Abstract
Background:
Research indicates that some children with autism spectrum disorder (ASD) experience a developmental regression.
Aims:
The study examined the percentage of children with autism, pervasive developmental disorder (PDD), ASD, and Asperger syndrome (AS) who were considered to be delayed (D), regressed (R), or delayed and later regressed (DR) and examined any relationship with autism severity, time of onset, factors associated with onset, gastrointestinal (GI) symptoms, race, age, and gender.
Materials and Methods:
The study reviewed developmental and medical information based on parental reports of 135 children with a diagnosis of autism, PDD, ASD, or AS.
Results:
The number of children in the D group was 53 (39.2%) with 19 (14.1%) in the DR group and 63 (46.7%) in the R group. Thus, 82 children (60.7%) were reported to have R. In regard to onset of symptoms, there was a significant difference between the D and R groups as well as between the DR and R groups. The analyses showed that there was no significant relationship between age, gender, race, severity, or GI symptoms and membership in any group; D, DR, or R. The majority of parents reported that the regression was preceded by or was associated with vaccinations (57.3%) or another medically related event (11.0%).
Conclusions:
The findings are consistent with previous research and reinforce our understanding of regression in those children with an ASD diagnosis.
Keywords: Autism spectrum disorder, asperger syndrome, childhood autism rating scale, delay, development, parental report, pervasive developmental disorder not otherwise specified, regression
Introduction
Autism spectrum disorder (ASD) is defined by persistent deficits in social communication and social interaction, and restricted, repetitive patterns of behavior, interests, or activities.[1] There have been recent changes made to the diagnostic criteria, including modifications to the core features. Although an ASD diagnosis is defined by the American Psychiatric Association, other features, more physical or health related, are associated with an ASD diagnosis. For example, children with ASD are more likely to have headaches/migraines, respiratory and food allergies,[2] gastrointestinal (GI) problems,[3,4] and infections[5] than typically developing children.
Another important aspect of those with any ASD diagnosis is that a significant number of children diagnosed with an ASD experience a developmental regression characterized by a loss of previously-acquired skills.[6,7] Many parents report that their child was developmentally normal until sometime after birth, typically 15-24 months, at which time the child began to regress or deteriorate.[8,9,10,11,12] The reported incidence of regression in autism varies in different studies from 15 to 62% of the cases.[6,7,13,14]
Typically, loss of verbal, nonverbal, and/or social abilities is reported.[7,9,11,12,14] For example, a study by Goldberg et al.,[14] found that children who lost verbal skills did so at an average age of 20.69 months; children who lost nonverbal skills did so at an average age of 18.58 months; and children who lost both skills, lost verbal skills at an average age of 21.2 months and nonverbal skills at an average age of 18.9 months. Malhi and Singhi[12] found that the mean age at regression was 22.43 months (standard deviation (SD) = 6.57) and that a large majority (66.7%) of the parents reported regression between 12 and 24 months of age. Similar to Goldberg et al.,[14] Malhi and Singhi[12] said that most (75%) of the parents of the regression-autistic group reported regression in the language domain, particularly in the expressive language sector, usually between 18 and 24 months of age. Another finding of Malhi and Singhi was that there were no significant differences between the two groups (children with ASD who had R and children with ASD who had not R) on the total Childhood Autism Rating Scale (CARS) score (a measure of autism severity) and the total number of Diagnostic and Statistical Manual - Fourth Edition (DSM IV) symptoms endorsed.[12]
Ozonoff et al.,[7] conducted a retrospective study of 60 children with autism (between 3 and 9 years of age) using the early development questionnaire (EDQ), to collect specific, parent-reported information about their children's development in the first 18 months. Ozonoff and colleagues found that the children could be divided into three groups: An early onset group (n = 29), a definite regression group (n = 23), and a heterogeneous mixed group (delays-plus-regression, n = 8). They reported that the children typically R at 15-24 months of age.
Several studies have sought to objectively evaluate the phenomenon of autistic regression early in life. For example, Werner and Dawson[15] evaluated home videotapes of children with autism between their first and second birthday parties with and without a reported history of regression, as well as videotapes of typically developing children. Analyses revealed that infants diagnosed with an ASD with regression show similar use of joint attention and more frequent use of words and babble compared with typical infants at 12 months of age. In contrast, infants diagnosed with an ASD characterized by early onset of symptoms and no regression displayed fewer joint attention and communicative behaviors at 12 months of age. By 24 months of age, both groups of toddlers diagnosed with an ASD displayed fewer instances of word use, vocalizations, declarative pointing, social gaze, and orienting to name as compared with typically developing 24-month-olds.
Similarly, Ozonoff et al.,[16] in a prospective longitudinal study, evaluated the emergence of the early behavioral signs of an ASD diagnosis, including gaze to faces, social smiles, and directed vocalization coded from video and rated by examiners evaluating study subjects at 6, 12, 18, 24, and 36 months of age. These investigators observed that the frequency of gaze to faces, shared smiles, and vocalizations to others were highly comparable between groups at 6 months of age, but in the group later diagnosed with an ASD, significantly declining trajectories were apparent over time. Group differences were significant by 12 months of age for most variables. These investigators concluded that their results suggest that behavioral signs of ASD are not present at birth, as once suggested by Kanner, but rather emerge over time through a process of diminishment of key social communication behaviors, and more children may present with a regressive course than previously thought.
Two recent studies examined brain differences between children with autism who regress and those who do not show symptoms of regression. One examined brain volume[17] and the other examined the activity and expression of protein kinase A (PKA), a cyclic AMP-dependent protein kinase.[13] Both studies found that children with autism who had R were different from both control subjects and individuals with non-regressive autism.
Many issues about regression in ASD remain in question or under debate and a better understanding of regression in ASD is needed. The purpose of the study was to examine the percentage of children who were considered to be D, R, or DR and to examine any relationship with autism severity, time of onset, GI symptoms, age, race, gender, and any factors associated with onset.
Materials and Methods
Overview
The study was a review of developmental and medical information based on parental reports of 135 children with autism, pervasive developmental disorder (PDD), ASD, or Asperger syndrome (AS) from data collected retrospectively and prospectively by the authors in previous studies. The current study examined whether the children were considered to be delayed (D), R, or delayed and later regressed (DR). D was defined as a significant lag in the appearance of normal developmental milestones or as any significant lag in a child's physical, cognitive, behavioral, emotional, or social development. R was defined as having lost previously acquired skills (including loss of language and social skills) and abilities. DR was defined as a significant lag in the appearance of normal developmental milestones with a later loss of previously acquired skills. The study used a Demographics and Medical Survey (DMS) to collect specific, parent-reported information about their child's developmental and medical history. The study also examined the percentage of children who were reported to have GI issues and the types of GI issues.
Institutional review board and consent
The studies from which this information was retrieved received IRB approval from the University of Texas Southwestern Medical Center IRB (Dallas, Texas); Liberty IRB, Inc. (Deland, Florida); or the Timberlawn Psychiatric Research Foundation, Inc., IRB (Dallas, Texas). All studies complied with the American Psychological Association ethical standards in the treatment of participants and in obtaining informed consent. All parents signed a consent and Health Insurance Portability and Accountability Act (HIPAA) form and all received a copy.
Study design
The study was an exploratory analysis using data that was collected retrospectively and prospectively from multiple studies conducted by Kern et al.,[18,19,20,21] from 2007 to 2012 using purposive sampling. Children with a diagnosis of autism, ASD, PDD, or AS were prospectively (at the time of each study) recruited from the community by using flyers and word of mouth. For example, autism organizations in the area were notified of the study and flyers were posted in pediatric neurology offices. All of this research was conducted in the greater Dallas Metroplex area. After explaining the study and obtaining informed consent from the parent (s), each child was evaluated by the principal investigator (PI; JKK) using the CARS. For the two children in the study who were one year of age, the Modified Checklist for Autism in Toddlers (M-CHAT) was completed by the child's parent. The parents also completed a DMS. The questions in that survey were designed to address whether the child should be categorized as D, R, or DR and if there was any relationship between the D, R, or DR groups and the child's age, gender, race, autism severity, or GI symptoms. A question regarding factors associated with onset was also included. Quantitative and qualitative examination of the combined data was conducted.
Participants
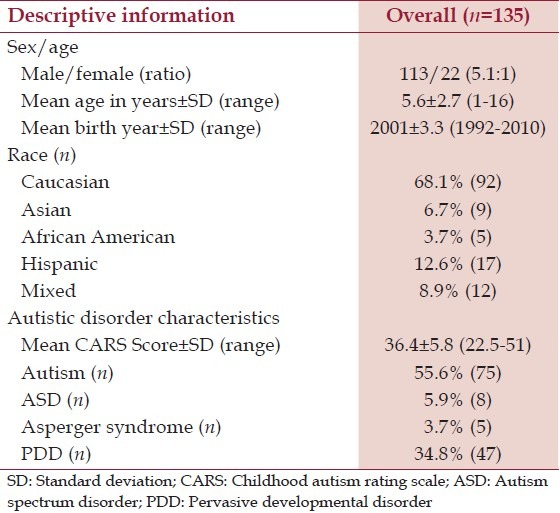
The children who participated in the Kern et al.,[18,19,20,21] studies, previously mentioned, were diagnosed with autism, ASD, PDD, or AS and were prospectively (at the time of each study) recruited from the community. Each child in the ASD group had been previously diagnosed by a professional. In the state of Texas, the only professionals who are allowed to diagnose ASD are either licensed clinical psychologists or medical doctors. To further evaluate each child's diagnostic accuracy, each child was observed by the PI (JKK), who has many years of experience in evaluating children with ASD, to make sure that they met the DSM-IV criteria for an ASD (this research was conducted prior to the adoption of DSM-5). In addition, the CARS evaluation was completed on each child by the PI who observed the participants and interviewed the parent (s). Although a total score of about 25 is considered to be the minimum cut off CARS score for an ASD diagnosis, a cut off of 22.5 was set to allow the children with a diagnosis of AS to participate. The study was designed to exclude children who had a history of Fragile X disorder, tuberous sclerosis, phenylketonuria (PKU), Lesch-Nyhan syndrome, seizure disorder, cerebral palsy, fetal alcohol syndrome, or any history of maternal illicit drug use. Detailed information was collected on each participant regarding age, race, gender, and year of birth. Table 1 summarizes the demographic information for the participants examined in the present study.
Table 1.
A summary of the participants
Clinical measures
DMS: The DMS asks basic demographic and medical questions. The demographic questions include queries about the child's name, address, age, race, gender, date of birth, diagnosis, age of diagnosis, and any comorbid diagnosis. The medical questions include queries about the child's allergies, medications, supplements, current, and past treatments, as well as whether the child was D, R, or DR, age of onset of symptoms, if anything preceded or was associated with the child's regression, GI problems, and any other medical issues. All questions are open-ended. The questionnaire was given to a parent to complete upon enrollment into a study. After the questionnaire was completed by a parent, the PI reviewed the answers with the parent to make sure that the answers were complete and clear.
Childhood Autism Rating Scale
Study participants were evaluated using a CARS test conducted only by a single study investigator (JKK) who observed the participants and interviewed the parent(s). The CARS was used to confirm the child's diagnosis and to determine the child's severity score.
The CARS is a 15-item behavioral rating scale developed to identify autism as well as to quantitatively describe the severity of the disorder.[22] The 15 items are: I. Relating to People; II. Imitation; III. Emotional Response; IV. Body Use; V. Object Use; VI. Adaptation to Change; VII. Visual Response; VIII. Listening Response; IX. Taste, Smell, and Touch Response and Use; X. Fear or Nervousness; XI. Verbal Communication; XII. Nonverbal Communication; XIII. Activity Level; XIV. Level and Consistency of Intellectual Response; and XV. General Impressions. Each item is scored from 1 (no pathology) to 4 (severe pathology) in 0.5 rating intervals. A total score of 15-29.5 is considered nonautistic; a score of 30-36.5 is considered mild to moderate autism; and a score from 37 to 60 is considered moderate to severe autism.[22] For CARS evaluation, a total score of about 25 is considered to be the minimum cut off CARS score for an ASD diagnosis.[23]
The CARS is a well-established measure. The internal consistency reliability alpha coefficient is 0.94; the inter-rater reliability correlation coefficient is 0.71; and the test-retest correlation coefficient is 0.88.[22,24] CARS scores have high criterion-related validity when compared to clinical ratings during the same diagnostic sessions, with a correlation of 0.84 (P < 0.001).[22] Other comparisons, based on information from records, parent interviews, and nonstructured clinical interviews with the child, report a correlation coefficient of 0.80 (P < 0.001). Many independent studies on the validity of the CARS indicate that it has high validity.[25,26,27,28,29,30]
MCHAT
The M-CHAT consists of 23 yes/no items that assess the child's attainment of developmental milestones.[31] The items address issues important in autism such as social relatedness, communication, pretend play, imitation, interaction, eye contact, response to name, interests, basic skills, and behavior. It is usually parent/caregiver completed. It is considered an instrument for the early detection of autism. A child either passes or fails the M-CHAT. A failed score is indicative of autism. Criteria for failure of the checklist is failing either two or more critical items, or failing three or more items. The internal reliability for the M-CHAT is adequate for the checklist as a whole (a = 0.85) as well as for the critical items (a = 0.83).[31] The M-CHAT has a sensitivity of 0.87, specificity of 0.99, positive predictive power of 0.80, and a negative predictive power of 0.99.[31] This measure was completed by a parent or guardian for those children less than 2 years of age.
Statistical analysis
The D, R, and DR groups were compared on continuous outcomes by analysis of variance. If the overall test was significant then pair-wise comparisons were tested using the Tukey-Kramer adjustment for multiple comparisons. Categorical outcomes were compared using a Chi-square test. A two-tailed P < 0.05 was considered statistically significant for all of the statistical tests in the present study.
Results
The number of children in the D group was 53 (39.2%) with 19 (14.1%) in the DR group and 63 (46.7%) in the R group. Thus, 82 children (60.7%) were reported to have R (lost previously acquired skills).
In regard to the onset of symptoms, there was a significant difference between the D and R (P = 0.0003) groups and between the DR and R (P = 0.0334) groups. The mean age of onset of initial symptoms was 10.9 (SD = 10.6) months in the D group, 11.4 (SD = 5.3) months in the DR group, and 17.0 (SD = 6.2) months in the R group. The mean age of onset of regression was 20.9 (SD = 16.0) months in the DR group and 17.0 (SD = 6.2) months in the R group. However, when one outlier was excluded from the analysis (a child in the DR group who was D and was reported to have R at 7 years of age), the mean age of onset of regression in the DR group was reduced to 17.4 (SD = 4.9) months. Thus, there was not a significant difference between the time of regression between the DR and R group when this anomalous child was excluded from the full DR group. For a summary of this information, see Table 2.
Table 2.
A summary of the regression information
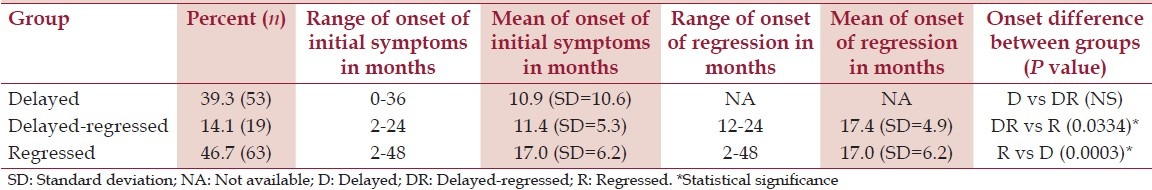
For the children in the D group, the age of onset of symptoms ranged from birth to 36 months. For the children in the DR group, the age of onset of initial symptoms ranged from 2 to 24 months, and the age of onset of regression ranged from 12 to 84 months. With the one outlier in the DR removed, the range for the age of onset was reduced to 12 to 24 months. In the children with R only, the age of onset of regression ranged from 2 to 48 months. For a summary of this information, see Table 2.
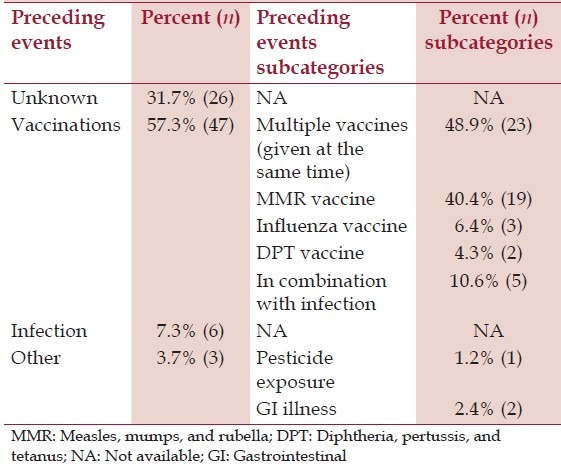
Turning to the question of what event preceded or was associated with the regression, the questionnaire data consisted of responses in four categories: Unknown (n = 26; 31.7%); vaccination (n = 47; 57.3%); infection (n = 6; 7.3%); or other (n = 3; 3.7%). In the group that reported regression after vaccination, the questionnaire responses fell into one of four categories: Multiple vaccines (n = 23; 48.9%); measles, mumps, and rubella (MMR) (n = 19; 40.4%); influenza (n = 3; 6.4%); and diphtheria, pertussis, and tetanus (DPT) (n = 2; 4.3%). Of those who listed vaccination as a factor, five (10.6%) stated that an infection was a cofactor. Of those who listed the DPT vaccine, one was prior to 1996 and one was after 1996. The questionnaires for those children in the ‘other’ category reported pesticide exposure (n = 1) and GI illness (n = 2) as the regression-associated factor. Being R or DR was not significantly related to any factors associated with the onset of regression. For a summary of this information, see Table 3.
Table 3.
Factors associated with regression
GI information was provided for 128 of the participants. About half, 63 (49.2%), were reported to have GI problems. Constipation was reported in 28 (21.9%) of these children (10 severe, 12 moderate, and six mild constipation). Diarrhea was reported in 23 (18.0%) of these children (five severe, six moderate, and 12 with chronic loose/soft stools). Both chronic diarrhea and constipation was reported in three (2.3%). Problems with GI yeast were reported in three of the children, discolored stool was reported in two of the children, and incontinence was reported in three of the children. About half, 65 (50.8%), were reported to have no GI problems.
The skills most frequently reported to be lost were speech, eye contact, pointing, and socialization. Other skills mentioned were nonverbal communication, responsiveness, interest in others, expression, ability to imitate, and, to a much lesser extent, motor skills. Problems noted were tantrums, behavioral issues, apparent deafness, and sensory issues (oversensitivity and undersensitivity).
Analyses showed that there was no significant relationship between the children's age, gender, race, severity, or GI symptoms, and their membership in the D, DR, or R groups.
Discussion
Similar to the Ozonoff et al.,[7] study of 60 children with autism that found that the children were divided into three groups: An early onset group, a definite regression group, and a heterogeneous mixed group (delays-plus-regression), the data from the current study also found three groups. The three groups found in the current study were children considered to be D, R, or DR. In the Ozonoff et al.,[7] study, 51.7% were reported to have R. In the current study, 60.7% of the children were found to have R. This finding is within the range of previous studies.[6,7,13,14,32]
Even though previous studies have found brain differences in children who are D as compared to children who have R, the results from the current study suggest that there was no significant relationship between the children's age, gender, race, severity, or GI symptoms and their subsequent membership in the D, DR, or R groups.[13,18] There were group differences in regard to onset. This finding is similar to previous research findings.[7] As mentioned in the Introduction, Malhi and Singhi[12] also found that there were no significant differences between the two groups in regard to autism severity based on the CARS. In contrast however, Xi et al.,[33] in a study of 152 children with autism, found that the regressive group scored significantly higher on the CARS (P < 0.05) and had a relatively higher proportion of severely ill children (66.7 vs. 45.4%; P < 0.05) compared with the non-regressive group.
In the current study about half the children, 49.2%, were reported to have GI problems (21.9% constipation, 18.0% diarrhea, and 9.3% with both). This finding is consistent with previous research. Wang et al.,[4] for example, as mentioned in the Introduction, similarly reported that 20% of the children diagnosed with an ASD had problems with constipation and 19% had chronic diarrhea.
The finding that 57.3% of the parents reported that the regression was preceded by or was associated with vaccination/immunization is consistent with previous research by Goldberg et al.[14] In that study, Goldberg et al.,[14] stated that the event mentioned by the majority of parents (67.6%) as concurrent with loss of skills was immunization. Also, consistent with the Goldberg study was that they found other medical events (e.g. various illnesses), were reported by about one-third of the parents. Zhang et al.,[34] found that febrile seizures and family history of neuropsychiatric disorders are correlated with autistic regression.
The results for the current study show that the probable mean age for the onset of regression was 17.4 months in the DR group and 17.0 months in the R group. These findings are consistent with previous studies that find the onset of regression resulting in an ASD diagnosis is typically 15-24 months of age.[7,8,9,10,11,12,14]
The results of the current study suggest that the children commonly lose speech, socialization, eye contact, and nonverbal skills. This is consistent with previous studies. In other studies, the loss of verbal, nonverbal, and social abilities is typically reported.[7,9,11,14,32] Significantly, two of the five children with a diagnosis of AS were reported to have R.
Both the 2004 Consensus Report issued by the Institute of Medicine (IOM)[35] and some studies, including Madsen et al.,[36] have failed to find an association between vaccines and autism. However, other studies have reported an association, including Gallagher et al.[37] The current study adds evidence to the latter findings.
Strengths and Limitations
The study limitations include issues with possible variability in parental and clinical recognition and reporting, and the possibility of some recall bias (a possible bias affected by respondent's memory). The study limitations also include issues regarding the validity of questionnaires.[7] In addition, the open-ended questions may be another limitation in that the information could have been more specific and/or quantitative by adding choices and time frames. However, a possible advantage to the open-ended questions could also be that they were not leading because providing choices may have been suggestive.
Another study limitation is that there was no verification of the parent report through medical records. However, many of the parents provided a vaccine record which did not conflict with their report.
Among the limitations of the present study is that participants examined were assumed to be on the autism spectrum based upon the fact that they were previously diagnosed with an ASD and a subsequent professional CARS evaluation. It is possible that other tests such as Autism Diagnostic Observation Schedule (ADOS) or Autism Diagnostic Interview, Revised (ADI-R) could have influenced whether the study participants were considered to be on the autism spectrum. Despite this potential limitation, the CARS evaluations are a well-recognized metric of helping to establish an ASD diagnosis and providing important quantitative measurements of ASD severity.
A strength of the present study was that the demographics of the cohort of participants diagnosed with an ASD examined in the present study appear to be similar to the recognized demographics of the general population diagnosed with an ASD, and therefore, the results observed should be expected to be extendible beyond the cohort of participants diagnosed with an ASD examined in the present study. In addition, since the participants diagnosed with an ASD examined in the present study were wide-ranging with respect to age, gender, racial composition, and severity, potential outlier skewing of the data should not have significantly impacted the results observed. Furthermore, the findings of the current study are consistent with previous research.
Conclusion
The findings from this study are consistent with previous research and reinforce our understanding of regression in ASD. The consistency of the findings on regression in ASD that are based on parental reports suggest that the benefit of studying parental reports may outweigh the limitations.
Footnotes
Source of Support: This research was funded by grants from the non-profit Autism Research Institute, the non-profit CoMeD, Inc., and the non-profit Institute of Chronic Illnesses, Inc. through a grant from the Brenen Hornstein Autism Research and Education Foundation,.
Conflict of Interest: The authors have been involved in vaccine/biologic litigation.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth edition. Washington, DC: American Psychiatric Association; 2013. Diagnostic criteria for autistic disorder. [Google Scholar]
- 2.Schieve LA, Gonzalez V, Boulet SL, Visser SN, Rice CE, Van Naarden Braun K, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006-2010. Res Dev Disabil. 2011;33:467–76. doi: 10.1016/j.ridd.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, Aman MG, et al. Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord. 2009;39:405–13. doi: 10.1007/s10803-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 4.Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr. 2011;32:351–60. doi: 10.1097/DBP.0b013e31821bd06a. [DOI] [PubMed] [Google Scholar]
- 5.Atladóttir HO, Thorsen P, Schendel DE, Østergaard L, pLemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: A Danish cohort study. Arch Pediatr Adolesc Med. 2010;164:470–7. doi: 10.1001/archpediatrics.2010.9. [DOI] [PubMed] [Google Scholar]
- 6.Stefanatos GA. Regression in autistic spectrum disorders. Neuropsychol Rev. 2008;18:305–19. doi: 10.1007/s11065-008-9073-y. [DOI] [PubMed] [Google Scholar]
- 7.Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: The delays-plus-regression phenotype. Autism. 2005;9:461–86. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- 8.Filipek PA, Accardo PJ, Baranek GT, Cook EH, Jr, Dawson G, Gordon B, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29:439–84. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 9.Davidovitch M, Glick L, Holtzman G, Tirosh E, Safir MP. Developmental regression in autism: Maternal perception. J Autism Dev Disord. 2000;30:11311–9. doi: 10.1023/a:1005403421141. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Karmakar P, Mohanan A. Language regression in children with Autism Spectrum Disorders. Int J Pediatr Otorhinolaryngol. 2013 Dec 12; doi: 10.1016/j.ijporl.2013.12.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Kern JK, Miller VS, Evans PA, Trivedi MH. Efficacy of porcine secretin in children with autism and pervasive developmental disorders. J Autism Dev Disord. 2002;32:153–60. doi: 10.1023/a:1015441428154. [DOI] [PubMed] [Google Scholar]
- 12.Malhi P, Singhi P. Regression in children with autism spectrum disorders. Indian J Pediatr. 2012;27:975–81. doi: 10.1007/s12098-012-0683-2. [DOI] [PubMed] [Google Scholar]
- 13.Ji L, Chauhan V, Flory MJ, Chauhan A. Brain region-specific decrease in the activity and expression of protein kinase a in the frontal cortex of regressive autism. PLoS One. 2011;6:e23751. doi: 10.1371/journal.pone.0023751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, et al. Language and other regression: Assessment and timing. J Autism Dev Disord. 2003;33:607–16. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- 15.Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry. 2005;62:889–95. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- 16.Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–66. [PMC free article] [PubMed] [Google Scholar]
- 17.Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A. 2011;108:20195–200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern JK, Grannemann BD, Trivedi MH, Adams J. Sulfhydryl-reactive metals in autism. J Toxicol Environ Health A. 2007;70:715–21. doi: 10.1080/15287390601188060. [DOI] [PubMed] [Google Scholar]
- 19.Kern JK, Grannemann BD, Gutman J, Trivedi MH. Oral tolerability of cysteine-rich whey protein isolate in autism - a pilot study. J Am Nutraceut Assoc. 2008;11:36–41. [Google Scholar]
- 20.Kern JK, Geier DA, Garver CR, Adams JB, Audhya T, Nataf R, et al. Efficacy of oral and transdermal glutathione in raising glutathione levels in ASD. Med Sci Monit. 2011;17:CR677–82. doi: 10.12659/MSM.882125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern JK, Geier DA, Adams JB, Troutman MR, Davis G, King PG, et al. Autism severity and muscle strength: A correlation analysis. Res Autism Spectr Disord. 2011;5:1011–5. [Google Scholar]
- 22.Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Western Psychological Services, 12031 Wilshire Boulevard, Los Angeles, California, 9002591251. 1994 [Google Scholar]
- 23.Chlebowski C, Green JA, Barton ML, Fein D. Using the Childhood Autism Rating Scale to diagnose autism spectrum disorders. J Autism Dev Disord. 2010;40:787–99. doi: 10.1007/s10803-009-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale. J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 25.Eaves R, Milner B. The criterion-related validity of the Childhood Autism Rating Scale and the Autism Behavior Checklist. J Abnorm Child Psychol. 1993;21:481–91. doi: 10.1007/BF00916315. [DOI] [PubMed] [Google Scholar]
- 26.Sevin J, Matson J, Coe D, Fee V, Sevin BM. A comparison and evaluation of three commonly used autism scales. J Autism Dev Disord. 1991;21:417–32. doi: 10.1007/BF02206868. [DOI] [PubMed] [Google Scholar]
- 27.Pereira A, Riesgo RS, Wagner MB. Childhood autism: Translation and validation of the Childhood Autism Rating Scale for use in Brazil. J Pediatr. 2008;84:487–94. doi: 10.2223/JPED.1828. [DOI] [PubMed] [Google Scholar]
- 28.Perry A, Condillac RA, Freeman NL, Dunn-Geier J, Belair J. Multi-site study of the Childhood Autism Rating Scale in five clinical groups of young children. J Autism Dev Disord. 2005;35:625–34. doi: 10.1007/s10803-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 29.Rellini E, Tortolani D, Trillo S, Carbone S, Montecchi F. Childhood Autism Rating Scale and Autism Behavior Checklist correspondence and conflicts with DSM-IV criteria in diagnosis of autism. J Autism Dev Disord. 2004;34:70370–8. doi: 10.1007/s10803-004-5290-2. [DOI] [PubMed] [Google Scholar]
- 30.Teal MB, Wiebe MJ. A validity analysis of selected instruments used to assess autism. J Autism Dev Disord. 1986;16:485–94. doi: 10.1007/BF01531713. [DOI] [PubMed] [Google Scholar]
- 31.Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31:131–44. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- 32.Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, Deprey LJ, et al. Regression in autism: Prevalence and associated factors in the CHARGE Study. Pediatrics. 2008;8:25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Xi CY, Hua TY, Zhao YJ, Liu XM. Characteristics of developmental regression in autistic children. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:781–3. [PubMed] [Google Scholar]
- 34.Zhang Y, Xu Q, Liu J, Li SC, Xu X. Risk factors for autistic regression: Results of an ambispective cohort study. J Child Neurol. 2012;27:975–81. doi: 10.1177/0883073811430163. [DOI] [PubMed] [Google Scholar]
- 35.Immunization Safety Review: Vaccines and Autism. Institute of Medicine: Consensus Report, 2004. [Accessed January 6, 2013]. at http://www.iom.edu/reports/2004/immunizationsafety-review-vaccines-and-autism.aspx .
- 36.Madsen KM, Lauritsen MB, Pedersen CB, Thorsen P, Plesner AM, Andersen PH, et al. Thimerosal and the occurrence of autism. Negative ecological evidence from Danish registry-data. Ugeskr Laeger. 2004;166:3291–3. [PubMed] [Google Scholar]
- 37.Gallagher CM, Goodman MS. Hepatitis B vaccination of male neonates and autism diagnosis, NHIS 1997-2002. J Toxicol Environ Health A. 2010;73:1665–77. doi: 10.1080/15287394.2010.519317. [DOI] [PubMed] [Google Scholar]


