expected to be 1.19 (1.12, 1.25) for IL-6 and 1.08 (0.97, 1.19) for MMP-9 in the absence of asymmetry.
Figure 2.
Forest plot for the updated meta-analysis of association of interleukin-6 and risk of non-fatal MI or coronary heart disease death in prospective studies adjusted for conventional risk factors*. *In most studies, these included age, sex, smoking status, adiposity markers, blood pressure, and/or lipid markers. †Current study. £The study reported total number of cardiovascular disease cases and gave relative risks for coronary heart disease without stating the number of coronary heart disease cases. ARIC, Atherosclerosis Risk in Communities; BRHS, British Regional Heart Study; BWHHS, British Women's Heart and Health Study; CaPS, Caerphilly Prospective Study; FINRISK, Finnish National Risk Factor Survey; HPFUS, Health Professionals' Follow-up Study; MONICA, MONItoring of trends and determinants in Cardiovascular disease; NSHDS, Northern Sweden Health and Disease Study; NSHS95, Canadian Nova Scotia Health Survey; PRIME, Prospective Epidemiological Study of Myocardial Infarction; RCPH, Research Centre for Prevention and Health; WHIOS, Women's Health Initiative Observational Study; WOSCOPS, West of Scotland Coronary Prevention Study.
Figure 3.
Meta-analysis of the associations of inflammatory cytokines and risk of non-fatal MI or coronary heart disease death in prospective studies adjusted for conventional risk factors*. *In most studies, these included age, sex, smoking status, adiposity markers, blood pressure, and/or lipid markers. †Current study. $The pooled estimate and heterogeneity statistics correspond to separate meta-analysis of 25 studies with IL-6 information (Figure 2). §Relative risk estimates were further adjusted for log CRP concentrations in this study in addition to conventional risk factors. ¶A proportion of the study participants had prevalent cardiovascular disease at baseline. £The study reported total number of cardiovascular disease cases and gave RRs for coronary heart disease without stating the number of coronary heart disease cases. Abbreviations: BHS, Busselton Health Study; BRHS, British Regional Heart Study; BWHHS, British Women's Heart and Health Study; FINRISK, Finnish National Risk Factor Survey; Health ABC, Health, Aging, and Body Composition Study; PRIME, Prospective Epidemiological Study of Myocardial Infarction; RCPH, Research Centre for Prevention and Health; ULSAM, Uppsala Longitudinal Study of Adult Men; WHI-HT, Women's Health Initiative Hormone Trials.
Correction for long-term within person variability
The extent of long-term within person variability in levels of the cytokines studied appeared to be substantial based on the generally low RDRs reported by some of the published studies (Supplementary material online, Table S6). Such variability suggests a potential underestimation of the true magnitude of association with non-fatal MI or CHD death, as indicated by comparison of the observed associations estimated with baseline levels against those inferred for long-term average (‘usual’) levels (Supplementary material online, Figure S4) based on the pooled RDRs available from the BRHS and Reykjavik studies (Supplementary material online, Table S6).
Discussion
The current results indicate that circulating levels of several different pro-inflammatory cytokines in initially healthy people are associated with risk of CHD outcomes in an approximately log-linear manner. These associations appear to be largely independent of several conventional and emerging cardiovascular risk factors. Furthermore, updated meta-analyses reinforce the validity and generalizability of these new data, suggesting that a 1-SD higher baseline level for each of IL-6, IL-18, and TNF-α is associated with ∼10–25% higher risk of non-fatal MI or CHD death. Analyses that make allowances for the considerable fluctuations that we observed of these analytes within individuals over a few years would increase the estimated size of these associations with CHD (see below). Similar associations have previously been reported for CRP and fibrinogen based on meta-analysis of individual participant data,3,4 hence we only analysed the limited data available on CRP in our primary study for making direct internal comparisons. Collectively, the current study establishes robust observational associations of several pro-inflammatory cytokines with the risk of CHD, which lends further support to the inflammation hypothesis in vascular disease. Although this does not establish causality in CHD for any of the analytes, the comprehensive evaluation of their associations with CHD should be important, given the evolving literature on cytokines as potential drug targets33,34 and gaps in translating basic scientific findings into clinical practice.1
Interleukin-6 is involved in the systemic inflammatory response, but also engages in local tissue inflammation35 and promotes differentiation of naive T-helper cells into Th17 cells,36 a cell type that has been implicated in auto-immune conditions such as rheumatoid arthritis. Tumour necrosis factor-α is a pro-inflammatory cytokine, which, similar to IL-6, has been implicated in auto-immune diseases.37 Both IL-6 and TNF-α signalling pathways are targets of drugs (such as tocilizumab and etanercept) which are used to treat auto-immune diseases.37,38 Moreover, there have been suggestions of an increased incidence of CVD in patients with rheumatoid arthritis,39 and non-randomized data from biological registries suggest TNF-α blocking therapy could reduce CVD risk in these patients,37 although such data have well-recognized limitations.40 It is of interest, therefore, that two of the agents (i.e. canakinumab, a monoclonal antibody to IL-1β, and low-dose methotrexate) being tested in phase 3 trials of CVD prevention lower circulating IL-6 levels,12,41 while low-dose methotrexate therapy also lowers TNF-α levels.12 Furthermore, consistent with previous studies, we found circulating IL-18 levels to be positively associated with CHD incidence. In contrast, we found no significant associations of sCD40L and MMP-9 levels with CHD in the aggregate of available prospective studies, suggesting that positive findings in previous non-prospective data may have been liable to potential biases, e.g. ‘reverse causality’.42–45
Our study had several strengths. First, whereas most previous studies have concurrently considered only a few cytokines, we were able to measure simultaneously multiple pro-inflammatory cytokines in a common subset of participants in one central laboratory, facilitating uniform comparison of the observed associations across markers. Furthermore, we excluded all individuals having baseline CVD to minimize any reverse association bias. Additionally, we combined data from previously published prospective studies, yielding qualitatively similar results to those observed in the new data. Moreover, to help identify independent associations, we adjusted for several conventional and emerging risk factors for cardiovascular disease, including all the inflammatory cytokines studied (although we acknowledge the possibility of ‘over-adjustment’).
Our study also had potential limitations. First, because we used stored samples, we cannot rule out protein degradation between sample collection and assay, especially for self-activable enzymes such as MMP-9.46 However, randomization of samples from CHD cases and non-cases within assay plates and blinding of laboratory technicians to case–control status of the samples should have ensured that samples were treated alike during sample handling and assay. Moreover independent studies employing accelerated stability testing protocols have concluded that serum samples for determination of cytokines such as IL-6 and sIL-6R can be stored at −20°C or less for several years without affecting recovery rates,47 although such evidence was lacking for serum levels of the other cytokines we studied. Furthermore, despite known diurnal variation in the concentrations of some of the cytokines studied,48 we believe that this would also have similarly affected both CHD cases and non-cases. Second, our principal analysis used a single baseline measurement of each cytokine to study its association with incident CHD. However, our reproducibility substudies of cytokine measurements made in the same individuals an average of 12 years apart in the Reykjavik9 cohort and 4 years apart in the British Regional Heart Study16,17,19,20 suggest that we could have substantially underestimated any underlying aetiological associations in CHD. Conversely, the poor reproducibility of some cytokines may limit their utility for use in clinical practice as reliable risk indicators based on just a single measurement. Third, information on some extensively studied downstream measures of inflammation (such as CRP) was only available in half of the participants in our primary study (due to exhaustion of serum samples), which limited the power for direct comparisons with other cytokines, besides inability to adequately adjust for the impact of differential measurement errors in different cytokines when making such aetiological comparisons. Fourth, atherosclerosis and plaque rupture are complex processes involving the interplay between several inflammatory substances at different stages of their evolution, hence the associations may somewhat differ according to the nature of the endpoints studied. To enhance power, we used a composite coronary endpoint as our primary outcome, but subanalyses focusing solely on non-fatal MI or CHD death yielded very similar findings to those overall. Owing to limited information, we could not assess the extent to which the duration of sample storage and differences in assay reproducibility may have influenced our meta-analysis findings; nevertheless, there was little variation seen in the few reported estimates of assay reproducibility. Finally, future observational analyses will wish to evaluate these cytokines in much larger prospective studies with extensive concomitant genetic data to enable causal evaluation (e.g. Mendelian randomization49).
In conclusion, several different pro-inflammatory cytokines are each associated with CHD risk independent of conventional risk factors and in an approximately log-linear manner. Although the current findings lend further support to the inflammation hypothesis in vascular disease, causality remains to be established for these cytokines in CHD.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by a grant from the British Heart Foundation (RG/08/014), the UK Medical Research Council, and the UK National Institute of Health Research Cambridge Biomedical Research Centre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
Dr Mathew Walker and Mr Carsten Agger provided assistance with data management. We thank Dr Paul Welsh and Ms Estelle Poorhang for technical assistance, and Dr Barbara Jefferis for providing clarifications on information sought about the BRHS and BWHHS studies.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. doi:10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. doi:10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. doi:10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, rocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, ez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De SB, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey SG, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. doi:10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 5.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, Coin L, Ashby D, Tzoulaki I, Brown IJ, Mt-Isa S, McCarthy MI, Peltonen L, Freimer NB, Farrall M, Ruokonen A, Hamsten A, Lim N, Froguel P, Waterworth DM, Vollenweider P, Waeber G, Jarvelin MR, Mooser V, Scott J, Hall AS, Schunkert H, Anand SS, Collins R, Samani NJ, Watkins H, Kooner JS. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. doi:10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wensley F, Gao P, Burgess S, Kaptoge S, Di AE, Shah T, Engert JC, Clarke R, vey-Smith G, Nordestgaard BG, Saleheen D, Samani NJ, Sandhu M, Anand S, Pepys MB, Smeeth L, Whittaker J, Casas JP, Thompson SG, Hingorani AD, Danesh J. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. doi:10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keavney B, Danesh J, Parish S, Palmer A, Clark S, Youngman L, Delepine M, Lathrop M, Peto R, Collins R. Fibrinogen and coronary heart disease: test of causality by ‘Mendelian randomization. Int J Epidemiol. 2006;35:935–943. doi: 10.1093/ije/dyl114. doi:10.1093/ije/dyl114. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65(Pt 2):S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. doi:10.1301/nr.2007.dec.S140-S146. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. doi:10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hingorani AD, Casas JP Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. doi:10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de FU, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, vey-Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di AE, Harari O, Danesh J IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. doi:10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7(Suppl. 1):332–339. doi: 10.1111/j.1538-7836.2009.03404.x. doi:10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. doi:10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Blankenberg S, Luc G, Ducimetiere P, Arveiler D, Ferrieres J, Amouyel P, Evans A, Cambien F, Tiret L. Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2003;108:2453–2459. doi: 10.1161/01.CIR.0000099509.76044.A2. doi:10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 15.Welsh P, Whincup PH, Papacosta O, Wannamethee SG, Lennon L, Thomson A, Rumley A, Lowe GD. Serum matrix metalloproteinase-9 and coronary heart disease: a prospective study in middle-aged men. QJM. 2008;101:785–791. doi: 10.1093/qjmed/hcn088. doi:10.1093/qjmed/hcn088. [DOI] [PubMed] [Google Scholar]
- 16.Jefferis BJ, Whincup PH, Welsh P, Wannamethee SG, Rumley A, Lawlor DA, Ebrahim S, Lowe GD. Prospective study of circulating soluble CD40 ligand concentrations and the incidence of cardiovascular disease in a nested prospective case-control study of older men and women. J Thromb Haemost. 2011;9:1452–1459. doi: 10.1111/j.1538-7836.2011.04415.x. doi:10.1111/j.1538-7836.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 17.Woodward M, Welsh P, Rumley A, Tunstall-Pedoe H, Lowe GD. Do inflammatory biomarkers add to the discrimination of cardiovascular disease after allowing for social deprivation? Results from a 10-year cohort study in Glasgow, Scotland. Eur Heart J. 2010;31:2669–2675. doi: 10.1093/eurheartj/ehp115. doi:10.1093/eurheartj/ehp115. [DOI] [PubMed] [Google Scholar]
- 18.Osler M, Linneberg A, Glumer C, Jorgensen T. The cohorts at the Research Centre for Prevention and Health, formerly ‘The Glostrup Population Studies. Int J Epidemiol. 2011;40:602–610. doi: 10.1093/ije/dyq041. doi:10.1093/ije/dyq041. [DOI] [PubMed] [Google Scholar]
- 19.Jefferis BJ, Whincup P, Welsh P, Wannamethee G, Rumley A, Lennon L, Thomson A, Lawlor D, Carson C, Ebrahim S, Lowe G. Prospective study of matrix metalloproteinase-9 and risk of myocardial infarction and stroke in older men and women. Atherosclerosis. 2010;208:557–563. doi: 10.1016/j.atherosclerosis.2009.08.018. doi:10.1016/j.atherosclerosis.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferis BJ, Whincup PH, Welsh P, Wannamethee SG, Rumley A, Lennon LT, Thomson AG, Carson C, Ebrahim S, Lowe GD. Circulating TNFalpha levels in older men and women do not show independent prospective relations with MI or stroke. Atherosclerosis. 2009;205:302–308. doi: 10.1016/j.atherosclerosis.2008.12.001. doi:10.1016/j.atherosclerosis.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdes LU, Bronnum-Hansen H, Madsen M, Borch-Johnsen K, Jorgensen T, Sjol A, Schroll M. Trends in selected biological risk factors for cardiovascular diseases in the Danish MONICA population, 1982–1992. J Clin Epidemiol. 2000;53:427–434. doi: 10.1016/s0895-4356(99)00193-6. doi:10.1016/S0895-4356(99)00193-6. [DOI] [PubMed] [Google Scholar]
- 22.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 23.Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull. 1999;46:354–357. [PubMed] [Google Scholar]
- 24.Joensen AM, Jensen MK, Overvad K, Dethlefsen C, Schmidt E, Rasmussen L, Tjonneland A, Johnsen S. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009;62:188–194. doi: 10.1016/j.jclinepi.2008.03.005. doi:10.1016/j.jclinepi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003;56:124–130. doi: 10.1016/s0895-4356(02)00591-7. doi:10.1016/S0895-4356(02)00591-7. [DOI] [PubMed] [Google Scholar]
- 26.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. doi:10.1016/S0895-4356(99)00102-X. [DOI] [PubMed] [Google Scholar]
- 27.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. doi:10.1093/biomet/73.1.1. [Google Scholar]
- 28.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. doi:10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 29.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–621. doi: 10.1093/oxfordjournals.aje.a008971. doi:10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. doi:10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 32.Wood AM, White I, Thompson SG, Lewington S, Danesh J Fibrinogen Studies Collaboration. Regression dilution methods for meta-analysis: assessing long-term variability in plasma fibrinogen among 27,247 adults in 15 prospective studies. Int J Epidemiol. 2006;35:1570–1578. doi: 10.1093/ije/dyl233. doi:10.1093/ije/dyl233. [DOI] [PubMed] [Google Scholar]
- 33.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. doi:10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9:703–718. doi: 10.1038/nrd2805. doi:10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- 35.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. doi:10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. doi:10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 37.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6:410–417. doi: 10.1038/nrcardio.2009.57. doi:10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 38.Navarro-Millan I, Singh JA, Curtis JR. Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin-6 receptor. Clin Ther. 2012;34:788–802. doi: 10.1016/j.clinthera.2012.02.014. doi:10.1016/j.clinthera.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. doi:10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 40.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56:2905–2912. doi: 10.1002/art.22809. doi:10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: A Phase IIb Randomized Placebo Controlled Trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. doi:10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 42.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600. doi:10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 43.Kinlay S, Schwartz GG, Olsson AG, Rifai N, Sasiela WJ, Szarek M, Ganz P, Libby P. Effect of atorvastatin on risk of recurrent cardiovascular events after an acute coronary syndrome associated with high soluble CD40 ligand in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) study. Circulation. 2004;110:386–391. doi: 10.1161/01.CIR.0000136588.62638.5E. doi:10.1161/01.CIR.0000136588.62638.5E. [DOI] [PubMed] [Google Scholar]
- 44.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. doi:10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 45.Hlatky MA, Ashley E, Quertermous T, Boothroyd DB, Ridker P, Southwick A, Myers RM, Iribarren C, Fortmann SP, Go AS. Matrix metalloproteinase circulating levels, genetic polymorphisms, and susceptibility to acute myocardial infarction among patients with coronary artery disease. Am Heart J. 2007;154:1043–1051. doi: 10.1016/j.ahj.2007.06.042. doi:10.1016/j.ahj.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 46.Rouy D, Ernens I, Jeanty C, Wagner DR. Plasma storage at -80 degrees C does not protect matrix metalloproteinase-9 from degradation. Anal Biochem. 2005;338:294–298. doi: 10.1016/j.ab.2004.10.052. doi:10.1016/j.ab.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 47.Kenis G, Teunissen C, De JR, Bosmans E, Steinbusch H, Maes M. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine. 2002;19:228–235. [PubMed] [Google Scholar]
- 48.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. doi:10.1161/01.CIR.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 49.Grisoni ML, Proust C, Alanne M, Desuremain M, Salomaa V, Kuulasmaa K, Cambien F, Nicaud V, Wiklund PG, Virtamo J, Kee F, Tiret L, Evans A, Tregouet DA. Lack of association between polymorphisms of the IL18R1 and IL18RAP genes and cardiovascular risk: the MORGAM Project. BMC Med Genet. 2009;10:44. doi: 10.1186/1471-2350-10-44. doi:10.1186/1471-2350-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.