Abstract
Background:
Vertical sleeve gastrectomy (VSG) was originally performed as the first-stage of biliopancreatic diversion with duodenal switch (BPD/DS) for superobesity as a strategy to reduce perioperative complications and morbidity. VSG is now considered a definitive procedure because of its technical simplicity and promising outcomes.
Aims:
To analyze the outcomes of laparoscopic VSG and to compare them with those of single-stage laparoscopic BPD/DS.
Materials and Methods:
A retrospective review of 200 consecutive patients who underwent VSG and BPD/DS between 2008 and 2011.
Results:
A total of 100 patients underwent laparoscopic VSG and 100 patients underwent laparoscopic BPD/DS. The patients in VSG group were older, but gender distribution and body mass index were comparable. Mean operative time for VSG was significantly shorter compared with that of BPD/DS. A single patient in each groups required open conversion. Staple line leak (n = 1) and intraluminal hemorrhage into the newly-created sleeve (n = 1) occurred in the BPD/DS group. Mean length of stay was shorter after VSG (3.1 vs. 3.9 days). At 6 months postoperatively, excess weight loss between the two groups revealed statistically significant difference, favoring BPD/DS.
Conclusions:
Despite promising outcomes and technical simplicity of VSG, BPD/DS provides significantly superior excess weight loss in morbidly obese patients.
Keywords: Biliopancreatic diversion, biliopancreatic diversion, comparison, sleeve gastrectomy
Introduction
Failure to achieve satisfactory weight loss has been reported in several series of morbidly obese patients who underwent bariatric procedures.[1,2] The result of inferior weight loss after Roux-en-Y gastric bypass (RYGB) in high body mass index (BMI) patients has led to the development of biliopancreatic diversion with duodenal switch (BPD/DS).[2] The technical complexity of BPD/DS and its significant perioperative risks in the supermorbidly obese patients have resulted in a two-stage approach. In this method, a vertical sleeve gastrectomy (VSG) is initially performed, followed by a second-stage malabsorptive procedure after the initial weight loss and resolution of the obesity-related comorbidities.
In the early era of minimally invasive surgery, laparoscopic VSG was proposed as a staged approach to the BPD/DS for high-risk super morbidly obese patients.[3,4] In the last decade, however, laparoscopic VSG has been increasingly considered by many bariatric surgeons as definitive procedure because of its promising early and midterm outcomes. The technical simplicity and more modest learning curve of laparoscopic VSG have contributed to its rapid adoption among bariatric surgeons.
As techniques in minimally invasive surgery improve, the previously high-risk BPD/DS is now performed as a single-stage operation with minimal complications and excellent outcomes. To our knowledge, there have been no reports that compare the complications and outcomes between laparoscopic VSG and laparoscopic single-stage BPD/DS. In this study, we investigated the outcomes of laparoscopic VSG as a stand-alone procedure and compare them with those of single-stage laparoscopic BPD/DS in morbidly obese patients.
Materials and Methods
A prospectively maintained database of 100 consecutive patients who underwent laparoscopic VSG (Group 1) and 100 consecutive patients who underwent laparoscopic BPD/DS (Group 2) by two bariatric surgeons in a large independent teaching hospital was retrospectively reviewed. This study was approved by Abington Memorial Hospital Institutional Review Board (IRB). Patient demographics (age, gender, BMI, and number of obesity-related comorbidities), intraoperative details, perioperative complications, length of hospital stay (LOS), weight loss outcome at 1-, 3-, 6-, 9-, 12-, 18- month intervals, and mortality were compared. Major complications were defined as potentially life-threatening events that were directly related to the operation. These include anastomotic or staple line leak, hemorrhage, intestinal obstruction, inadvertent injury to other organs, venous thromboembolism, and all events that required return to the operating room. Minor complications were defined as nonlife-threatening events that result in prolongation of the typical postoperative recovery course. These include superficial skin or soft tissue infection, minor incisional hematoma, urinary tract infection, or musculoskeletal problems.
The standard criteria for bariatric surgery selection included BMI above 40 kg/m2 without comorbidities, or BMI above 35 kg/m2 with at least one obesity-related comorbidity. All patients underwent comprehensive preoperative medical evaluation, detailed psychological assessment, relevant laboratory, and radiologic testing, as well as esophagogastroduodenoscopy. A sleep apnea test was performed in patients with clinical suspicion of obstructive sleep apnea. All patients were counseled about other surgical options, including laparoscopic RYGB and laparoscopic adjustable gastric banding. Postoperatively, patients were encouraged to maintain an exercise program and regularly attend the bariatric patient-support group meetings. Standard postoperative follow-up included visits to the outpatient clinic at 1-, 3-, 6-, 9-, 12-, 18- month intervals and then annually thereafter. Continuous dietary counseling was provided. In this study, statistical analysis was performed using student's t-test with P ≤ 0.05 considered statistically significant.
Results
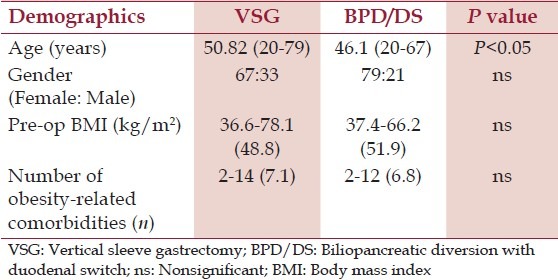
A total of 100 patients from each group were included. Patients in group 1 were older than those in group 2 (50.8 vs. 46.4 years; P < 0.05), although gender distribution (female predominance), average BMI (48.8 vs. 51.9 kg/m2 ; P > 0.05), and number of obesity-related comorbidities (7.1 vs. 6.8; P > 0.05) were comparable [Table 1].
Table 1.
Patients demographics
One patient in each group required open conversion because of dense intraabdominal adhesions from prior laparotomy. Compared to the BPD/DS group, the VSG group had a significantly shorter mean operative time (107 vs. 296.8 min, P < 0.05). Average blood loss was minimal (<50 mL) in both groups, without intraoperative complications. In the BPD/DS group, one patient developed a staple line leak, which resulted in perigastric air-fluid collection 14 days postoperatively. The leak resolved spontaneously after percutaneous placement of a drain and intravenous antibiotic treatment. Another patient developed intraluminal bleeding from the gastric staple line. This patient experienced significant postoperative nausea and hematemesis, which required placement of a decompressive nasogastric tube and blood transfusion. The bleeding resolved on postoperative day 2, without the need for endoscopic or surgical intervention [Table 2].
Table 2.
30-day perioperative outcomes and complications
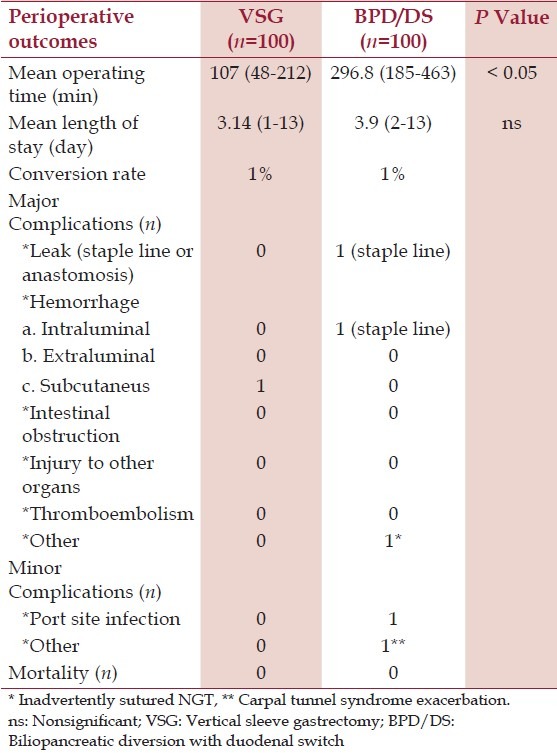
One patient in the VSG group was returned to the operating room for surgical hemostasis, related to a port-site hemorrhage. In the BPD/DS group, two patients were returned to the operating room, one on postoperative day 1 for an endoscopic release of a nasogastric tube that had been inadvertently sutured during the robotically-assisted creation of the duodenoileal anastomosis,[5] and the other patient returned on postoperative day 2 because of port-site infection. One patient experienced a minor postoperative complication (exacerbation of carpal tunnel syndrome). The average LOS after VSG and BPD/DS was statistically comparable. In the VSG group, two patients had an extended hospital stay because of prosthetic heart valve complications, as well as urologic complications related to recurrent hematuria and urinary retention. In the BPD/DS group, two other patients stayed longer in the hospital because of the exacerbation of carpal tunnel syndrome and skin/soft tissue infection at one of the trocar insertion sites (9 and 13 days, respectively).
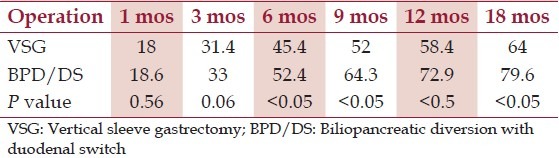
In the first 3 months postoperatively, patients in the VSG group and BPD/DS group achieved a comparable percentage of excess weight loss. The weight loss, however, promptly reached statistical difference at the 6-month interval (45.4 vs. 52.4%, P < 0.05), with the BPD/DS served as the more effective weight loss operation. The difference became even more significant at 9, 12, and 18 months, postoperatively [Table 3]. Percentage of patients who reached BMI < 35 kg/m2 at 18-months postoperatively (final weight loss) was 60% in the VSG group and 85% in the BPD/DS group. At the end of study (18 months postoperatively), approximately 35% of VSG patients were lost to follow-up. In the BPD/DS group, 30% of the patients were similarly unavailable for their follow-up. No mortality occurred in this study.
Table 3.
Excess weight loss aft er laparoscopic vertical sleeve gastrectomy and biliopancreatic diversion with duodenal switch
Discussion
BPD/DS is often referred to as the DS operation. It is a modification of the original biliopancreatic diversion operation described by Scopinaro et al., in 1979[6] and the DS operation described by Demeester et al., in 1987.[7] Ideally, bariatric procedure should be technically simple with acceptable low morbidity and mortality, sustained weight loss, as well as excellent resolution of obesity-related comorbidities. Greater technical challenges and perioperative risks associated with the BPD/DS have led to the adoption of VSG as the initial and potentially definitive procedure for treatment of morbid obesity.[3] Patients with unsatisfactory weight loss and poor resolution of obesity-related comorbidities after VSG then proceeded with the DS during a second operation. Many of the superobese and high-risk patients underwent the second stage within 2 years with improved comorbidities and surgical risk status.[4] In contrast, patients with sufficient weight loss and excellent resolution of obesity-related comorbidities after VSG do not require the second stage of the BPD/DS.
The primary goal of bariatric surgery is to find a technically simple, safe, and effective operation for the treatment of morbid obesity. When perioperative variables were compared between the two groups, the VSG group had a significantly shorter operative time, which correlated to its technical simplicity. Literature showed that the operative mortality rate in the first reported laparoscopic BPD/DS series was 2.5% and specifically in a subgroup of patients with preoperative BMI greater than 60 kg/m2, the mortality rate reached 6.5%.[3,8] When compared to the 0% mortality rate reported by Mukherjee et al.,[9] after laparoscopic VSG, BPD/DS was clearly a procedure with significant risks of death. Additionally, in two large open BPD/DS series in 1993 and 2007 by Marceau et al.,[10,11] the anastomotic leak rate was 2.7% and 3.75%, respectively.
In our series of VSG and BPD/DS, however, the overall morbidity (1% staple line leak and 1% bleeding rate after BPD/DS, vs. 0% after VSG) and mortality (0% after both procedures) are significantly lower, compared with those reported in the literature. These findings suggested that in the current era of widely used minimally invasive approach, both VSG and single-stage BPD/DS can be performed laparoscopically, with a low complication rate and a relatively equal safety profile.
Our patients experienced excess weight loss of 18% and 31.4% at 1 and 3 months after the VSG, respectively. This early result was comparable with that achieved after the BPD/DS. At 6 months postoperatively, however, the BPD/DS group showed a significantly higher percentage of weight loss compared with the VSG. This difference may represent the role of malabsorptive component in medium and long-term weight reduction in postbariatric surgery patients.
The Third National Health and Nutrition Examination Survey 1999 reported that morbidly obese patients with BMI greater than 35 kg/m2 were found to have relative risks (RRs) of 1.97, 6.16, and 3.77 to experience heart diseases, diabetes mellitus, and hypertension, respectively, when compared to the nonobese population.[12] In addition to a higher prevalence of obesity-related medical comorbidities, a significant increased risk of mortality was observed in patients with BMI >35 kg/m2 (RR = 1.36, P < 0.05).[13] This information led us to conclude that although modest weight loss has been shown to improve many of the metabolic complications of obesity, greater degree of weight loss (target BMI <35 kg/m2 ) is necessary to achieve all of the benefits of bariatric surgery.
Our study demonstrated that the VSG group experienced a 64% excess weight loss, while BPD/DS group experienced 79.6% excess weight loss at 18 months postoperatively. There have been different opinions among bariatric surgeons on the degree of postoperative excess weight loss to be considered adequate, but we believe that BMI should fall below 35 kg/m2, in order to optimally benefit from a bariatric operation, based on the published Third National Health and Nutrition Survey data. Hypothetically, if a morbidly obese male with BMI of 57 kg/m2 and body weight of 400 lbs were to undergo a laparoscopic VSG, his BMI would only fall to 36 kg/m2 at 18 months postoperatively. With this degree of weight loss (postoperative BMI still above 35 kg/m2 ), the patient will continue to have an increased risk for developing heart disease, diabetes mellitus, and hypertension, compared to their nonobese counterparts, as mentioned above. When the same patient were to undergo laparoscopic BPD/DS, his BMI would fall to 31 kg/m2 postoperatively, which gives him the maximum benefit of the bariatric surgery. In this study, we found that after BPD/DS, a significantly higher percentage of patients were able to reach final BMI of <35 kg/m2 compared with the VSG group (85% vs. 60%, respectively). Therefore, we believe a higher percentage of excess weight loss to achieve final BMI less than 35 kg/m2 using BPD/DS, should be sought in an appropriate clinical setting.
There are several limitations in this study, such as its retrospective nature with relatively small sample size. Our follow-up rate at 18 months postoperatively was only approximately 65%-70%, as described previously. Similar problem, however, has been reported by Himpens et al., where 22% of their patients were unavailable for medium-term follow-up, even in a country using socialized medical care system.[14]
Conclusions
Laparoscopic VSG is a lower-risk and a technically simpler operation with promising weight loss outcome. The single-stage laparoscopic BPD/DS, however, produces superior weight loss with acceptable complication rates. The statistically significant difference in weight loss between the two operations became evident at 6 months postoperatively and persisted thereafter. We, therefore, recommend single-stage laparoscopic BPD/DS in patients with a high BMI, in an attempt to achieve the target BMI of less than 35 kg/m2 postoperatively.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Bloomston M, Zervos EE, Camps MA, Goode SE, Rosemurgy AS. Outcome following bariatric surgery in super versus morbidly obese patients: Does weight matter? Obes Surg. 1997;7:414–9. doi: 10.1381/096089297765555395. [DOI] [PubMed] [Google Scholar]
- 2.Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: Correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg. 2008;12:250–5. doi: 10.1007/s11605-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 3.Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–82. doi: 10.1381/096089298765554476. [DOI] [PubMed] [Google Scholar]
- 4.Silecchia G, Boru C, Pecchia A, Rizzello M, Casella G, Leonetti F, et al. Effectiveness of laparoscopic sleeve gastrectomy (first stage of biliopancreatic diversion with duodenal switch) on co-morbidities in super-obese high-risk patients. Obes Surg. 2006;16:1138–44. doi: 10.1381/096089206778392275. [DOI] [PubMed] [Google Scholar]
- 5.Sucandy I, Antanavicius G. A novel use of endoscopic cutter: Endoscopic retrieval of a retained nasogastric tube following a robotically assisted laparoscopic biliopancreatic diversion with duodenal switch. N Am J Med Sci. 2011;3:486–8. doi: 10.4297/najms.2011.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66:618–20. doi: 10.1002/bjs.1800660906. [DOI] [PubMed] [Google Scholar]
- 7.Demeester TR, Fuchs KH, Ball CS, Albertucci M, Smyrk TC, Marcus JN. Experimental and Clinical Results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–26. doi: 10.1097/00000658-198710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WW, Gagner M, Kini S, Inabnet WB, Quinn T, Herron D, et al. Laparoscopic vs open biliopancreatic diversion with duodenal switch: A comparative study. J Gastrointest Surg. 2003;7:552–7. doi: 10.1016/S1091-255X(02)00149-X. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee S, Devalia K, Rahman MG, Mannur KR. Sleeve gastrectomy as a bridge to a second bariatric procedure in superobese patients: A single institution experience. Surg Obes Relat Dis. 2012;8:140–4. doi: 10.1016/j.soard.2011.04.232. [DOI] [PubMed] [Google Scholar]
- 10.Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, Simard S. Biliopancreatic diversion with a New Type of Gastrectomy. Obes Surg. 1993;3:29–35. doi: 10.1381/096089293765559728. [DOI] [PubMed] [Google Scholar]
- 11.Marceau P, Biron S, Hould FS, Lebel S, Marceau S, Lescelleur O, et al. Duodenal switch: Long term results. Obes Surg. 2007;17:1421–30. doi: 10.1007/s11695-008-9435-9. [DOI] [PubMed] [Google Scholar]
- 12.The Third National Health and Nutrition Examination Survey 4 December 1999. Centers for Disease Control and Prevention, Atlanta, GA. [Accessed July 1, 2013]. at http://www.cdc.gov/nchs/nhanes.htm .
- 13.Orpana H, Berthelot JM, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: Results from a national longitudinal study of Canadian adults. Obesity. 2010;18:214–8. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- 14.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–24. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]


