Abstract
Background:
Usually the chest tube removal (CTR) has been described as one of the worst experiences by patients in the intensive care unit.
Aim:
This study aimed to compare the effects of cold therapy and relaxation on pain of CTR among the patients undergoes coronary artery bypass graft surgery.
Materials and Methods:
This single-blinded clinical trial was done on 80 post-cardiac surgery patients in the heart hospital of Sari-Iran. The patients were assigned to three randomized groups that included cold therapy, relaxation, and control groups. Data analysis was done by T-test, Chi-square, generalized estimating equations and repeated measures analysis variance tests.
Results:
The groups had no significant differences in pain intensity before CTR (P = 0.84), but immediately after CTR there was a significant difference between the treatment (cold application and relaxation groups) and control groups (P = 0.001). There was no significant difference between relaxation and cold therapy groups.
Conclusion:
Regarding the relaxation and cold application methods showed relatively equal effects on reducing the pain owing to CTR. Thus, the use of relaxation because of economics, without side effects, easy to use and effective is recommended by the authors to the practitioners.
Keywords: Chest tube removal, cold application, coronary artery bypass grafting, pain, relaxation
Introduction
According to American Heart Association, annually more than 448,000 patients underwent cardiothoracic surgery including coronary artery bypass grafting (CABG), valve replacement or repair, or repair of structural defects.[1] Inserting chest tubes (CT) after CABG was aimed to maintain heart and lung functioning and essential to prevent from pleural effusion, pneumothorax and hemothorax.[2,3] Keeping CTs in place, however, is associated with increased pain and discomfort for the patient, mechanical irritation of the heart and pericardium, and an increased incidence of infection.[4]
The chest tubes are typically removed within 24-48 hours after surgery or when the excess air, blood, or fluid has been properly drained.[5,6] Chest tubes removal (CTR) after surgery has been described as one of the worst experiences among these patients.[2,5,7,8] Studies showed that moderate to severe pain has been reported by patients who experienced CTR[6,7,8,9] and unfortunately their pain was managed very poorly.[5,6,9,10]
Unrelieved pain in addition to causing suffering, may be associated with the absence of sound breath, distress, decreased chest expansion, hyper-resonance on the injured side, impaired respiratory performance producing hypoxemia, increased sympathetic response provoking myocardial ischemia, and activation of a generalized stress response that can trigger tachycardia, increased cardiac output, and vasoconstriction.[11] Cardiovascular surgical patients may be particularly vulnerable to the physiological effects.[9] Although analgesic drugs are the most effective tool available to nurses but there are many other ways to relieve pain. Analgesics have some side effects and there are individual differences in their effects, so non-pharmacological methods may attract some attention.[12,13]
Cold application is a non-pharmacological pain-relieving method. It has been utilized for years.[9,10,11,12,13,14] Regarding the activity of inflammatory enzymes rises with increasing temperatures.[15] Ice is believed to help control pain by inducing local anesthesia around the treatment area. Investigators have also shown that it decreases edema, cellular metabolism, and local blood flow.[16] The main beneficial effect of cold during recovery is the cold-related vasoconstriction that may limit vessel permeability and thus inflammatory processes, reducing muscle pain.[17] Based upon this framework, also, based on laboratory and clinical studies, cold decreases the nerve conduction velocity and increases the pain threshold.[15,18,19] Several studies have shown a decrease in peripheral blood flow caused by different methods of application of cold.[20,21] The reasons put forward to explain this decrease in blood flow are vasoconstriction caused by the sympathetic nervous system reflex and the affinity caused by the cold of the postjunctional alpha-2 receptors of the vessel walls. The result of the two factors referred to above is a reduction in the activity of the noradrenaline enzymatic metabolites, an increase in blood viscosity, the activation of the platelet aggregates that release 5HT and thromboxane A2.[22]
Relaxation is another non-pharmacological technique in pain management keeping in mind that anxiety can cause pain and pain also can cause anxiety.[23] Relaxation is defined as the absence of physical, mental, emotional tension, and can assist in the management of pain both physiologically and psychologically. Physiologically, relaxation leads to a reduction in or reversal of the sympathetic response to pain leading to a decrease in oxygen consumption, blood pressure, heart rate, and respiration. Psychologically, distraction is a built-in component of relaxation and impacts pain management by decreasing the cognitive awareness of pain.[2]
Considering the importance of pain management as the highest nursing priority, there is no consensus about controlling pain following CTR.[18] While the usual way to pain management is prescription of analgesics but research has showed that despite of using analgesics and anesthetics, CTR is still painful.[5,9] In addition, pharmacological methods are costly and may leads to complications.
There have been few studies about the effectiveness of cold application and relaxation, which achieved contradictory results. In addition, there is no research about comparing these methods on pain owing to CTR. Therefore, this study aimed to examine the effectiveness of relaxation and cold therapy on the patient's pain severity after CTR. Based on aim of study the following research questions considered:
Is relaxation would be effective in pain reducation after CTR preoperative bypass surgery?
Is cold therapy would be effective in pain reducation after CTR preoperative bypass surgery?
Which method works better in pain reduction (cold therapy-relaxation)?
Materials and Methods
Approval for the study obtained from the relevant ethics committee at the University of Mazandaran, Sari, Iran. Before the beginning of the study, written informed consent was obtained from each patient.
This randomized, observer-blind, prospective experimental study was conducted during February-March 2013 in the Mazandaran Heart Center. The subjects include 80 patients underwent open heart surgery. The sample size was determined based on a similar study[2] with a 0.99% confidence coefficient. Based on mean and standard deviation of pain intensity immediately after CTR in experimental and control groups, respectively, 3.85 ± 1.75, and 5.6 ± 1.94, the sample size was calculated 30 in each group (intervention and control) that according to effect size of 1.33 the samples increased to 40 in each group.
Data collection tools included a demographic questionnaire and Visual Analogue Scale (VAS) to evaluate pain. VAS comprised a 100-mm baseline that is indicative a continuum with the ends marked “0 = no pain” and “100 = unbearable pain”.[24] Reliability and validity of this scale is approved internationally.[25,26] Various researches have used this scale to rate pain severity in patients underwent CABG surgery.[27]
Inclusion criteria included: Willingness to participate, upper 18-years old, full consciousness, ability to understand visual analog pain scale, first-time experience with CABG and chest tube, BMI less than 30 kg/m218 and having two chest tubes for 24 hours at least (one mediastinal tube and one left pleural). Exclusion criteria included unwillingness to participate, oversensitivity to cold, received mechanical ventilation support, visual or auditory defects, received opioid analgesic during less than 4 hours before intervention and drug dependency.
On the first day after operation, the researcher offered some explanation about the procedure to patients eligible to participate in study. Patients randomly were assigned to groups by Rand between function in Excel software (this function used to generate random numbers). As the participants in the study had two chest tubes, the act of assignment in intervention groups was done twice (one assignment for each tube). The case group was divided to seven bulks comprising 6 cases and within each bulk 6 cards were divided to 1-2 then randomly selected (three indicated by cod[1] for left pleural cold therapy- mediastinal relaxation and three cod number[2] considered for left pleural relaxation- mediastinal cold therapy). So each chest tube of patient assigned in one group and finally patients were divided randomly in the groups of cold therapy, relaxation, or control.
After the first assignment, the subjects were taught to rate the pain intensity by using VAS and got enough information about CTR procedure. In addition, we presented some information about cold application and relaxation for experimental groups that was repeated just before the CTR process.
All above procedures are supervised by a physician. For every group subjects’ all tight clothes were loosened, they were placed in semi-fowler position and a pillow was set below their head and knees in order to ensure their comfort. The usual intervention, as used for the control group, was nothing more than acetaminophen pills each 6 hours. All subjects received the intervention. In cold therapy group, cooling gel packs with 0°C temperature (made in Iranian Bespar Javidan Ghostar company) was used to reduce the body temperature around the chest tube during undressing. The bandage was removed from chest area and body temperature measured and recorded by an infrared thermometer at the same time by non-contact infrared thermometers UNI-T 912 (Hong Kong) then three cooling packs (8 × 10 cm) twisted in gauze was placed around the tube so that it would locate in the center. The packs would remain in their position, after 10 minutes till the temperature reached 13°C.[28,29] At this moment, all packs were removed and the tube was exit according to the usual method. The temperature was recorded immediately and 15 minutes after extubation. According to the evidence, effectiveness of cooling treatments sustains around 30 minutes.[30,31] There is no other evidence to confirm the effectiveness time duration for relaxation. Therefore, 1 hour after cooling therapy and extubation-related tube, the next tube extubated while patients exposed to relaxation technique. In the relaxation group, patients were encouraged to breathe calmly and deeply. In this exercise, they should inhale calm and deep through their nose and exhale calm through semi-closed lips, all with closed eyes for 15 minutes.[5] The tube was removed after 5 minutes of exercising.
In control group, the tubes of the first patient were removed randomly with 1-hour intervals. Then the procedure was repeated for all patients in the control group as routine. The chest tubes in all groups were removed by the same nurse and procedure lasted on 1-2 minutes. The pain intensity was recorded by a nurse who trained to record VAS and was blind to the conducted different CTR procedure. The pain severity was recorded in three phases (before, immediately, and 15 minutes after CTR).
Statistical analysis
SPSS version 20 was used to analyse the recorded data. Descriptive statistics were used to summarize demographic characteristics of study participants. The analysis included all randomized participants for whom data were available (N = 90). To assess comparability of the study groups at baseline on sociodemographic and pain levels, we used t-test (for continued and ordinal variables) and Chi-square (for categorical variables). To compare the two study groups on the outcome and process measures overtime we fit multiple marginal linear regression analysis. Since the self-reported pain was measured repeatedly overtime (before intervention, immediately after removing and after 15 minutes), the generalized estimating equations method for longitudinal data was applied to adjust the standard errors of the regression coefficient estimates. Generalized estimating equations (GEE) employed to adjust for possible correlation over the assessment time points by controlling for baseline values of the dependent variable examined. The independent variables considered in the regression analysis were delivered treatments in two groups. A P value less than 0.05 was considered statistically significant.
Results
The subjects’ mean old age was 58.11 ± 9.53 (control group 58.48 ± 9.78 and experimental group was 57.75 ± 9.38). Results showed that the age variable had no considerable differences in subgroups (P = 0.8). The body mass index (BMI) mean was 26.16 ± 2.61 kg/cm2 for the experimental group and it was 25.51 ± 3.16 kg/cm2 in the control group, it was not significantly different in the groups (P = 0.155).
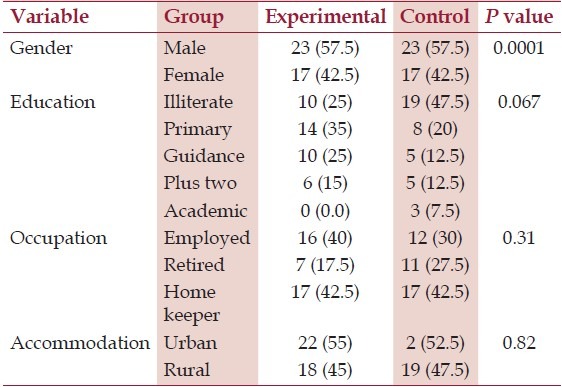
Tubes remaining time duration for the control group were 41.97 ± 2.35 and for the experimental group was 42.17 ± 2.35. The groups had no significant differences with regard to holding duration (P = 0. 7). The subjects were all married. Table 1 displays other demographic data for each group. As it is presented, the groups had no differences related to these variables.
Table 1.
Comparing some demographic characteristics in experimental and control groups
To investigate tube type (left pleural, mediastinal) effect on pain rating and to compare pain severity in control, cold therapy, and relaxation groups at tree phases (before, during, and after extubation), we used GEE model. Wald Chi-square test showed no significant difference in pain severity in the phases. These results are displayed in Table 2. Figure 1 depicted pain severity rating of all groups in the three phases.
Table 2.
Comparing means related to pain severity between the three groups in three phases
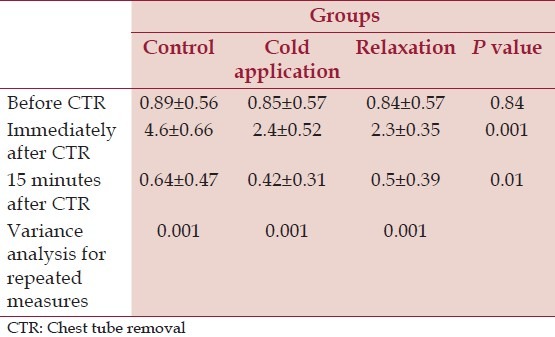
Figure 1.
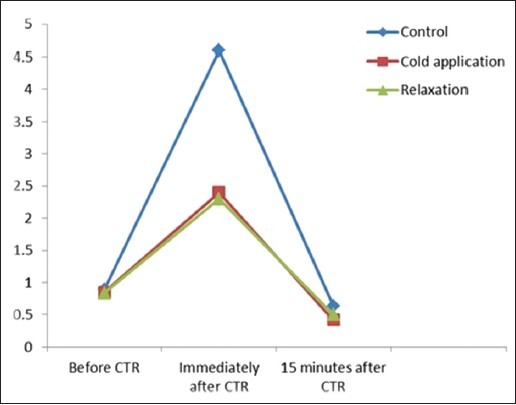
The difference of three groups at three time of assessment
Wald Chi-square test showed that there were no significant differences between pain severity before CTR in the three groups (P = 0.84, df = 2, F = 0.058). This test also showed that the groups had a considerable difference in phases two (just after CTR, P = 0.001, df = 2, F = 468.35) and three (15 minutes after CTR, P = 0.01, df =, F = 93.3). We used Bonferroni pairwise test for after experience evaluations.
In Bonferroni post-hoc test paired comparison results showed that cold application and relaxation group had a significant difference comparing with a control group in phase two (just after CTR, P = 0.001). The cold application and relaxation groups were not significantly different (P = 0.4). There was also a considerable difference between control group and cold therapy group in phase three (15 min after CTR, P = 0.01), but this difference was not significant between relaxation and control groups (P = 0.11) or between cold application and relaxation groups (P = 0.6).
Pain levels in the groups at three time frames (before, immediately and after CTR) showed in Figure 1 pain is in the same condition before CTR in all groups although immediately after CTR there is considerable difference between the control and experiment a group so pain in lower in patients who were subjected for relaxation and cold therapy. They reported same pain level in two experimental groups (cold therapy and relaxation) and 15 minutes after CTR these differences sustained between groups.
According to the results acquired from repeated measures analysis of variance also, there was a significant difference for within groups measures in all three phases (P = 0.001). Multiple comparisons of Bonferroni tests showed that the maximum difference was observed between immediately after CTR and after 15 minutes (P = 0.001).
Discussion
Our finding has implicated that therapy could relieve pain after CTR. The results are consistent with Mazloum,[32] Ertug[2] and Demir[18] findings. According to our findings, pain severity of cold application group at phase 3 (15 minutes after extubing) was lower than the control group. This is also consistent with Mazloum and Demir's results. The results similarity could be due to full covering of ice packs into the tubes nearby areas. Sauls, on the other hand, reported the cold application as a not very efficient method to relieve pain at just after and 15 minutes later phases.[10] The shape of ice packs, cold therapy duration (10 minutes), or sample size could account for these differences.
Cold gel packs used in our study were flexible so that they cover around tubes easily and lead to effective cooling around the tissue. Research showed that physiological effects of cold therapy appear when it continues for 20 minutes or temperature tube surrounding skin decreases to 13°C or less.[28,29] Our results showed that pain severity in phase two (just immediatly after CTR) was considerably lower in relaxation group than control group. It is consistent with Friesner et al.[5] In another study the relaxation method was not recognized as an effective way to reduce CTR-related pain.[23] Apparently these contradictory results could be explained with shorter breathing exercise before CTR, and smaller sample size in Houston's work.
We found no significant difference between relaxation and control groups in phase 3 (15 min after extubation) regarding pain severity. This result is not consistent with Friesner's findings.[5] It may be due to continuing relaxation exercise after CTR in his work. We did not find any significant difference between relaxation and cold application groups at phase 3. We could not find any published studies about relaxation or cold application effects on CTR induced pain after coronary artery bypass graft surgery, but there were some studies using these techniques to decrease pain after other surgeries such as muscular pain syndrome with delayed onset,[32] labor pain,[33,34] and blood injection for thalassemia patients.[35] The overall results indicate a beneficial outcome to pain and pain management.
Our results indicate a relatively equal effect of cold therapy and relaxation on CTR-related pain after coronary artery bypass grafting. As the relaxation requires any expense, it could be a preferred technique for managing pain in CTR patients.
This study was done on a relatively small sample, so the findings could not be generalized without caution. Repeating these findings with a bigger sample size and for the other patients experiencing any king of chest tubes could be useful in order to a comprehensive conclusion. Comparing the other nonpharmacological ways of controlling pain could also develop our knowledge and may propose some cheaper and more effective ways of pain management.
Acknowledgment
This paper is a result of a dissertation submitted to award master degree on critical care nursing. The ethical committee of Mazandaran University approved this study in 2012 with registered number of 91-288. We appreciate the financial support of Mazandaran University, corporation of staffs of Sari Nursing and Midwifery Faculty, the subjects and staffs of Mazandaran Heart Hospital.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Writing Group Members. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics -2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Ertug N, Ulker S. The effect of cold application on pain due to chest tube removal. J Clin Nurs. 2012;21:784–90. doi: 10.1111/j.1365-2702.2011.03955.x. [DOI] [PubMed] [Google Scholar]
- 3.Wynne R, Botti M, Copley D, Bailey M. The normative distribution of chest tube drainage volume after coronary artery bypass grafting. Heart Lung. 2007;36:35–42. doi: 10.1016/j.hrtlng.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Mirmohammad-Sadeghi M, Etesampour A, Gharipour M, Shariat Z, Nilforoush P, Saeidiv M, et al. Early chest tube removal after coronary artery bypass graft surgery. N Am J Med Sci. 2009;1:333–7. doi: 10.4297/najms.2009.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friesner SA, Curry DM, Moddeman GR. Comparison of two pain management strategies during chest tube removal: Relaxation exercise with opioids and opioids alone. Heart Lung. 2006;35:269–76. doi: 10.1016/j.hrtlng.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Bruce EA, Howard RF, Franck LS. Chest drain removal pain and its management: A literature review. J Clin Nurs. 2006;15:145–54. doi: 10.1111/j.1365-2702.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 7.Akrofi M, Miller S, Colfar S, Corry PR, Fabri BM, Pullan MD, et al. A randomized comparison of three methods of analgesia for chest drain removal in postcardiac surgical patients. Anesth Analg. 2005;100:205–9. doi: 10.1213/01.ANE.0000140237.96510.E5. [DOI] [PubMed] [Google Scholar]
- 8.Hessami MA, Najafi F, Hatami S. Volume threshold for chest tube removal: A randomized controlled trial. J Inj Violence Res. 2009;1:33–6. doi: 10.5249/jivr.v1i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh M, Gopinath R. Topical analgesia for chest tube removal in cardiac patients. J Cardiothorac Vasc Anesth. 2005;19:719–22. doi: 10.1053/j.jvca.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Houston S, Jesurum J. The quick relaxation technique: Effect on pain associated with chest tube removal. Appl Nurs Res. 1999;12:196–205. doi: 10.1016/s0897-1897(99)80261-4. [DOI] [PubMed] [Google Scholar]
- 11.Sauls J. The use of ice for pain associated with chest tube removal. Pain Manag Nurs. 2002;3:44–52. doi: 10.1053/jpmn.2002.123017. [DOI] [PubMed] [Google Scholar]
- 12.Genzyme Corporation (2001) . Understanding Chest Drainage: From the manufacturers of Pleur-Evac. MA, USA.
- 13.Layzell M. Improving the management of postopera tive pain. Nurs Times. 2005;101:34–6. [PubMed] [Google Scholar]
- 14.Fayyazi S, Shariati A, Momeni M. The efficacy of Benson's relaxation technique on postoperative pain in coronary artery bypass graft. Sci Med J. 2010;8:478–89. [Google Scholar]
- 15.Abedian Z, Navabi Rigi Sh, Dadgar S, Esmaeili HA. Comparing the effect of colling gel pads and ice pack, after episiotomy on the intensity of perineal pain. IJOGI. 2008;10(2):79–86. [Google Scholar]
- 16.Modabber A, Rana M, Ghassemi A, Gerressen M, Gellrich N, Hölzle F, et al. Three-dimensional evaluation of postoperative swelling in treatment of zygomatic bone fractures using two different cooling therapy methods: A randomized, observer-blind, prospective study. Trails. 2013;14:238. doi: 10.1186/1745-6215-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dykstra JH, Hill HM, Miller MG, Cheatham CC, Michael TJ, Baker RJ. Comparisons of cubed ice, crushed ice and wetted ice on intramascular and surface temperature changes. J Athl Train. 2009;44:136–41. doi: 10.4085/1062-6050-44.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausswirth C, Louis J, Bieuzen F, Pournot H, Fournier J, Filliard JR, et al. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS One. 2011;6:e27749. doi: 10.1371/journal.pone.0027749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demir Y, Khorshid L. The effect of cold applicationin combination with standard analgesic administration on pain and anxiety during chest tube removal: A single-blinded, randomized, double-controlled study. Pain Manag Nurs. 2010;11:186–96. doi: 10.1016/j.pmn.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Kol E, Erdogan A, Karsh B, Erbil N. Evaluation of the outcomes of ice application for the control of pain associated with chest tube irritation. Pain Manag Nurs. 2013;14:29–35. doi: 10.1016/j.pmn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Barcoft H, Edholm O. The effct of temperature on blood flow and deep temperature in the human forearm. J Physiol. 1943;102:5–20. doi: 10.1113/jphysiol.1943.sp004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snedeker J, Recine V, MacCartee C. Cryotherapy and the athletic injury. Athl J. 1974;55:16–8. [Google Scholar]
- 23.Taber C, Countryman K, Fahrenbruch J, LaCount K, Cornwall MW. Measurement of reactive vasodilatation during cold gel pack application to non-traumatized ankles. Phys Ther. 1992;72:294–9. doi: 10.1093/ptj/72.4.294. [DOI] [PubMed] [Google Scholar]
- 24.Rafii F, Mohammadi Fakhar F, Jamshidi Orak R, Inanloo M. Effect of Jaw relaxation on pain intensity of burn dressing. Iran J Critical Care Nurs. 2010;3:51–6. [Google Scholar]
- 25.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–2. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 26.Tamiya N, Araki S, Ohi G, Inagaki K, Urano N, Hirano W, et al. Assessment of pain, depression, and anxiety by visual analogue scale in Japanese women with rheumatoid arthritis. Scand J Caring Sci. 2002;16:137–41. doi: 10.1046/j.1471-6712.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- 27.van Valen R, van Vuuren H, van Domburg RT, van der Woerd D, Hofland J, Bogers AJ. Pain management after cardiac surgery: Experience with a nurse-driven pain protocol. Eur J Cardiovasc Nurs. 2012;11:62–9. doi: 10.1177/1474515111430879. [DOI] [PubMed] [Google Scholar]
- 28.Forouzan Nia SKh, Hosseini HA, Mir Hosseini SJ, Abdollahi MH, Moshtaghion SH, Shahrad A, et al. Analgesic effects of cryotherapy to reduce pain and paresthesia after median sternotomy after coronary artery bypass graft surgery. Yazd Univ Med J. 2009;17:115–21. [Google Scholar]
- 29.Janwatanakul P. The effect of quabtity of ice and size of contact area on ice pack/skin interface temperature. Physiotheapy. 2009;95:120–5. doi: 10.1016/j.physio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Chesterton LS, Foster NE, Ross L. Skin temperature response to cryotherapy. Arch Phys Med Rehabil. 2002;83:543–9. doi: 10.1053/apmr.2002.30926. [DOI] [PubMed] [Google Scholar]
- 31.Moti Allah T, Ghaffari Nejad F. Reflex vasodilatation of the vessels and skin impedance measurments after using gel ice bag on your forearm. Tabib Shargh J. 2004;6:23–9. [Google Scholar]
- 32.Hedayati R, Salavati M, Ghadiri P. Comparing the effects of heat and cold on the base stimulation thresholds in normal subjects. Rafsanjan Univ Med J. 2005;4:248–54. [Google Scholar]
- 33.Mazloum SR, Abbasi Teshnizi M, Kianinejad A, Gandomkar F. Effect of applyig ice bag on pain intensity associated with chest tube removal after cardiac surgery. Q Ofoghe Danesh. 2012;18:109–14. [Google Scholar]
- 34.Rahimi A. Effect of massage, cold and exercise in redusing symptoms of delayed onset muscle pain syndrome. Rafsanjan Univ Med J. 2005;4:276–85. [Google Scholar]
- 35.Ivan Bagha R, Fardi Azar Z, Kamranpour B, Ghojazadeh M. Comparison of acetaminophen tab, diclofenac supp and ice packs on perineal pain after episiotomy incision the patients referred to Tabriz Al-Zahra hospital. Sabzevar Univ Med J. 2006;13:145–51. [Google Scholar]


