Abstract
Background:
The increase in invasive fungal infections (IFIs) in neonatal intensive care unit (NICU) is jeopardizing the survival of preterm neonates. Probiotics modulating the intestinal microflora of preterm neonates may minimize enteral fungal colonization.
Aims:
This study was to examine whether probiotic supplementation in neonates reduced fungal septicemia.
Materials and Methods:
This prospective, randomized, double blind trial investigating the supplementation of preterm infants with a probiotic was done from May 2012 to April 2013, with 112 subjects randomized into two groups. Primary outcome: Decreased fungal colonization in gastrointestinal tract. Others: Incidence of late onset septicemia; duration of the primary hospital admission; number of days until full enteral feeds established.
Results:
Full feed establishment was earlier in probiotics group compared to placebo group (P = 0.016). The duration of hospitalization was less in the probiotic group (P = 0.002). Stool fungal colonization, an important outcome parameter was 3.03 ± 2.33 × 105 colony formation units (CFU) in the probiotics group compared to 3 ± 1.5 × 105 CFU in the placebo group (P = 0.03). Fungal infection is less in the study group (P = 0.001).
Conclusion:
The key features of our study were reduced enteral fungal colonization, reduce invasive fungal sepsis, earlier establishment of full enteral feeds, and reduced duration of hospital stay in the probiotics group.
Keywords: Fungal, neonates, preterm, probiotics, sepsis
Introduction
Candidemia in the neonatal intensive care unit (NICU) is the third most frequent causal agent of late-onset sepsis in preterm neonates affecting 1.6-9% very low birth weight (VLBW) and 15% extremely low birth weight (ELBW) neonates.[1,2,3,4] The increased frequency of invasive fungal infections (IFIs) in NICU is questioning the survival of preterm neonates and neurodevelopmental outcome.[5]
In healthy term infants, colonizations of the aseptic intestine are acquired from the birth canal, subsequently modified by diet.[6] However, preterms in NICU acquire colonizing bacteria from the intensive care microenvironment rather than their mother.[7]
In NICU preterms, intestinal functional immaturity, broad-spectrum antibiotics, and delay in initiating enteral feeding prevent the enteric colonization with normal commensal microorganisms. Thus, they harbor aerobes like Staphylococci (coagulase negative and Staphylococcus aureus), Enterobacteria (Klebsiella), Enterococci, and anaerobes like Clostridia.[8] Normal commensal bacterial flora inhibits Candida growth by competing for adhesion sites and nutrients.[9] The use of H2 blockers is another risk factor for Candida infection.[10] Preterms have an abnormal pattern of gut colonization with bifidobacteria and lactobacilli which normally colonize healthy full-term infants.[7,11] This altered intestinal flora increase their susceptibility to necrotizing enterocolitis and risk of bacterial translocation.[12,13]
Of the risk factors, fungal colonization by Candida species is common for any IFI.[4,5,6,7,8,9,10,11,12,13,14] Sixty percent of VLBW neonates become colonized by fungi during the 1st month of NICU life and 21% of them progress to become infected.[14]
Of all colonization sites[14,15] the gastrointestinal tract is most frequently implicated in subsequent systemic fungal dissemination.[16] Thus, reducing fungal colonization can prevent IFI. Systemic antifungal drugs have shown promising results, but antifungal prophylaxis raises concerns about selection of resistant strains.[17,18,19]
Probiotics, defined as live microbial supplements providing health benefits to the host may modulate the intestinal microbiota in preterms.[20] The bacteria most frequently used as probiotics are the bifidobacteria and lactobacilli. Probiotics may prevent gastrointestinal and urinary infections by: Increasing resistance of mucosal barrier to migration of bacteria and their toxins by strengthening intestinal cell junctions, increased host response to microbes, and increased mucosal immunoglobulin A response, inhibit the growth of pathogens, production of bacteriocins, and competitive exclusion of potential pathogens.[21,22] Two meta-analyses based on an aggregate of seven and nine clinical trials concluded that neonatal probiotic supplementation reduces the incidence of necrotizing enterocolitis (NEC) in preterm infants, diarrhea, colon distension, and abdominal cramps and less time required to reach full enteral feeding.[23,24,25,26,27,28] But, the safety and efficacy of probiotics in preterms especially ELBW is yet to be proven.[24]
Our hypothesis was that increased colonization with beneficial microflora like probiotics would protect the neonate host from the expansion of fungal colonies in the gastrointestinal tract.[29] In this field, some trials have been conducted, but some do not include preterms weighing less than 1,000 g and before 28 weeks’ gestation. Data regarding the optimal strain (s), dose, time to start, and duration of treatment of currently available probiotics are lacking.
The objective of this study was to evaluate the hypothesis that supplementation with probiotics may reduce the colonization and expansion of fungal colonies in the gastrointestinal tract, and reduce the risk of bacterial and/or fungal late onset sepsis in the NICU.
Materials and Methods
This is a single-center, prospective, randomized, double blind, placebo-controlled trial investigating the supplementation of preterm, LBW infants with a probiotic combination comprising Bifidobacterium infantis, Lactobacillus, and B. lactis.
Institutional approval was obtained from the ethics committee of our institution and Clinical Trial Registry of India (CTRI) registration number REF/2012/12/004378. Written informed consent was obtained from the parents prior to study and confidentiality was maintained throughout the study.
In the NICU of a tertiary care hospital of eastern India from May 2012 to April 2013, a total of 341 subjects are assessed for eligibility and 112 satisfying the inclusion and exclusion criteria (mentioned below) were randomized into two groups [Figure 1]. The mean delivery per year was 9,000 with average NICU admission of 600 per year.
Figure 1.
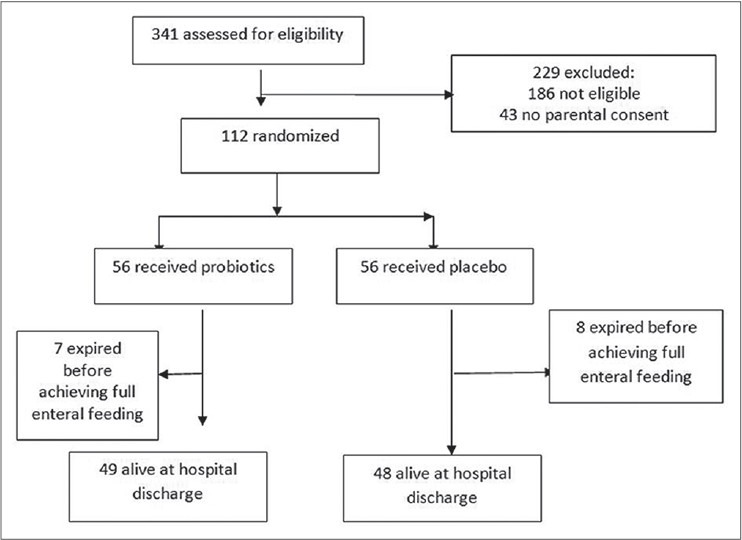
Flow of patients through the trial
Inclusion criteria were admission to the NICU, a stable oral feeding within 72 h of birth and an informed parental consent; gestational age (GA) < 37 weeks; birth weight < 2,500 g; adequate renal and liver function; a postnatal age < 2 week; did not have baseline fungal colonization at enrollment (with colonization defined by isolation of fungi from a culture specimen obtained from any site during the first 3 days of life); did not receive any form of antifungal prophylaxis other than the probiotic used.
Exclusion criteria were the presence of major congenital malformation; antenatal and perinatal risk factors for sepsis, major congenital malformation; stigma of congenital infection; severe lesions diagnosed by cranial ultrasound (e.g. intraventricular hemorrhage (IVH) grade 3 and 4 and major ischemic lesions); altered liver and renal function; likely to die within 72 h of birth; and babies of mothers taking supplemental probiotics by capsule/powder.
The primary outcome is decreased fungal colonization in gastrointestinal tract (GIT) with probiotics. The secondary outcomes are: Incidence of late onset septicemia; NEC graded by modified Bell's criteria;[30] mortality; duration of the primary hospital admission; number of days until full enteral feeds established (120 ml/kg/day or more for 3 consecutive days).
The probiotic used was Prowel by Alkem Batch PWS3002C containing Lactobacillus acidophilus 1.25 billion, B. longum 0.125 billion, B. bifidum 0.125 billion, and B. lactis 1.0 billion per 1 g sachet.
For quality control and safety, study batch and placebo was tested for the presence of pathogens using standard microbiological techniques and for the presence and quantitation of the probiotic organisms.
The newborns were randomized into two groups by a random-generated (computer-generated), predetermined number table. Group I (probiotic group) (n = 56) received supplementation with probiotics daily from the first 72 h for 6 weeks or until discharged as long as minimal enteral nutrition was not contraindicated. Group II (control) (n = 56) received sterile water as placebo. The dose of 6 × 109 colony forming units (CFU)/day of lactobacillus, that is, half of 1 g sachet was chosen on the basis of published data from previous studies of VLBW neonates.[31] For ELBW and <32 weeks, the starting dose should be 1.5 × 109 CFU/day for ELBW neonates until they reach enteral feeds of 50-60 ml/kg/day when the dose was increased to 3 × 109 CFU/day.[32] Three hundred and forty-one assessed for eligibility. All doctors, nurses, laboratory staff, and parents are blind to the randomized allocation.
The study intervention/placebo is given orally or via gastric tube twice daily with expressed breast milk in infants receiving minimal enteral nutrition. Freshly expressed mother's breast milk is the feed of choice followed by frozen breast milk, if fresh is not available. Intravenous fluids and nutrition are used until approximately 120 ml/kg of milk is tolerated per 24 h period.
Antimycotic treatment consisted of liposomal amphotericin B at the initial dose of 1 mg kg/day, with a gradual increase up to a maximum of 6 mg kg/day.[33] Treatment was stopped 7 days after a negative culture for Candida and three consecutive negative C-reactive protein. Antibiotic treatment was carried out after antibiotic assays. Treatment was stopped 7-12 days after negative assay of C-reactive protein and the absence of clinical signs of infection.[34]
Clinical procedures
Within few hours after delivery, enteral nutrition was started with 1 ml of human milk given every 2 or 3 h, and the amount of milk given was increased by 1.0 ml every 3-6 h, as tolerated; human milk was supplemented with parenteral glucose administered from day 1 of life and with amino acids and lipids administered from day 2 of life through a Premicath catheter. Nutrition administered with intermittent meals was progressively increased, if tolerated, and parenteral nutrition was progressively decreased and stopped, following the same protocols as reported by the international guidelines.[35]
Infants were weighed daily and examined by doctors at least twice daily for gastrointestinal symptoms.[36] Such as regurgitation (defined as the passage of refluxed gastric contents into the oral pharynx), vomiting/feeding intolerance (defined as the expulsion of the refluxed gastric contents from the mouth), abdominal distension, and characteristics of the feces. Length of hospitalization was also recorded.
Clinical signs of infection were monitored, including fever, desaturation, apnea, bradycardia, pallor or cyanosis, necessity of oxygen supplementation, and intubation. The laboratory parameters monitored were C-reactive protein and blood count. Investigations to detect any mycotic involvement of the organs included ultrasounds (renal, cardiac, abdominal, and transfontanellar), examination of the fundus oculi, and chest X-rays. Cranial ultrasound was performed for each preterm infant in the 1st week of life and another between 15 and 21 days after birth and at least one at term age.
Microbiology
Gastric aspirations were cultured for Candida detection at birth and after 7, 14, 21, 28, 35, and 42 days; quantitative fungal stool cultures were also examined at the same time.[37] For fungal culture, 0.2 g of specimen was diluted with 1.8 ml of sterile saline. Ten microliter aliquot was then plated on Sabouraud's dextrose agar containing 300 μg/ml chloramphenicol and 10 μg/ml gentamicin and incubated in air at 35°C for 48 h. C albicans was identified by germtubes and chlamydospore formation. Species identification of germ tube negative yeasts was done by by commercial API C20 AUX yeast kit. Yeast counts were obtained by colony counting 48 h after incubation. Infants were considered at high level of colonization if they presented >104 CFU/g of feces.[38] Blood cultures and Platelia Candida test were conducted for the diagnosis of invasive candidiasis and to evaluate antifungal chemotherapy efficacy.[39]
Proven fungal or bacterial infection was defined as a positive culture: (1) From blood (drawn from peripheral sites); (2) from urine (collected by suprapubic sterile puncture or sterile bladder catheterization, with a growth of 10,000 fungal organisms per ml); (3) from cerebrospinal fluid; or (4) from intravascular catheter tip (only considered proof of microbiologically documented fungal infection in patients with previous peripheral colonization by the same species).
Statistical methods
Sample size calculation
0To reduce the incidence of culture proven sepsis from 33% (from local epidemiological data) to 16% (a 51.5% reduction) with a power of 0.8, the estimated sample size is 83 per group. The trial will therefore recruit 163 infants over 1 year.
Intention-to-treat analysis
Data from all randomized participants will be considered in the intention-to-treat model (for the primary outcome).
The primary end point was to evaluate the incidence of enteric fungal colonization. Statistical Package for Social Sciences (SPSS) statistical software for Windows, version 17.0 (SPSS Inc.), was used for all statistical computations. Student's t-test or the Mann-Whitney U test, when appropriate, was used for comparison of continuous variables; and a Chi-square test or Fisher's exact test, when appropriate, was used for comparison of categorical variables. The anthropometric variables (weight at birth and gestational age) were reported as mean ± standard deviation (SD). The intergroup comparisons for all variables were performed by independent sample's t-test. All tests were two-tailed. The level of significance was set at P < 0.05.
Results
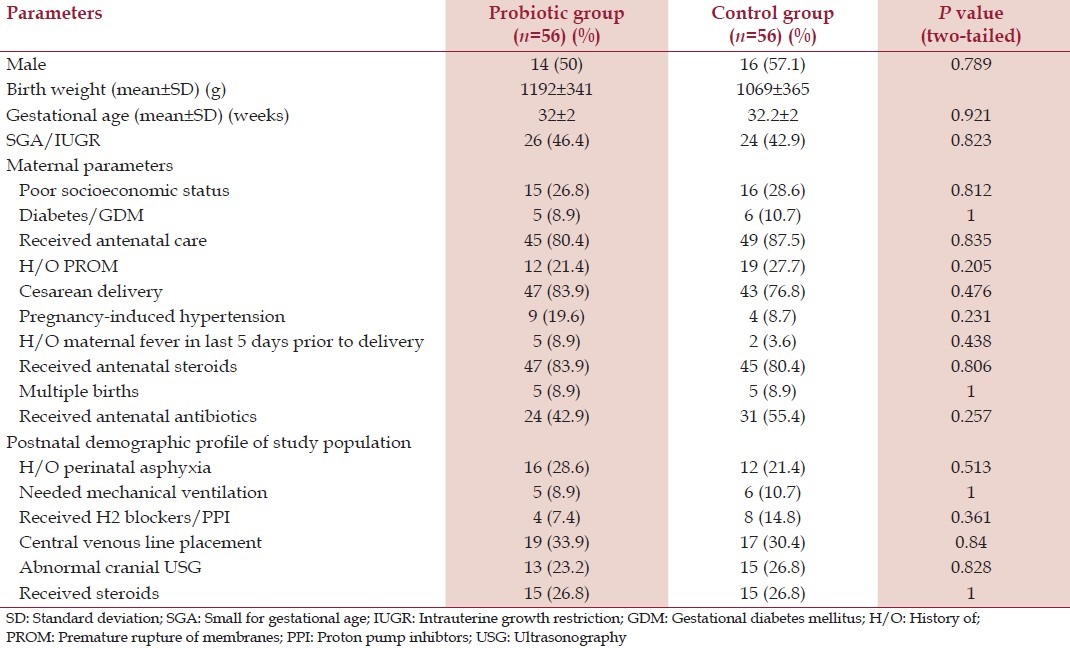
A total of 112 preterm neonates were enrolled, 56 were randomly assigned to the study group and 56 to the placebo group. The baseline demographic characteristics between the two groups are reported in Table 1 and no significant differences are found between the two groups.
Table 1.
Baseline demographic profile of the study patients of the two groups
Enteral feeding was started at a similar postnatal age in probiotic (9.96 ± 5.20d) and placebo (11.22 ± 5.04d) groups (P = 0.2) and consisted of human milk in all neonates in both the groups. Oral supplementation with probiotics or placebo started in parallel with enteral feeding: 52.14 ± 17.14 and 50.68 ± 16.8 h and there is no significant difference (P > 0.05). Feeding advancement in the probiotics group was done by 4.51 ± 3.0 ml daily and by 3.27 ± 2.0 ml in the control group (P = 0.012). Full feed establishment was in 11.22 ± 5.04 days in probiotics group compared to 15.41 ± 8.07 days in the placebo group (P = 0.016) which is significantly earlier in the probiotic group.
The total number of days on total parenteral nutrition in the probiotics group was 6.91 ± 5.9 days compared with 6.36 ± 5.30 days in the placebo group (P = 0.6). The duration of hospitalization was 25.77 ± 9.16 days in the probiotic group compared to 31.21 ± 12.67 days in the placebo group (P = 0.002).
Stool fungal colonization, one of the important outcome parameters was 3.03 ± 2.33 × 105 CFU in the probiotics group compared to 3 ± 1.5 × 105 CFU in the placebo group (P = 0.03).
The total leukocyte count (TLC) on day 3 of treatment in the probiotics group was 7090 ± 2148.006 cells/mm3 compared to 6626.79 ± 1888.674 cells/mm3 in the placebo group (P = 0.22). TLC on day 9 of treatment is 5553.52 ± 1854.58 cells/mm3 in the probiotics group compared to 4277.36 ± 1376.68 cells/mm3 in the placebo group (P = 0). TLC on day 15 of treatment is 6190.57 ± 1295 cells/mm3 in the probiotics group compared to 5572.55 ± 1269.658 cells/mm3 in the placebo group (P = 0.001). The C-reactive protein (CRP) on day 3 of treatment 0.773 ± 0.3498 mg/dl in the probiotics group compared to 0.784 ± 0.2682 mg/dl in the placebo group (P = 0.8). The CRP values on day 9 of treatment was 1.167 ± 0.4568 mg/dl in the probiotics group compared to 1.498 ± 0.4865 mg/dl in the placebo group (P = 0). The CRP values on day 15 of treatment in the probiotic group 0.742 ± 0.2635 mg/dl compared to 0.808 ± 0.29 mg/dl in the placebo group (P = 0.23).
Duration of antimycotic treatment in the probiotics group 7.59 ± 8.551 days compared to 13.5 ± 8.94 days in the placebo group (P = 0.001). Other significant outcome parameters are given in Table 2. The incidence of infections is significantly more in the placebo group than the probiotics group (P = 0.017).
Table 2.
Outcome parameters
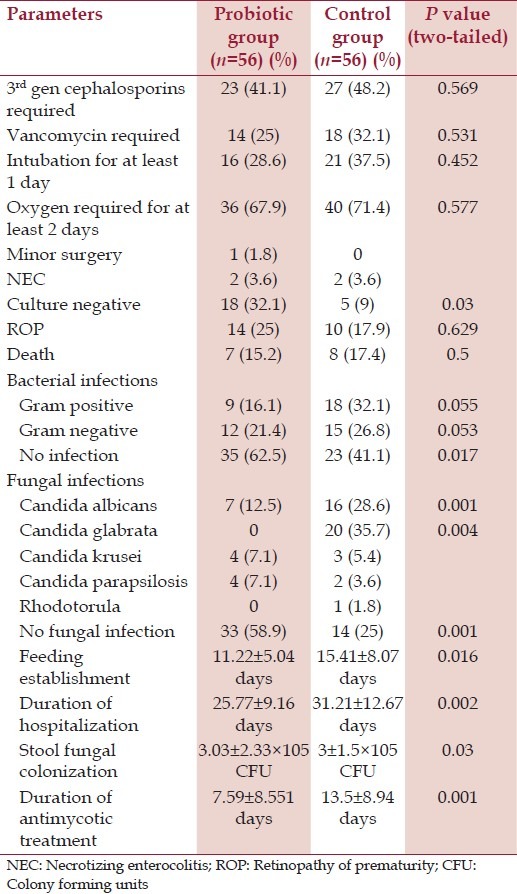
The occurrence of late onset sepsis (inclusive of fungal sepsis) is 55.4% in the probiotics group and 75% in the control group (P = 0.02). There is no significant difference between the occurrence of gram negative and gram positive infections in the two groups.
There is absence of fungal sepsis in 58.9% in the probiotics group compared to absence of fungal infection in 25% of the control group (P = 0.001). 12.5% in the probiotics group had Candida albicans infection compared to 28.6% in the control group (P = 0.001%). None of the neonates in the probiotics group suffered from C. glabrata infection, while 35.7% of the neonates in the control group suffered from C. glabrata infection (P = 0.004). No significance differences were found between the two groups in colonization rates of C. krusei and C. parapsilosis. There are no significant differences in outcome parameters of death, NEC, third generation cepahalosporin requirement, vancomycin requirement, retinopathy of prematurity, etc.
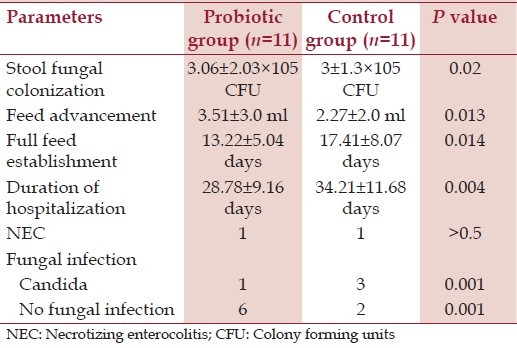
There were no gastrointestinal symptoms in 71.4% of neonates in the probiotics group and no symptoms in 30.4% of neonates (P = 0.0001). Abdominal distension was present in 10.7% of neonates in the probiotics group and 37.5% of the neonates in the control group (P = 0.001), there were no significant differences between the groups in terms of vomiting and diarrhea. The neonates under 1000 g are the most vulnerable and the outcome parameters concerning them is depicted in Table 3.
Table 3.
Outcome parameters in<1,000 g babies
Discussion
The presence of fungal colonization at various sites of the gastrointestinal tract is a well-known risk factor for subsequent dissemination of fungal sepsis in preterm neonates.[10,40] To reduce the development of invasive fungal sepsis due to Candida species it will be helpful if the gut colonization is reduced.
At birth, intestinal colonization is derived from vaginal mucoses of the mother and fecal microflora. Diet can influence microflora and in breastfed neonates gut microflora is dominated by Bifidobacteria. Probiotics have wide ranged effects including modulation of gut microflora, promoting mucosal barrier functions, inhibiting mucosal pathogen adherence, and interacting with innate and adaptive immune systems of the host. The intestinal mucosal barrier consists of the intestinal microbiota that restrict mucosal colonization by pathogens and also resist penetration by pathogens. The direct effects of probiotics on the lumen are competition with the pathogens for nutrients, production of antimicrobial substances, receptorial hydrolysis, and nitric oxide (NO); while the indirect effects are based on site of interaction of the probiotics and the effectors of immune response topographically located in the intestinal tract.[41]
Premature neonates in the NICU are highly prone to develop disorders of gut microecology with an overgrowth of pathogenic microflora including fungi,[38] as they are often treated with long courses of antibiotics and also this group experience difficulty in receiving full enteral nutrition.[7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] The gut is a reservoir site as well as a major colonization site for all types of pathogens and probably the most important site from where fungal dissemination occurs.[14,15,16,17]
Our study examined the effectiveness of probiotics (Lactobacillus acidophilus and B. longum in this case) in preventing the gastrointestinal colonization by Candida species in preterm low birth weight neonates.
The results show a significant reduction in gastrointestinal colonization by Candida species among low and VLBW preterm neonates given probiotics and gastrointestinal colonization being demonstrated by measuring stool fungal colonization. The presumed mechanisms by which the given probiotics modify the microecology including the fungal ecology in the gut are by competitive exclusion of fungi and the reduction in their ability to colonize the gut mucosa by increased mucosal IgA responses;[43] changes in intestinal permeability with an increased gut mucosal barrier to fungi and modifications of host response to fungi.[44,45] As in our study we used human milk for the neonates in both the groups, the results of the study are not attributable to the type of milk used. A study by Sims et al., described four-fold reduction in overall colonization of the gut by administering oral nystatin to preterm neonates with a birth weight of <1,250 g.[46] Studies by Kicklighter et al., and Kaufman et al., showed three-fold reduction in stool or rectal fungal colonization in neonates with birth weight of <1250 or 1000 g, respectively by administering prophylactic intravenous fluconazole.[17,18] However, the promising results obtained with fluconazole have to be weighed against the increased risk of emergence of resistant strains. Also the use of fluconazole as prophylactic treatment raises concerns about cost of the treatment.[26]
So strategies must be thought upon that reduce fungal colonization of gut with minimum cost and minimum adverse effects and minimum potential to cause emergence of drug resistant strains of fungi.
Our study shows the potential beneficial effects of probiotics on clinical and physiological variables related to gut function and probiotics administration in our study leads to earlier full feed establishment and increased rate of feeding advancement in the study group. These findings were corroborated by the findings from study by Rouge et al.,[47] Our study also showed significant reduction in the duration of hospital stay in the neonates belonging to the study group compared to the placebo group, which however is different from result by Rouge et al.,[47] but corroborated by the findings of Romeo et al.[1]
Our study showed that the incidence of infection is more in the placebo group than the probiotics group which is corroborated by studies by Lin et al., and Sims et al., and negated by findings of Rouge et al.[46,47,48] Our study shows that there is a significant increase in fungal sepsis in the placebo group compared to the study group and significant increase in the infection by C. albicans and C. glabrata infection corroborated by findings of Romeo et al.[1]
So the key features of our study were reduced enteral fungal colonization, reduced invasive fungal sepsis, earlier establishment of full enteral feeds, and reduced duration of hospital stay in the study group. Probiotics had good safety profile and did not show any side effects in preterm neonates, furthermore their use reduced gastrointestinal symptoms in the study group. Limitations of our study are that we could not compare between the different types of probiotics and we did not follow-up the neonates neurologically on long-term basis.
Conclusion
To date only a few clinical trials have reported the outcomes for preterm neonates given probiotics. Although our findings regarding fungal colonization and prevention of IFI are encouraging larger, more definitive randomized control trials are required to establish or negate the use of probiotics as an additive treatment to prevent IFI in preterm neonates.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Romeo MG, Romeo DM, Trovato L, Oliveri S, Palermo F, Cota F, et al. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J Perinatol. 2011;31:63–9. doi: 10.1038/jp.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: Trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J. 1998;17:504–8. doi: 10.1097/00006454-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk Factors For Candidemia In Neonatal Intensive Care Unit Patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19:319–24. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: Risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 6.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–7. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Schwiertz A, Gruhl B, Löbnitz M, Michel P, Radke M, Blaut M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res. 2003;54:393–9. doi: 10.1203/01.PDR.0000078274.74607.7A. [DOI] [PubMed] [Google Scholar]
- 8.Gewold IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birth weight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F167–73. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy MJ, Volz PA. Ecology of Candida albicans gut colonization: Inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985;49:654–63. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, et al. National Epidemiology of Mycoses Study Group. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20:1119–24. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Butel MJ, Suau A, Campeotto F, Magne F, Aires J, Ferraris L, et al. Conditions of bifidobacterial colonization in preterm infants: A prospective analysis. J Pediatr Gastroenterol Nutr. 2007;44:577–82. doi: 10.1097/MPG.0b013e3180406b20. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: A systematic review of randomised controlled trials. Lancet. 2007;369:1614–20. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 13.Dai D, Walker WA. Protective nutrients and bacterial colonization in the immature human gut. Adv Pediatr. 1999;46:353–82. [PubMed] [Google Scholar]
- 14.Baley JE, Kliegman RM, Boxerbaum B, Fanaroff AA. Fungal colonization in the very low birth weight infant. Pediatrics. 1986;78:225–32. [PubMed] [Google Scholar]
- 15.Slotman GJ, Saphiro R, Moffa SM. Fungal sepsis: Multisite colonization versus fungemia. Am Surg. 1994;60:107–13. [PubMed] [Google Scholar]
- 16.Ibanez-Nolla J, Nolla-Salas M, Leon MA, Garcia F, Marrugat J, Soria G, et al. Early diagnosis of candidiasis in non-neutropenic critically ill patients. J Infect. 2004;48:181–92. doi: 10.1016/s0163-4453(03)00120-8. [DOI] [PubMed] [Google Scholar]
- 17.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics. 2001;107:293–8. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–6. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 19.McGuire W, Clerihew L, Austin N. Prophylactic intravenous antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2004:CD003850. doi: 10.1002/14651858.CD003850.pub2. [DOI] [PubMed] [Google Scholar]
- 20.FAO/WHO. Health and nutrition properties of probiotics in food including powder milk with life lactic acid bacteria. . Food and Agriculture Administration of the United Nations and World Health Organization Expert Consultation Report. [Accessed August 1, 2013]. at http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf .
- 21.Mattar AF, Drongowski RA, Coran AG, Harmon CM. Effect of probiotics on enterocyte bacterial translocation in vitro. Pediatr Surg Int. 2001;17:265–8. doi: 10.1007/s003830100591. [DOI] [PubMed] [Google Scholar]
- 22.Neu J, Caicedo R. Probiotics: Protecting the intestinal ecosystem? J Pediatr. 2005;147:143–6. doi: 10.1016/j.jpeds.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Wagner RD, Pierson C, Warner T, Dohnalek M, Hilty M, Balish E. Probiotic effects of feeding heat-killed Lactobacillus acidophilus and Lactobacillus casei to Candida albicans-colonized immunodeficient mice. J Food Prot. 2000;63:638–44. doi: 10.4315/0362-028x-63.5.638. [DOI] [PubMed] [Google Scholar]
- 24.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008:CD005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Sims ME A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–6. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 26.Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonisation by candida species in preterm neonates: A randomised study. Clin Infect Dis. 2006;42:1735–42. doi: 10.1086/504324. [DOI] [PubMed] [Google Scholar]
- 27.Millar MR, Bacon C, Smith SL, Walker V, Hall MA. Enteral feeding of premature infants with Lactobacillus GG. Arch Dis Child. 1993;69:483–7. doi: 10.1136/adc.69.5_spec_no.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Shimizu T, Hosaka A, Kaneko N, Ohtsuka Y, Yamashiro Y. Effects of bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr Int. 2004;46:509–15. doi: 10.1111/j.1442-200x.2004.01953.x. [DOI] [PubMed] [Google Scholar]
- 29.Millar M, Wilks M, Costeloe K. Probiotics for preterm infants? Arch Dis Child Fetal Neonatal Ed. 2003;88:F354–8. doi: 10.1136/fn.88.5.F354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants: A prospective double-blind study. Biol Neonate. 2002;82:103–8. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande GC, Rao SC, Keil AD, Patole SK. Evidence-based guidelines for use of probiotics in preterm neonates. BMC Med. 2011;9:92. doi: 10.1186/1741-7015-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, et al. Guidelines for treatment of Candidiasis. Clin Infect Dis. 2004;38:161–89. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very low-birth-weight infant. Clin Microbiol Rev. 2004;17:638–80. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang RC, Uauy R, Koletzko B, Zlotkin SH. Scientific Basis and Pratical Guidelines. Cincinnati: Digital Educational Publishing; 2012. Nutrition of the preterm infant. [Google Scholar]
- 36.Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: A prospective randomized study. Pediatrics. 2007;119:e124–30. doi: 10.1542/peds.2006-1222. [DOI] [PubMed] [Google Scholar]
- 37.Gray JW. Surveillance of infection in neonatal intensive care units. Early Hum Dev. 2007;83:157–63. doi: 10.1016/j.earlhumdev.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Pappu-Katikaneni LD, Rao KP, Banister E. Gastrointestinal colonization with yeast species and Candida septicemia in very low birth weight infants. Mycoses. 1990;33:20–3. doi: 10.1111/myc.1990.33.1.20. [DOI] [PubMed] [Google Scholar]
- 39.Oliveri S, Trovato L, Betta P, Romeo MG, Nicoletti G. Experience with the Platelia Candida ELISA for the diagnosis of invasive candidosis in neonatal patients. Clin Microbiol Infect. 2008;14:377–3. doi: 10.1111/j.1469-0691.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang YC, Li CC, Lin TY, Lien RI, Chou YH, Wu JL, et al. Association of fungal colonization and invasive disease in very low birth weight infants. Pediatr Infect Dis J. 1998;17:819–22. doi: 10.1097/00006454-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Betta P, Vitaliti G. Intestinal Microflora-Effects of probiotics in newborns. New Adv Basic Clin Gastroenterol. 2012 [Google Scholar]
- 42.Collins MD, Gibson GR. Probiotics, prebiotics and synbiotics: Approaches for modulating the microbial ecology of gut. Am J Clin Nutr. 1999;69:1052–7. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 43.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: A randomized placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 44.Brand S, Reinecker HC. An enhanced barrier is a better defense: Effects of probiotics on intestinal barrier function. Inflammatory Bowel Diseases. 2002;8:67–9. [Google Scholar]
- 45.Caplan MS, Jilling T. Neonatal necrotizing enterocolitis: Possible role of probiotic supplementation. J Pediatr Gastroenterol Nutr. 2000;30:18–22. [PubMed] [Google Scholar]
- 46.Sims ME, Yoo Y, You H, Salminen C, Walther FJ. Prophylactic oral nystatin and fungal infections in very low birthweight infants. Am J Perinatol. 1988;5:33–6. doi: 10.1055/s-2007-999649. [DOI] [PubMed] [Google Scholar]
- 47.Rougé C, Piloquet H, Butel MJ, Berger B, Rochat F, Ferraris L, et al. Oral supplementation with probiotics in very low birth weight preterm infants: A randomized, double blind, placebo controlled trial. Am J Clin Nutr. 2009;89:1828–35. doi: 10.3945/ajcn.2008.26919. [DOI] [PubMed] [Google Scholar]
- 48.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]


